Abstract
Dysregulation of apoptosis through the Fas–Fas ligand pathway is associated with the onset of autoimmune disease. Since autoantibodies directed against unknown antigens are present in the sera of these patients, sera samples were examined for the presence of autoantibodies directed against the Fas molecule. Using Western blotting and a ProteinChip analysis, autoantibodies against Fas were detected in patients with silicosis, systemic lupus erythematosus (SLE) and systemic sclerosis (SSc), and weakly detected in healthy individuals. Using epitope mapping employing 12-amino-acid polypeptides with the SPOTs system, a minimum of four epitopes and a maximum of 10 epitopes were found. Several amino acid residues involved in binding FasL, such as C66, R87, L90, E93 and H126, were presented within the epitopes. Serum containing a large amount of anti-Fas autoantibody from silicosis patients inhibited the growth of a Fas-expressing human cell line, but did not inhibit the growth of a low Fas-expresser nor a Fas-expresser in which the Fas gene had been silenced by small interference RNA. All epitopes in the intracellular region of Fas were located in the death domain. The possible roles of anti-Fas autoantibody detected in healthy volunteers and patients with silicosis or autoimmune diseases are discussed here.
Keywords: anti-Fas autoantibody, autoimmune diseases, silicosis, siRNA
Introduction
Disruption of apoptosis through the Fas–Fas ligand (FasL) pathway is related to the onset of autoimmune disease. Previously, we reported elevated serum levels of soluble Fas (sFas) and dominant expression of sFas message in patients suffering from silicosis (SIL), systemic sclerosis (SSc) and systemic lupus erythematosus (SLE), and proposed that the blocking of apoptosis is involved in the pathogenesis of these diseases.1,2
Since autoantibodies against unknown antigens are present in the serum of these patients, their sera have been analysed for autoantibodies directed against the Fas molecule, and here we report the detection of anti-Fas autoantibodies. In previous studies, we found autoantibodies directed against DNA topoisomerase I,3 desmogleins4 and caspase-8.5
The SPOTs system is a technology that allows the solid-phase synthesis of an extensive series of short peptides for the systematic analysis of antibody epitopes.6 ProteinChip surface enhanced laser desorption/ionization (SELDI) mass spectrometry can be used to detect a few femtomoles of a specific protein from a crude solution and determine its molecular mass with an error rate of less than 0·2%.7 Recombinant human Fas was linked to the PS2 chips (the surface of the PS2 chip contains an epoxy surface which covalently reacts with amine and thiol groups; Cipahgen Biosystems, Fremont, CA) then autoantibodies were captured from the serum samples and detected.
In addition to the detection and epitope-mapping of the anti-Fas autoantibody, we examined the function of the anti-Fas autoantibody derived from the serum of an SIL patient using Fas-expressing and non-expressing sister human myeloma lines, both of which were established from the same myeloma patient.
Patients and methods
Patients and sera
Serum samples were obtained from 52 patients with SIL (age range 52–84 years, mean ± standard deviation 67·8 ± 6·9 years; male (M) : female (F) ratio 46 : 6) with no clinical symptoms of autoimmune diseases such as sclerotic skin, Raynaud's phenomenon, facial erythema or arthralgia; 15 patients with SLE (18–60 years, mean 41·1 ± 12·1 years, M : F = 2 : 13, 11 out of 14 had been treated using steroids); 15 patients with SSc (14–67 years, 56·2 ± 14·0 years, all females, four out of 14 had been treated using steroids); and 10 healthy volunteers (HV) (22–72 years, 50·4 ± 18·4 years, M : F = 4 : 6). Specimens were taken only in cases where informed consent had been obtained. The criteria for diagnosis of SIL, SLE, and SSc were in accordance with the International Labor Office (ILO) 2000 International classification of radiographs of pneumoconiosis,8 the 1982 revised criteria for the classification of systemic lupus erythematosus,9 and the preliminary criteria for the classification of systemic sclerosis (scleroderma),10 All the SIL patients were workers from the brickyards in Bizen City and Hinase in Okayama prefecture, Japan.
Western blot analysis
Human Fas linked to glutathione-s-transferase (Fas, Intracellular Death Domain, Human, Recombinant, Escherichia coli; Calbiochem, La Jolla, CA) was separated using 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis. Proteins were electrotransferred to polyvinylidene difluoride membranes (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, UK), and then saturated with Block Ace (Dainihon Pharm. Co., Osaka, Japan) overnight at 4°. After washing with 0·1% Tween 20 in phosphate-buffered saline (Tween-PBS), the blots were cut into pieces (testing strips) and incubated at room temperature for 1·5 hr with a 1/100 dilution of serum samples. The strips were washed with Tween-PBS and incubated at room temperature for 1 hr with a 1/15 000 dilution of horseradish peroxidase (HRP)-conjugated sheep anti-human immunoglobulin (Amersham Pharmacia Biotech). After further washing, the bound antibodies were detected by the enhanced chemiluminescence method using ECL Plus Western blotting detection reagents (Amersham Pharmacia Biotech). The ratio of the intensity of the detected band in patients was calculated relative to that of a healthy volunteer whose serum was analysed as a background standard at the same time as the other specimens. Band intensities were calculated using an IBAS 2000 image analysis system (Zeiss-Kontron, Munich, Germany). The cut-off point among the calculated relative ratios was determined by drawing a receiver operating characteristic curve, and a ratio greater than 1·2 was considered positive.
