Abstract
Glucocorticoid-induced leucine zipper (GILZ) is a 137 amino acid protein, rapidly induced by treatment with glucocorticoids (GC), characterized by a leucine zipper (LZ) domain (76–97 amino acids), an N-terminal domain (1–75 amino acids) and a C-terminal PER domain (98–137 amino acids) rich in proline and glutamic acid residues. We have previously shown that GILZ binds to and inhibits NF-κB activity. In the present study we used a number of mutants with the aim of defining the GILZ molecular domains responsible for GILZ/p65NF-κB interaction. Results, obtained by in vitro and in vivo co-immunoprecipitation (Co-IP) and by transcriptional activity experiments, indicate that GILZ homo-dimerization, through the LZ domain, as well as the C-terminal PER domain, particularly the 121–123 amino acids, are both necessary for GILZ interaction with NF-κB, inhibition of transcriptional activity and of IL-2 synthesis.
INTRODUCTION
Glucocorticoids (GC) are involved in the physiological regulation of a variety of biological processes including inflammation, immune response, metabolism, cell growth and development. They are of extraordinary therapeutic value in a wide-range of pathologies including inflammatory and autoimmune diseases (1–4). In particular, immunosuppressive and anti-inflammatory effects are consequences of GC complex action on innate and adaptive immunity that reflects GC capacity to inhibit T lymphocyte activation. For these reasons, GC are used as therapeutic agents in several acute and chronic inflammatory/autoimmune diseases and in organ transplantation where activation and development of T-cell mediated immunity plays an important role (2,5–7).
Genomic and non-genomic effects are induced by GC treatment. However, most of the effects are mediated by modulation of gene transcription, through GC interaction with the glucocorticoid receptor (GR), which functions as a ligand-dependent transcription factor and regulates gene expression directly, by binding to DNA, or indirectly through protein–to–protein interaction with other transcription factors (8–11). Thus, GC inhibit T lymphocyte activation and proliferation, cytokine production and trans-activation of several transcription factors involved in T-cell activation processes, such as AP-1 and NF-κB (11–14).
GC inhibit NF-κB activity through different mechanisms including augmentation of I-κB expression, competition for co-activator proteins, such as CBP/p300, and direct association of GR with NF-κB subunits (8,12,14). It has also been reported that treatment with dexamethasone (DEX), a synthetic GC, down-modulates p65NF-κB trans-activation potential in the absence of any I-κB up-regulation, thus further suggesting that GC-mediated NF-κB activity inhibition is a complex mechanism also characterized by some redundancy (15).
Glucocorticoid-induced leucine zipper (GILZ), a GC-induced gene, was first isolated as a DEX-responsive gene from a thymus subtraction library (16). Based on protein motifs, GILZ belongs to the leucine zipper (LZ) family and shares significant homology with other members of the same family (17). The GILZ gene encodes a 137 amino acid protein characterized by an LZ domain, present in the central portion (76–97 amino acids) of the molecule, by an N-terminal domain (1–75 amino acids) that, contrary to other family members, does not contain an obvious DNA-binding sequence (18,19) and by a C-terminal domain (98–137 amino acids), proline (P) and glutamic acid (E) rich (PER) region, containing eight glutamic acid residues, eight proline residues and five PxxP hypothetical SH3-binding consensus sequences (20). Similar to other protein families, GILZ LZ is characterized by a heptad repeat of leucine residues (21,22). The leucine residues are found in position d of conventional nomenclature for LZ heptad repeats (abcdefg) (23) and constitute a widely observed structural motif that serves to promote both homo- and hetero-dimerization (24–26). In particular, dimerization of LZ proteins occurs via the formation of a short parallel coiled-coil α-helices (27).
