Abstract
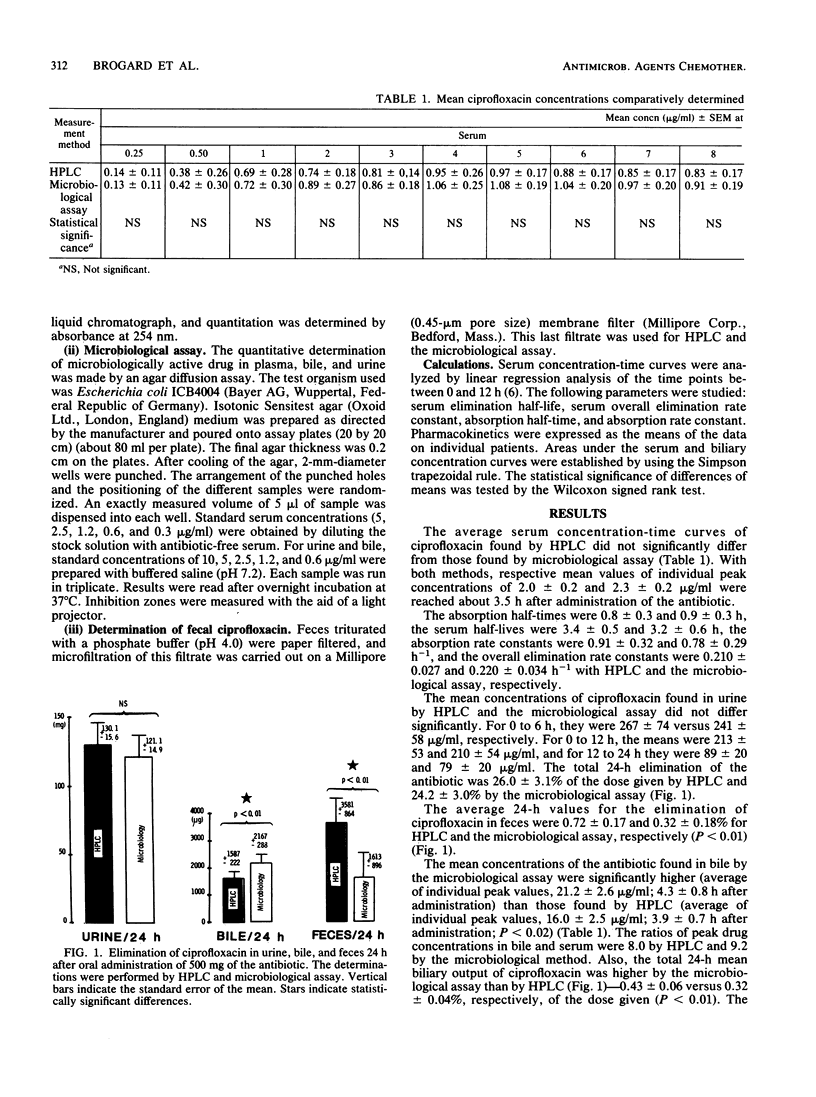
Serum kinetics and biliary, urinary, and fecal elimination of ciprofloxacin, a new quinolone derivative, were studied in 12 recently cholecystectomized patients provided with T-tube drainage during 24 h after oral administration of a single 500-mg dose of this substance. Drug concentrations were measured by both high-pressure liquid chromatography (HPLC) and microbiological assay. The results were comparable for the concentrations in serum (average of peaks, 2.0 +/- 0.2 micrograms/ml by HPLC and 2.3 +/- 0.3 micrograms/ml by the microbiological method) and urine (0 to 6 h, 267 +/- 74 and 241 +/- 58 micrograms/ml, respectively). This was not the case for biliary values, for which the microbiological assay yielded significantly higher concentrations than did HPLC (average of peak concentrations, 21.2 +/- 2.6 and 16.0 +/- 2.5 micrograms/ml, respectively [P less than 0.02]), nor for total 24-h biliary output (2,167 +/- 288 and 1,587 +/- 222 micrograms, respectively [P less than 0.01]). This suggests hepatic biotransformation of ciprofloxacin into microbiologically active metabolites. The apparent broad antibacterial spectrum of ciprofloxacin and its higher biliary levels than simultaneously determined serum concentrations suggest that this derivative is suitable for the treatment of biliary tract infections.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brogard J. M., Arnaud J. P., Blickle J. F., Lavillaureix J. Biliary elimination of apalcillin in humans. Antimicrob Agents Chemother. 1984 Sep;26(3):428–430. doi: 10.1128/aac.26.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogard J. M., Comte F., Lavillaureix J. Comparative pharmacokinetic profiles of cinoxacin and pipemidic acid in humans. Eur J Drug Metab Pharmacokinet. 1983 Jul-Sep;8(3):251–259. doi: 10.1007/BF03188755. [DOI] [PubMed] [Google Scholar]
- Brogard J. M., Kopferschmitt J., Arnaud J. P., Dorner M., La Villaureix J. Biliary elimination of mezlocillin: an experimental and clinical study. Antimicrob Agents Chemother. 1980 Jul;18(1):69–76. doi: 10.1128/aac.18.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump B., Wise R., Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983 Nov;24(5):784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fass R. J. In vitro activity of ciprofloxacin (Bay o 9867). Antimicrob Agents Chemother. 1983 Oct;24(4):568–574. doi: 10.1128/aac.24.4.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffken G., Lode H., Prinzing C., Borner K., Koeppe P. Pharmacokinetics of ciprofloxacin after oral and parenteral administration. Antimicrob Agents Chemother. 1985 Mar;27(3):375–379. doi: 10.1128/aac.27.3.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höffler D., Dalhoff A., Gau W., Beermann D., Michl A. Dose- and sex-independent disposition of ciprofloxacin. Eur J Clin Microbiol. 1984 Aug;3(4):363–366. doi: 10.1007/BF01977496. [DOI] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J., van Veldhuizen G. Comparative activities of ciprofloxacin (Bay o 9867), norfloxacin, pipemidic acid, and nalidixic acid. Antimicrob Agents Chemother. 1983 Aug;24(2):302–304. doi: 10.1128/aac.24.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUNDLE F. F., CASS M. H., ROBSON B., MIDDLETON M. Bile drainage after choledochostomy in man, with some observations on biliary fistula. Surgery. 1955 Jun;37(6):903–910. [PubMed] [Google Scholar]
- Van Caekenberghe D. L., Pattyn S. R. In vitro activity of ciprofloxacin compared with those of other new fluorinated piperazinyl-substituted quinoline derivatives. Antimicrob Agents Chemother. 1984 Apr;25(4):518–521. doi: 10.1128/aac.25.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingender W., Graefe K. H., Gau W., Förster D., Beermann D., Schacht P. Pharmacokinetics of ciprofloxacin after oral and intravenous administration in healthy volunteers. Eur J Clin Microbiol. 1984 Aug;3(4):355–359. doi: 10.1007/BF01977494. [DOI] [PubMed] [Google Scholar]
- Wise R., Lockley R. M., Webberly M., Dent J. Pharmacokinetics of intravenously administered ciprofloxacin. Antimicrob Agents Chemother. 1984 Aug;26(2):208–210. doi: 10.1128/aac.26.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]