Autoantibody immunoglobulin subclass specificity
The immunoglobulin subclass specificity of the autoantibodies in patients was determined by Western blotting using the sera of anti-Fas positive patients and HRP-conjugated sheep anti-human immunoglobulin G1 (IgG1), IgG2, IgG3, IgG4, IgM, or IgA (The Binding Site Limited, Birmingham, UK) at the same time, followed by detection using enhanced chemiluminescence. The intensity of each band and each background was calculated. Then, the relative intensity of each immunoglobulin subclass in each patient was calculated relative to the total amount obtained from the six bands of the immunoglobulin subclasses.
SELDI ProteinChip analysis
ProteinChip surface enhanced laser desorption/ionization (SELDI) mass spectrometry was performed to detect and quantify the autoantibodies captured on the antigencoated ProteinChip arrays.7 NP (normal phase) and PS2 (epoxy) ProteinChips® (Ciphagen Biosystems, Fremont, CA) were used for the analysis. The protocol details for individual arrays were based on the manufacturer's recommendations. Briefly, Fas (450 ng/2 μl/spot) was loaded on the spots of a pre-activated SELDI ProteinChip and incubated at room temperature for 1 hr. The chip was blocked at room temperature for 20 min with 1 m ethanolamine and washed three times with 0·5% Triton X-100 in PBS. Then, 6 μl serum sample was applied to the spots and the chips were incubated overnight at 4°. The whole chip array was washed three times with 0·1% Triton X-100 in PBS, then twice with distilled water, and air dried. Next, 0·5 μl saturated sinapinic acid (an energy absorbing molecule) in 50% acetonitrile containing 0·5% trifluoroacetic acid was applied. Following a final air-drying, the chip was analysed using the SELDI ProteinChip System (PBS-II, Ciphagen Biosystems). A rabbit polyclonal anti-human Fas IgG (FL-335, Santa Cruz Biotechnology, Santa Cruz, CA) was used as a positive control. The data were normalized with internal standards before making a comparison between the spots.
Epitope mapping
Oligopeptides were synthesized on activated membranes using the SPOTs system (Sigma-Genosys, The Woodlands, TX).6 The peptides were 12 amino acids in length and had a sequential overlap of nine amino acids. The SPOTs spanned the Fas amino acid sequence from residues 1–319. The membranes with immobilized peptides attached were blocked at room temperature for 8 hr in 10% concentrated blocking buffer (Sigma-Genosys) dissolved in Tris-buffered saline containing 0·05% Tween 20 (TBS-T). After three washes of 10 min each with TBS-T, the SPOTs were incubated overnight at 4° with a 1/50 dilution of serum samples. After further washing, the bound antibodies were detected using the secondary antibody β-galactosidase-conjugated goat anti-human immunoglobulin (American Qualex Antibodies, San Clemente, CA) and the substrate 5-bromo-4-chloro-3-indoyl-β-d-galactopyranoside, according to the manufacturer's instructions.
Cell lines
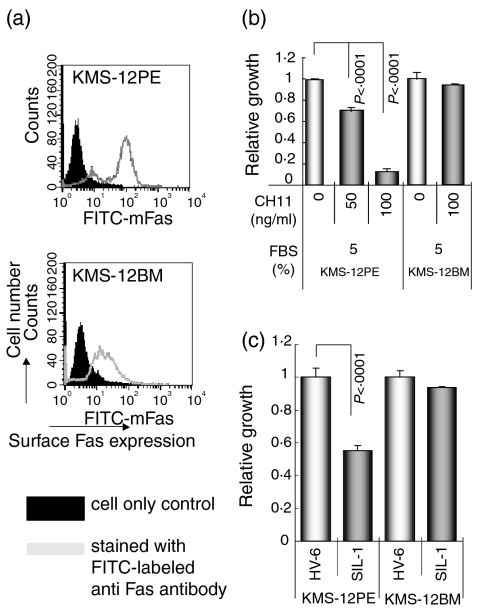
Two human sister myeloma cell lines designated KMS-12PE and KMS-12BM were established from the pleural effusion (PE) and bone marrow (BM) of the same patient.11 Both lines showed the same IgH rearrangement and the same chromosomal marker including t(11;14) (q13;q32) with overexpression of cyclin D1.12 However, as we reported11,12 several cellular biological differences such as sensitivity to anti-myelomatous agents, surface expression of several CD markers such as 7, 11a, 20, 27 and 95/Fas, and apoptosis-related protein expression were found between the two lines. One of these differences was in CD95/Fas surface expression. KMS-12PE showed a high level of expression and KMS-12BM demonstrated a very low level of expression.13 To confirm this finding, both cell lines were incubated with fluorescein isothiocyanate-labelled anti-human Fas antibody (MBL Co., Nagoya, Japan) for 30 min at room temperature in the dark, washed with PBS containing 0·1% sodium azide, and analysed by flow cytometry. The original patients for both lines, HV-6 and SIL-1, whose sera were used in the in vitro assay were of the same ABO blood type.
Culture with CH11, Fas-stimulating anti-Fas antibody, and serum from HV or SIL
The Fas-expressing KMS-12PE cells and low Fas-expressing KMS-12BM cells were cultured with or without 50 or 100 ng/ml of CH11 (anti-human Fas antibody, which stimulates Fas-mediated apoptosis, MBL Co.)14 in RPMI-1640 medium plus 5% fetal bovine serum. After 2 days, cell growth was estimated with a WST-1 Proliferation Assay System (Takara Biochem., Tokyo, Japan) as reported previously.15 Briefly, cells were applied to a Premix water-soluble tetrazolium salt, 2-(4-iodophenyl)-3(4-nitrophenyl)-5-(2,4-disulphophenyl)-2H-tetrazolium, a monosodium salt (WST-1), and were cultured for the final 4 hr. Then, the absorbance (A450nm to A600nm) of Formosan, which is the product of the reduction of WST-1 by mitochondrial dehydrogenase, was measured by a microplate reader and cell growth was determined as a percentage of the control.
