Abstract
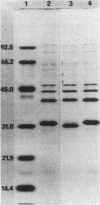
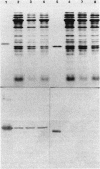
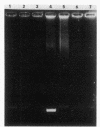
Three clinical isolates of Haemophilus ducreyi, representing at least two subtypes, were shown to be resistant to streptomycin and kanamycin. They also produced a beta-lactamase and chloramphenicol acetyltransferase and were resistant to tetracycline. In the three strains the resistance to both aminoglycoside antibiotics was encoded by a plasmid of ca. 4.7 kilobases which apparently did not carry ampicillin, chloramphenicol, or tetracycline resistance genes, as determined after transfer to Escherichia coli by transformation. Resistance to streptomycin and kanamycin was due to the presence of two aminoglycoside phosphotransferases (APH). The enzyme modifying kanamycin was a 3',5"-APH of type I [APH(3',5")-I], as inferred from its substrate profile and immunological cross-reactivity with the APH(3',5")-I encoded by the transposable element Tn903. However, the APH(3',5")-I gene in H. ducreyi did not appear to be carried by Tn903.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASIN J. Chancroid; a report of 1,402 cases. Am J Syph Gonorrhea Vener Dis. 1952 Sep;36(5):483–487. [PubMed] [Google Scholar]
- Albritton W. L., Brunton J. L., Slaney L., MacLean I. Plasmid-mediated sulfonamide resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Jan;21(1):159–165. doi: 10.1128/aac.21.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton W. L., Maclean I. W., Slaney L. A., Ronald A. R., Deneer H. G. Plasmid-mediated tetracycline resistance in Haemophilus ducreyi. Antimicrob Agents Chemother. 1984 Feb;25(2):187–190. doi: 10.1128/aac.25.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B., Albritton W. L., Biddle J., Johnson S. R. Common beta-lactamase-specifying plasmid in Haemophilus ducreyi and Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1984 Feb;25(2):296–297. doi: 10.1128/aac.25.2.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. E., Davies J., Allet B., Rochaix J. D. Transposition of R factor genes to bacteriophage lambda. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3628–3632. doi: 10.1073/pnas.72.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton J. L., Clare D., Ehrman N., Meier M. A. Evolution of antibiotic resistance plasmids in Neisseria gonorrhoeae and Haemophilus species. Clin Invest Med. 1983;6(3):221–228. [PubMed] [Google Scholar]
- Brunton J., Meier M., Ehrman N., Maclean I., Slaney L., Albritton W. L. Molecular epidemiology of beta-lactamase-specifying plasmids of Haemophilus ducreyi. Antimicrob Agents Chemother. 1982 Jun;21(6):857–863. doi: 10.1128/aac.21.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casin I. M., Sanson-Le Pors M. J., Gorce M. F., Ortenberg M., Pérol Y. The enzymatic profile of Haemophilus ducreyi. Ann Microbiol (Paris) 1982 Nov-Dec;133(3):379–388. [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Deneer H. G., Slaney L., Maclean I. W., Albritton W. L. Mobilization of nonconjugative antibiotic resistance plasmids in Haemophilus ducreyi. J Bacteriol. 1982 Feb;149(2):726–732. doi: 10.1128/jb.149.2.726-732.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffic F. L., Moreau N., Siegrist S., Goldstein F. W., Acar J. C. La resistance plasmidique de Haemophilus sp. aux antibiotiques aminoglycosidiques : isolement et etude d'une nouvelle phosphotransférase. Ann Microbiol (Paris) 1977 May-Jun;128A(4):383–391. [PubMed] [Google Scholar]
- Grindley N. D., Joyce C. M. Genetic and DNA sequence analysis of the kanamycin resistance transposon Tn903. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7176–7180. doi: 10.1073/pnas.77.12.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Hammond G. W., Slutchuk M., Scatliff J., Sherman E., Wilt J. C., Ronald A. R. Epidemiologic, clinical, laboratory, and therapeutic features of an urban outbreak of chancroid in North America. Rev Infect Dis. 1980 Nov-Dec;2(6):867–879. doi: 10.1093/clinids/2.6.867. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Briaux-Gerbaud S., Courvalin P. Tn1525, a kanamycin R determinant flanked by two direct copies of IS15. Mol Gen Genet. 1983;189(1):90–101. doi: 10.1007/BF00326060. [DOI] [PubMed] [Google Scholar]
- Labigne-Roussel A., Gerbaud G., Courvalin P. Translocation of sequences encoding antibiotic resistance from the chromosome to a receptor plasmid in Salmonella ordonez. Mol Gen Genet. 1981;182(3):390–408. doi: 10.1007/BF00293927. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Latif A. S. Thiamphenicol in the treatment of chancroid in men. Br J Vener Dis. 1982 Feb;58(1):54–55. doi: 10.1136/sti.58.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüders G., Braun J., Pietzcker F., Schüle D. Neue therapeutische Gesichtspunkte beim Ulcus molle. Hautarzt. 1975 Jan;26(1):35–40. [PubMed] [Google Scholar]
- Marmar J. L. The management of resistant chancroid in Vietnam. J Urol. 1972 May;107(5):807–808. doi: 10.1016/s0022-5347(17)61144-3. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odumeru J. A., Ronald A. R., Albritton W. L. Characterization of cell proteins of Haemophilus ducreyi by polyacrylamide gel electrophoresis. J Infect Dis. 1983 Oct;148(4):710–714. doi: 10.1093/infdis/148.4.710. [DOI] [PubMed] [Google Scholar]
- Plummer F. A., Nsanze H., D'Costa L. J., Karasira P., Maclean I. W., Ellison R. H., Ronald A. R. Single-dose therapy of chancroid with trimethoprim-sulfametrole. N Engl J Med. 1983 Jul 14;309(2):67–71. doi: 10.1056/NEJM198307143090202. [DOI] [PubMed] [Google Scholar]
- Rajan V. S., Sng E. H. Streptomycin-resistant Haemophilus ducreyi. Lancet. 1982 Nov 6;2(8306):1043–1043. doi: 10.1016/s0140-6736(82)90072-1. [DOI] [PubMed] [Google Scholar]
- Sanson-Le Pors M. J., Casin I. M., Ortenberg M., Perol Y. Detection of chloramphenicol acetyltransferase (CAT) activity in a strain of Haemophilus ducreyi. Ann Microbiol (Paris) 1982 Sep-Oct;133(2):311–315. [PubMed] [Google Scholar]
- Sanson-Le Pors M. J., Casin I., Ortenberg M., Perol Y. In-vitro susceptibility of thirty strains of Haemophilus ducreyi to several antibiotics including six cephalosporins. J Antimicrob Chemother. 1983 Mar;11(3):271–280. doi: 10.1093/jac/11.3.271. [DOI] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Sturm A. W., Zanen H. C. Drug of choice for chancroid. Lancet. 1983 Jan 15;1(8316):125–125. doi: 10.1016/s0140-6736(83)91766-x. [DOI] [PubMed] [Google Scholar]
- Taylor D. N., Echeverria P., Hanchalay S., Pitarangsi C., Slootmans L., Piot P. Antimicrobial susceptibility and characterization of outer membrane proteins of Haemophilus ducreyi isolated in Thailand. J Clin Microbiol. 1985 Mar;21(3):442–444. doi: 10.1128/jcm.21.3.442-444.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van A. D., Goldstein F., Acar J. F., Bouanchaud D. H. A transferable kanamycin resistance plasmid isolated from Haemophilus influenzae. Ann Microbiol (Paris) 1975 Apr;126(3):397–399. [PubMed] [Google Scholar]