Small interference (si) RNA, RNA extraction, cDNA synthesis, multiplex-reverse transcription-polymerase chain reaction (MP-RT-PCR)
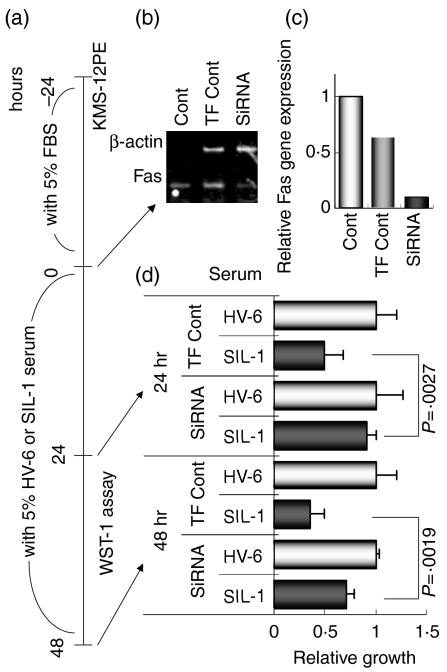
To clarify whether the growth inhibition found in Fas-expressing KMS-12PE cells, but not low Fas-expressing KMS-12BM cells, caused by SIL-patients' serum, but not HVs' serum, was mediated by the Fas molecule, the siRNA procedure was employed to silence the Fas gene in KMS-12PE cells. KMS-12PE cells were cultured with RPMI-1640 medium plus 5% fetal bovine serum with control medium (no transfection), transfection control medium (TransIT-TKO transfection reagent, Mirus, Madison, WI) (i.e. transfection performed without siRNA) or siRNA medium [i.e. transfection performed with siRNA for the Fas gene (GUGGAAAUAAACUGCAUUU(TT), TAKARA BIO Inc., Tokyo, Japan] according to the manufacturer's protocol at −24 hr. At time 0, cells were washed with PBS and resuspended in RPMI-1640 medium plus 5% serum derived from HV-6 or SIL-1 (whose serum contained a large amount of anti-Fas autoantibody) with either control, transfection control, or siRNA medium for 48 hr. At the same time, cells were harvested and total RNA was extracted using RNA-Bee reagent (Tel.Test Inc., Friendswood, TX). RNA extraction, cDNA synthesis, and MP-RT-PCR were performed as described previously.15 The primers for Fas and β-actin, the housekeeping control gene, were as follows; (Fas; forward: TTCACTTCGGAGGATTGCTC, reverse: GGCTTATGGCAGAATTGGCC, size of amplicon: 212 base pairs; β-actin; forward: TGACGGGGTCACCCACACTGTGCCCATCTA, reverse: CTAGAAGCATTTG CGGTGGACGATGGAGGG, size; 661 base pairs). The ratio and number of PCR cycles were determined to amplify both products logarithmically and in relatively similar amounts. The procedure followed for MP-RT-PCR was also reported previously. After visualization of the MP-RT-PCR products electrophoresed on a 1·2% agarose gel stained with ethidium bromide, gel images were obtained using a FAS-II UV-image analyser (TOYOBO Co. Ltd, Tokyo, Japan), and the densities of the products were quantified using Quantity One™ version 2·5 (PDI Inc., Huntington Station, NY). The relative Fas gene expression in individual samples was calculated as the density of the product of that gene divided by that of the β-actin gene derived from the same MP-RT-PCR as a control culture being 1·0. Thereafter, the WST-1 assay was employed every 24 hr to estimate whether or not siRNA for the Fas gene rescues the growth inhibition induced by serum from the SIL-1 patient.
Statistical analysis for WST-1 assay
The growth inhibitory effects of CH11 and serum from the SIL-1 patient on two human myeloma cell lines and the rates of recovery from the growth inhibition when KMS-12PE cells were cultured with transfection control or siRNA medium supplemented with serum derived from HV-6 or SIL-1 patients were analysed using Fisher's protected least significant difference (PLSD) test.
Results
Detection of autoantibodies against human Fas by Western blotting
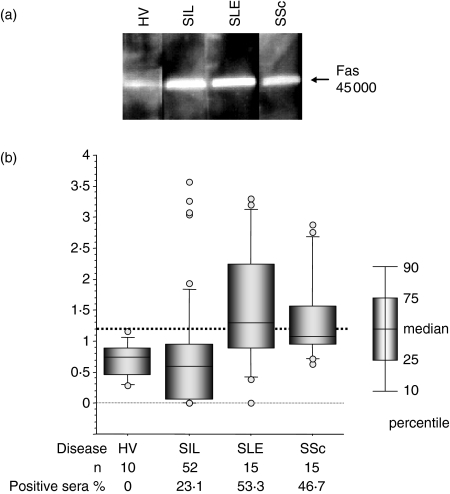
As shown in Fig. 1(a), anti-Fas autoantibodies were detected in the sera of patients with SIL, SLE and SSc, as well as in HV. The percentage of positive (≥ 1·2) sera in patients with SIL, SLE and SSc was 23·1%, 53·3% and 46·7%, respectively (Fig. 1b). The positive sera from the patients with high relative ratios (four SIL, three SLE and three SSc) were used for the SELDI ProteinChip analysis, epitope mapping and analysis of the autoantibody immunoglobulin subclass. In addition, the sera from HV-6 and SIL-1 were used for in vitro Fas-functional assays.
Figure 1.
Detection of anti-Fas autoantibodies by Western blot analysis. (a) Human Fas linked to glutathione-s-transferase was separated by 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis and electrotransferred to a polyvinylidene difluoride membrane. The blots were incubated with sera (1/100 dilution) from either a healthy volunteer (HV) or patients with silicosis (SIL), systemic lupus erythematosus (SLE) or systemic sclerosis (SSc). The blot was then incubated with horseradish peroxidase-conjugated sheep anti-human immunoglobulin (1/15 000 dilution). The bound antibodies were detected using the enhanced chemiluminescence method. (b) The ratio of the intensity of the detected band in the patient samples was calculated relative to that of a healthy volunteer (HV) analysed at the same time. The cut-off point was determined to be 1·2 by the receiver operating characteristic curve (indicated by the thick dotted line). The percentage of positive sera for each patient is also shown. Open circles represent outlier values.
Capture of anti-Fas autoantibodies by ProteinChip
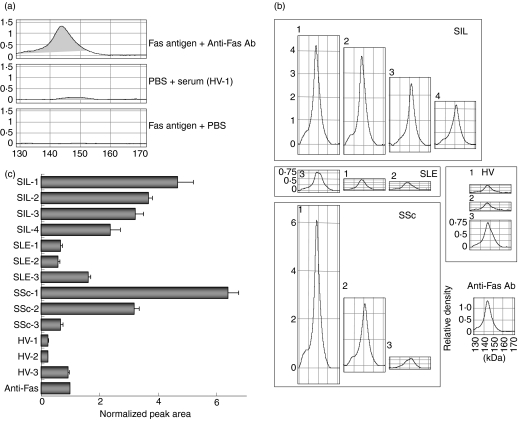
The autoantibodies were examined using SELDI ProteinChip analysis.7 Recombinant human Fas antigen was linked to the NP1 chip, and a purity check of the antigen was performed (data not shown). Then, Fas antigen was linked to the PS2 chip, and incubated with rabbit polyclonal anti-human Fas IgG. The chip was washed and treated with the energy-absorbing molecule sinapinic acid, and analysed with the SELDI ProteinChip system. Captured antibodies were detectable, and the average molecular weight of the IgG in the main peak was about 144 000 (Fig. 2a). No non-specific binding was found in the treatment with PBS (without Fas antigen) and serum of HV-1 or with Fas antigen and PBS (Fig. 2a). The peak detected by treatment with PBS and a serum sample was used as the background. Then, the sera from HV, SIL, SLE and SSc patients (three, four, three and three samples, respectively) were analysed for anti-Fas autoantibodies using PS2 chips. Autoantibodies directed against recombinant human Fas antigen were captured on the PS2 chips (Fig. 2b). Anti-Fas autoantibodies with MW about 148 000 were found in eight serum samples (SIL-1, SIL-2, SLE-1, SLE-2, SSc-1 and HV-1, HV-2, HV-3). Anti-Fas autoantibodies of about 152 000 MW were also found in three serum samples (SIL-3, SIL-4 and SSc-2). Moreover, anti-Fas autoantibodies of both 148 000 and 152 000 MW were found in two serum samples (SLE-3 and SSc-3). To make a comparison between the samples, the integrated value of the peak area at 130 000–170 000 for each sample was calculated (Fig. 2c). The anti-Fas autoantibodies were detected in greatest amounts in the sera of SIL or SSc, and moderate amounts were detected in SLE patients. Anti-Fas autoantibodies were also observed in HV, but only in small amounts. These findings are consistent with the results obtained by Western blotting.
Figure 2.
Surface enhanced laser desorption/ionization (SELDI) ProteinChip analysis of anti-Fas autoantibodies. (a) Human Fas antigen (450 ng/2 μl/spot) was loaded on the spots of a preactivated SELDI ProteinChip and incubated at room temperature for 1 hr. The chip was blocked with 1 m ethanolamine and incubated with rabbit anti-human Fas polyclonal antibody as a positive control (top panel) overnight at 4°. The whole chip array was treated with saturated sinapinic acid (an energy absorbing molecule), and analysed using the SELDI ProteinChip System. To clarify whether non-specific binding is found under the same conditions, another chip was treated with PBS (without Fas antigen) and serum of a healthy volunteer (HV-1) (middle panel), or with Fas antigen and PBS (bottom panel). (b) ProteinChip arrays were treated in the same way as in (a), except that the arrays were incubated with human sera instead of rabbit antibody. The x-axes in each figure indicate a molecular weight of 130 000–170 000, and the y-axes show the relative density of the arrays. The molecular weight of the antibody in serum was determined from the peak of the reacted density. Antibodies of approximately 148 000 MW in eight serum samples (SIL-1, SIL-2, SLE-1, SLE-2, SSc-1 and HV-1, HV-2, HV-3), 152 000 in three serum samples (SIL-3, SIL-4 and SSc-2), and two molecular weight antibodies, 148 000 and 152 000, in two serum samples (SLE-3 and SSc-3) were found. (c) The integrated value of the 130 000–170 000 peak area for each sample was calculated from the graph obtained in (b) and was normalized against that of the positive control.
Identification of amino acids involved in antibody
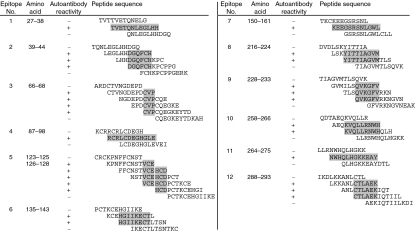
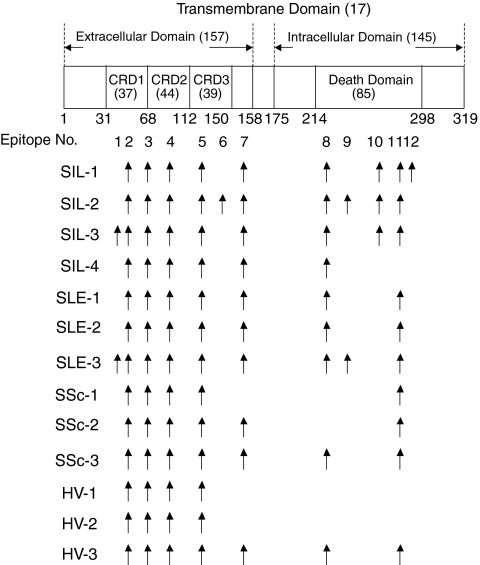
Twelve epitopes in total were recognized by the autoantibodies (Fig. 3). The locations of each epitope on the Fas molecule and the reactive autoantibodies are summarized in Fig. 4. A large number of epitopes were recognized by the autoantibodies derived from patients, specifically SIL compared with HV. Most of the epitopes in the extracellular domain were localized to the cysteine-rich domain (CRD1, -2, -3). Epitopes 3, 4 and 5 included amino acid residues of Fas, C66; R87, R89, L90, E93 and G94; V123 and H126, respectively. All of these residues were assumed to be involved in the binding of FasL to the Fas molecule. In addition, all epitopes in the intracellular domain (epitopes 8–12) were localized to the death domain. Epitopes 10 and 12 were recognized only in SIL, and epitope 9 was recognized only in SIL or SLE patients, and not in SSc patients or HV.
Figure 3.
Epitope mapping of autoantibodies directed against human Fas. Oligopeptides were synthesized on activated membranes using the SPOTs system. The peptides were 12 amino acids in length and had a sequential overlap of nine amino acids. The membranes were blocked, incubated with 1/50 diluted sample sera, then detected using β-galactosidase-conjugated goat anti-human immunoglobulin antibody. Hatched sequences indicate each epitope recognized by the autoantibodies.
Figure 4.
The location of each epitope on the Fas molecule recognized by the anti-Fas autoantibodies in sera from patients with silicosis (SIL), systemic lupus erythematosus (SLE), systemic sclerosis (SSc) and healthy volunteers (HV). The numbers with and without parentheses on the Fas molecule indicate the number of amino acids within the specified domains and the amino acid positioning of the domains within the Fas molecule, respectively. CRD, cysteine-rich domain.
Autoantibody immunoglobulin subclass specificity
The main immunoglobulin class of anti-Fas autoantibodies in SIL, SLE or SSc was found to be IgG, and that in HV was IgM or IgA, although IgG, IgM and IgA could be detected in all cases (data not shown). The major IgG subclass in SIL was IgG1, and that in SLE and SSc patients was IgG1 or IgG2 (data not shown).
Growth inhibition induced by serum from SIL-1 in Fas-expressing KMS-12PE but not low Fas-expressing KMS-12BM cells
As shown in Fig. 5(a), and in previous reports,11–13 KMS-12PE cells show a high level of surface Fas expression; however, KMS-12BM cells, a sister line of KMS-12PE, demonstrate a very low level of expression. To confirm these findings, the Fas-stimulating and Fas-mediated apoptosis-inducing anti-Fas antibody CH1114 was added to the cultures of both lines. As shown in Fig. 5(b), CH11 antibody reduced the growth of Fas-expressing KMS-12PE cells, but not that of KMS-12BM cells. In addition, the serum derived from the SIL-1 patient, which contained a large amount of anti-Fas autoantibody as detected by Western blotting and ProteinChip analyses, also reduced KMS-12PE cell growth as compared with the HV-6 serum; however, the growth of KMS-12BM cells was unaffected by both HV-6 and SIL-1 sera (Fig. 5c).
Figure 5.
(a) Surface Fas expression levels of KMS-12PE and KMS-12BM human myeloma sister cell lines. The filled histogram indicates the cell only control and the open histogram indicates cells stained with fluorescein isothiocyanate-labelled anti-Fas monocloal antibody. (b) The relative growth of KMS-12PE and KMS-12BM cells cultured with RPMI-1640 medium plus 5% FBS with or without (control) the Fas-mediated apoptosis inducing CH11 antibody (50 and 100 ng/ml) as analysed by the WST-1 assay with the control value being set at 1·0. Although growth of the Fas-expressing KMS-12PE cell line was inhibited by CH11, growth of KMS-12BM cells showed no change. (c) KMS-12PE and KMS-12BM cell lines were cultured with serum from HV-6 or SIL-1 (the SIL-1 serum contained a large amount of anti-Fas autoantibodies as analysed by Western blotting and ProteinChip System). The serum from SIL-1 inhibited growth of KMS-12PE, a Fas-expresser, but not KMS-12BM, a low Fas-expresser.
siRNA for Fas gene rescues the growth inhibition of KMS-12PE caused by SIL-1 patient's serum
Figure 6(a) shows the timing of the experiments carried out to determine whether or not silencing of the Fas gene by siRNA rescues the growth inhibition induced by the SIL-1 patient's serum in Fas-expressing KMS-12PE cells. Gene silencing was estimated to have occurred by time 0, i.e. 24 hr after cultivation of KMS-12PE cells with RPMI-1640 medium plus 5% fetal bovine serum with either control, transfection control, or siRNA medium. As shown in Fig. 6(b) (an actual gel image of MP-RTR-PCR) and Fig. 6(c) (Fas gene expression relative to the control culture value of 1·0), although the transfection control reduced Fas gene expression slightly, siRNA greatly reduced expression in KMS-12PE cells. Thereafter, KMS-12PE cells, cultured with either transfection control or siRNA medium, were continuously cultured with HV-6 or SIL-1 serum together with the same transfection control or siRNA medium and the WST-1 assay was employed to monitor cell growth. Figure 6(d) demonstrates that cultivation with siRNA medium plus SIL-1 serum overcomes the growth inhibition observed when KMS-12PE cells are cultured in transfection control medium plus SIL-1 serum for either 24 or 48 hr. These results suggest that the anti-Fas autoantibody detected in the serum of SIL functions as a Fas-mediated apoptosis-inducing antibody similar to CH11. This is in agreement with the epitope mapping studies which indicated that most of the extracellular epitopes recognized by SIL-1 were involved in binding Fas and FasL.
Figure 6.
(a) Time schedule of siRNA experiments. Details are given in the Materials and methods. (b) The gel image of MP-RT-PCR for the Fas gene in KMS-12PE cells cultured for −24–0 hr with RPMI-1640 medium supplemented with 5% fetal bovine serum with (TF Cont) or without (Cont) transfection control medium or siRNA medium. (c) The relative Fas gene expression level was analysed from the gel-image shown in (b). Although the TF Cont showed a slight reduction, the siRNA sample demonstrated a marked decrease in Fas gene expression. (d) After harvesting RNA samples, each group of cells was resuspended in the appropriate medium containing 5% serum from either HV-6 or SIL-1. After 24 and 48 hr, the WST-1 assay was performed. At both time points, silencing the Fas gene led to a statistically significant recovery of the SIL-1 serum-induced growth inhibition.
Discussion
A significant finding of the present study is that the anti-Fas autoantibodies characterize the recognized amino acid residues involved in binding FasL, and function as Fas-mediated apoptosis-inducing antibodies. The extracellular portion of Fas contains three cysteine-rich domains (CRD1, -2, -3) that appear to be necessary for the binding of FasL.16–19 The replacement of two amino acid residues, R86 and R87 in CRD2, with serine totally abolished the interaction of Fas with FasL.16 In addition, mutations of four amino acid residues, K84, L90 and E93 in CRD2 and H126 in CRD3, to serines reduced the Fas–FasL binding activity.16 Moreover, the change of C66 in CRD1 to arginine led to a loss of binding to FasL.19 In another study, the residues of human Fas involved in binding FasL were predicted to be F81, S82, S83, R89 and G94 in CRD2, and Y122 and V123 in CRD3.17 In the present study, we found that epitopes 3, 4 and 5 included several amino acid residues involved in binding FasL, such as C66 in CRD1, R87, R89, L90, E93 and G94 in CRD2, and V123 and H126 in CRD3. Two additional sequences of Fas (amino acids 40–59 in CRD1 and amino acids 130–149 in CRD3) can bind to the CH-11 monoclonal antibody.20 The cysteine residues in these peptides were reported to be important for anti-Fas monoclonal antibody CH-11 binding.21 Amino acids 39–44 (epitope 2) and amino acids 135–143 (epitope 6) within these peptides were recognized by the anti-Fas autoantibodies detected in our experiments. Epitopes 2 and 6 include cysteine residues (C43 and C141), which suggests the importance of these residues for antibody binding. Therefore, anti-Fas autoantibodies directed against these epitopes may induce the Fas-triggered apoptosis. Certainly, the SIL serum that contained the largest amount of anti-Fas autoantibody reduced the growth of Fas-expressing KMS-12PE cells, but not of low Fas-expressing KMS-12BM cells, or KMS-12PE cells whose Fas gene expression was silenced by siRNA.
All epitopes detected by autoantibodies in the intracellular region of Fas (epitope 8–12) were located in the death domain. Signalling for apoptosis involves the self association of a conserved death domain of Fas22–24 and interaction with another protein, FADD.25 The residues of the Fas death domain (Fas-DD), R234 (in helix α2), V238 (between α2 and α3), E240 and D244 (in α3) are involved in the self association and binding to FADD.25 Although epitope 9 contained most of the helix α2 amino acids (i.e. 228–233) in the present study, the other residues mentioned above were not recognized by the anti-Fas autoantibodies. Charged residues are present in Fas-DD26 suggesting the importance of electrostatic interactions involving the death domain. Seven of these residues, K230, K258, K271, K272, E273, E292 and K293 were included in epitopes 9–12. In addition, four residues in the TNF-R1 death domain are conserved in Fas (F232, V238, W265 and I294), which will probably alter the protein structure when mutated.25 Epitope 9 included F232, and epitopes 10 and 11 contained W265. Therefore, the anti-Fas autoantibodies directed against these epitopes may affect the self association of Fas-DD, in addition to the Fas–FADD interaction.
Sera from 26% of patients with sporadic amyotrophic lateral sclerosis induced apoptosis in a human neuroblastoma cell line, and most contained anti-Fas autoantibodies belonging to both the IgG and IgM classes.27 Apoptosis-inducing anti-Fas antibodies were also detected in therapeutic preparations of normal human IgG with reactivity toward both the extracellular and intracellular regions of Fas.28 This evidence is consistent with the present results that the anti-Fas autoantibodies recognized the epitopes in both the extracellular and intracellular domains, and most of the autoantibodies belong to the IgG class, and certainly these autoantibodies can induce apoptosis.
It is well known that a properly functioning immune system is dependent on programmed cell death at virtually every stage of lymphocyte development and activity. Apoptosis of peripheral T cells during the down phase of an immune response is a critical feature to maintain homeostasis. Two major pathways have been identified so far: a death receptor-dependent pathway, triggered upon repeated antigen stimulation, and a death receptor-independent pathway, which is induced upon cytokine deprivation. A number of effector molecules interfering with apoptotic pathways have been described. In addition, the majority of antigen-specific T cells are eliminated to maintain the homeostasis of the T-cell population. However, a certain number of antigen-specific T cells may survive, forming a pool of memory T cells. Elimination of T cells during the termination phase of an immune response is called activation-induced cell death (AICD) and occurs by apoptosis. Regarding these circumstances, it may be difficult to define the physiological role of the anti-Fas autoantibodies detected in the healthy volunteers (HV). Production of an anti-Fas autoantibody may be triggered when antigen-presenting cells encounter the Fas molecule derived from T cells after AICD. Then, the produced anti-Fas autoantibody could function as one of the inducible factors for the death-receptor-dependent apoptotic pathway during the down-phase of an immune response to maintain homeostasis. However, as the amounts of anti-Fas autoantibody found in the HV in our study were relatively low, their effect in the regulation of homeostasis may be weak when compared with the amount of death-pathway-activating molecules such as Fas ligand during repeated antigen stimulation.
If the HV have a certain amount of anti-Fas autoantibody, the pathological roles of the increased amounts of this autoantibody found in SIL and patients with autoimmune diseases as investigated in this study may be important. Our results showed that only significant amounts of anti-Fas autoantibody can trigger Fas-mediated apoptotic signal transduction and subsequent apoptosis in surface Fas-expressing cells. If so, and if the patients with SIL and autoimmune diseases contain higher numbers of Fas-expressing self-recognizing T cells in their bodies, these cells may be killed by self-anti-Fas autoantibody and undoubtedly pathological progression will be terminated by a decrease in self-recognizing T cells.
However, it should be emphasized that patients with SIL showed increased levels of soluble Fas (sFas) in the serum and higher sFas mRNA expression levels in peripheral blood mononuclear cells when compared with HV.1,2 These phenomena have also been reported in patients with autoimmune diseases. In addition, the lymphocytes in SIL patients showed a higher percentage of surface FasLow cells than HV, even though the total positivity of surface Fas including FasLow and FasHigh fractions showed no significant differences between SIL patients and HV.1 The higher levels of expression and translation of sFas in T cells may result in relatively reduced numbers of surface Fas molecules and consequently a reduction in the number of FasHigh T cells and an increase in the FasLow fraction. Thus, the self-anti-Fas autoantibody may not be able to react with a high number of Fas-expressing T cells in SIL patients and this may cause other progressive steps of dysregulation of autoimmunity.
The present study provides evidence that anti-Fas autoantibodies occur in patients with SIL, SLE and SSc, in addition to HV. At least 12 epitopes on the Fas molecule were recognized by the autoantibodies. The epitopes in the extracellular domain included several amino acids involved in binding FasL, and all epitopes in the intracellular domain were located in the death domain. These findings represent, to our knowledge, the first mapping of epitopes recognized by anti-Fas autoantibodies. In addition, these anti-Fas autoantibodies act to stimulate Fas-triggered apoptosis. Future studies are required to elucidate the physiological and pathological roles of the anti-Fas autoantibodies found in HV and also in diseases in which immune tolerance has been dysregulated.
Acknowledgments
This work was supported by Kawasaki Medical School Grants (#13–402), and by a Grant-in-Aid (#11670355) for Scientific Research from the Ministry of Education, Science, Sports and Culture, Japan.
References
- 1.Tomokuni A, Aikoh T, Matsuki T, et al. Elevated soluble Fas/APO-1 (CD95) levels in silicosis patients without clinical symptoms of autoimmune diseases or malignant tumours. Clin Exp Immunol. 1997;110:303. doi: 10.1111/j.1365-2249.1997.tb08332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otsuki T, Sakaguchi H, Tomokuni A, et al. Soluble Fas mRNA is dominantly expressed in cases with silicosis. Immunology. 1998;94:258. doi: 10.1046/j.1365-2567.1998.00509.x. 10.1046/j.1365-2567.1998.00509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tomokuni A, Otsuki T, Sakaguchi H, Isozaki Y, Hyodoh F, Kusaka M, Ueki A. Detection of anti-topoisomerase I autoantibody in patients with silicosis. Environ Health Prev Med. 2002;7:7. doi: 10.1007/BF02898059. 10.1265/ehpm.2002.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueki H, Kohda M, Nobutoh T, et al. Antidesmoglein autoantibodies in silicosis patients with no bullous diseases. Dermatology. 2001;202:16. doi: 10.1159/000051578. 10.1159/000051578. [DOI] [PubMed] [Google Scholar]
- 5.Ueki A, Isozaki Y, Tomokuni A, et al. Intramolecular epitope spreading among anti-caspase-8 autoantibodies in patients with silicosis, systemic sclerosis and systemic lupus erythematosus, as well as in healthy individuals. Clin Exp Immunol. 2002;129:556. doi: 10.1046/j.1365-2249.2002.01939.x. 10.1046/j.1365-2249.2002.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frank R. Spot synthesis. an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron. 1992;48:9217. 10.1016/S0040-4020(01)85612-X. [Google Scholar]
- 7.Austen BM, Frears ER, Davies H. The use of seldi proteinchip arrays to monitor production of Alzheimer's betaamyloid in transfected cells. J Pept Sci. 2000;6:459. doi: 10.1002/1099-1387(200009)6:9<459::AID-PSC286>3.0.CO;2-B. 10.1002/1099-1387(200009)6:9<459::AID-PSC286>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 8.International Labour Organization (ILO) Guidelines for the Use of the ILO International Classification of Radiographs of Pneumoconioses. Revised. Geneva, Switzerland: ILO Publications; 2000. [Google Scholar]
- 9.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 10.Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma) Arthritis Rheum. 1980;23:581. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 11.Ohtsuki T, Yawata Y, Wada H, Sugihara T, Mori M, Namba M. Two human myeloma cell lines, amylase-producing KMS-12-PE and amylase-non-producing KMS-12-BM, were established from a patient, having the same chromosome marker, t(11;14)(q13;q32) Br J Haematol. 1989;73:199. doi: 10.1111/j.1365-2141.1989.tb00252.x. [DOI] [PubMed] [Google Scholar]
- 12.Otsuki T, Hata H, Harada N, et al. Cellular biological differences between human myeloma cell lines KMS-12-PE and KMS-12-BM established from a single patient. Int J Hematol. 2000;72:216. [PubMed] [Google Scholar]
- 13.Otsuki T, Yamada O, Sakaguchi H, Tomokuni A, Wada H, Yawata Y, Ueki A. Human myeloma cell apoptosis induced by interferon-alpha. Br J Haematol. 1998;103:518. doi: 10.1046/j.1365-2141.1998.01000.x. 10.1046/j.1365-2141.1998.01000.x. [DOI] [PubMed] [Google Scholar]
- 14.Fadeel B, Thorpe J, Chiodi F. Mapping of the linear site on the Fas/APO-1 molecule targeted by the prototypic anti-Fas mAb. Int Immunol. 1995;7:1967. doi: 10.1093/intimm/7.12.1967. [DOI] [PubMed] [Google Scholar]
- 15.Otsuki T, Yamada O, Kurebayashi J, et al. Estrogen receptors in human myeloma cells. Cancer Res. 2000;60:1434. [PubMed] [Google Scholar]
- 16.Starling GC, Bajorath J, Emswiler J, Ledbetter JA, Aruffo A, Kiener PA. Identification of amino acid residues important for ligand binding to Fas. J Exp Med. 1997;185:1487. doi: 10.1084/jem.185.8.1487. 10.1084/jem.185.8.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider P, Bodmer JI, Holler N, Mattmann C, Scuderi P, Terskikh A, Peitsch MC, Tschopp J. Characterization of Fas (Apo-1, CD95) –Fas ligand interaction. J Biol Chem. 1997;272:18827. doi: 10.1074/jbc.272.30.18827. 10.1074/jbc.272.30.18827. [DOI] [PubMed] [Google Scholar]
- 18.Vaishnaw AK, Orlinick JR, Chu JL, Krammer PH, Chao MV, Elkon KB. The molecular basis for apoptotic defects in patients with CD95 (Fas/Apo-1) mutations. J Clin Invest. 1999;103:355. doi: 10.1172/JCI5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlinick JR, Vaishnaw A, Elkon KB, Chao MV. Requirement of cysteine-rich repeats of the Fas receptor for binding by the Fas ligand. J Biol Chem. 1997;272:28889. doi: 10.1074/jbc.272.46.28889. 10.1074/jbc.272.46.28889. [DOI] [PubMed] [Google Scholar]
- 20.Fadeel B, Thorpe CJ, Yonehara S, Chiodi F. Anti-Fas IgG1 antibodies recognizing the same epitope of Fas/APO-1 mediate different biological effects in vitro. Int Immunol. 1997;9:201. doi: 10.1093/intimm/9.2.201. 10.1093/intimm/9.2.201. [DOI] [PubMed] [Google Scholar]
- 21.Fadeel B, Lindberg J, Achour A, Chiodi F. A three-dimensional model of the Fas/APO-1 molecule: cross-reactivity of anti-Fas antibodies explained by structural mimicry of antigenic sites. Int Immunol. 1998;10:131. doi: 10.1093/intimm/10.2.131. 10.1093/intimm/10.2.131. [DOI] [PubMed] [Google Scholar]
- 22.Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 23.Itoh N, Nagata S. A novel protein domain required for apoptosis. Mutational analysis of human Fas antigen. J Biol Chem. 1993;268:10932. [PubMed] [Google Scholar]
- 24.Boldin MP, Mett IL, Varfolomeev EE, Chumakov I, Shemer-Avni Y, Camonis JH, Wallach D. Self association of the ‘death domains’ of the p55 tumor necrosis factor (TNF) receptor and Fas/APO1 prompts signaling for TNF and Fas/APO1 effects. J Biol Chem. 1995;270:387. doi: 10.1074/jbc.270.1.387. 10.1074/jbc.270.1.387. [DOI] [PubMed] [Google Scholar]
- 25.Huang B, Eberstadt M, Olejniczak ET, Meadows RP, Fesik SW. NMR structure and mutagenesis of the Fas (APO-1/CD95) death domain. Nature. 1996;384:638. doi: 10.1038/384638a0. 10.1038/384638a0. [DOI] [PubMed] [Google Scholar]
- 26.Berglund H, Olerenshaw D, Sankar S, Federwisch M, McDonald NQ, Driscoll PC. The three-dimensional solution structure and dynamic properties of the human FADD death domain. J Mol Biol. 2000;302:171. doi: 10.1006/jmbi.2000.4011. [DOI] [PubMed] [Google Scholar]
- 27.Yi FH, Lautrett C, Vermot-Desroches C, Bordessoule D, Couratier F, Wijdenes J, Preud'homme JL, Jauberteau MO. In vitro induction of neuronal apoptosis by anti-Fas antibody-containing sera from amyotrophic lateral sclerosis patients. J Neuroimmunol. 2000;109:211. doi: 10.1016/s0165-5728(00)00288-5. 10.1016/S0165-5728(00)00288-5. [DOI] [PubMed] [Google Scholar]
- 28.Prasad NK, Papoff G, Zeuner A, Bonnin E, Kazatchkin MD, Ruberti G, Kaveri SV. Therapeutic preparations of normal polyspecificIgG (IVIg) induce apoptosis in human lymphocytes and monocytes. a novel mechanism of action of IVIg involving the Fas apoptotic pathway. J Immunol. 1998;161:3781. [PubMed] [Google Scholar]