GILZ is present in different cell types including T lymphocytes and macrophages (28). Treatment with GC induces a rapid increase of GILZ expression in T lymphocytes, where it inhibits anti-CD3-induced IL-2 production, IL-2 receptor (IL-2R) expression, Fas and FasL up-regulation and cell-death consequent to CD3-induced activation (11,16,29,30), thus indicating that GILZ mimics GC-mediated effects in T lymphocytes. Moreover, GILZ expression is down-regulated by anti-CD3 stimulation, further suggesting that GILZ contributes to the control of T-cell activation and development (11,29). In macrophages, DEX up-regulates GILZ (28) and GILZ over-expression, like GC, inhibits production of inflammatory mediators and pro-inflammatory chemokines as well as Toll-like receptor expression (31). Most of these functions, such as T-cell activation, interleukin and chemokine production, are under control of various transcription factors including NF-κB.
In our previous studies we have shown that GILZ, when over-expressed by GC treatment or transfection, both functionally and physically interacts with p65NF-κB and inhibits NF-κB transcriptional activity in T lymphocytes and in macrophages (29–32). In particular, we have shown that GILZ associates with and inhibits NF-κB (p65/p52) transcriptional activity, but not with other LZ, such as Fra-1 (29). Moreover, these effects are independent from I-κB and in fact GILZ does not affect expression of I-κB and NF-κB subunits, I-κB phosphorylation and degradation or I-κB/NF-κB binding. Similarly, I-κB does not interfere with GILZ/NF-κB interaction (29). In addition, GILZ/NF-κB co-immunoprecipitation (Co-IP) and inhibition of in vivo trans-activation activity are also detected in transfected cell lines defective for the entire NF-κB system, including I-κB, thus indicating that GILZ interaction with NF-κB (p65/p52) does not require other Rel- or I-κB-related proteins (29). In the present paper, we have analyzed GILZ/p65NF-κB interaction with the aim to identify the GILZ molecular characteristics responsible for this interaction. Results indicate that GILZ homo-dimerization and the PER region are necessary for in vitro and in vivo GILZ interaction with NF-κB and for in vivo transcriptional activity inhibition.
MATERIALS AND METHODS
Cell lines and plasmid construction
Human kidney epithelial carcinoma cell line 293T and Cos-1 cells were cultured in DMEM supplemented with 10% FBS (Invitrogen, San Diego, CA) and antibiotics. Mouse hybridoma T-cell line 3DO, was maintained in suspension in RPMI medium supplemented with 10% FBS, 10 mM HEPES buffer and antibiotics. Eukariotic expression plasmids pCR3.1-p65, pCR3.1-GILZ, were constructed as described previously by cloning into the EcoRI site of pCR3.1 expression vector (Invitrogen), p65, murine GILZ open reading frame (ORF) cDNA. pCR3.1-p52 was constructed by inserting the p52 cDNA ORF into pCR3.1 expression vector at the EcoRI and PstI sites. Reporter plasmids containing tandem repeats of either the murine IgK/HIV-κB (pBIIXLUC) sites linked upstream to a minimal murine c-fos promoter and luciferase coding sequences were obtained from R. Dalla Favera (Columbia University, NY). pEGFP-N1 vector was purchased from Clontech (Palo Alto, CA). The plasmids encoding GILZ mutants (LZ1-7, ΔN-GILZ, ΔC-GILZ and PER1-10) were constructed by inserting in EcoRI-digested pCR3.1 fragments generated by PCR, using appropriate synthetic oligonucleotides as primers and pCR3.1-GILZ as template. LZ-GILZ mutant was constructed by substituting the GILZ LZ domain coding sequence with the CREB LZ domain. Briefly, the murine CREB LZ coding sequence was reconstructed using two synthetic oligonucleotides (for: 5′-AACTGTTTAGAGAACAGAGTGGCAGTGCTTGAAAACCAAAACAAAACATTGATTGAGGAGCTAAAAGCACTG-3′; rev: 5′-TCGACAGTGCTTTTAGCTCCTCAATCAATGTTTTGTTTTGGTTTTCAAGCACTGCCACTCTGTTCTCTAAACAGTT-3′; Invitrogen), annealed with the following conditions: 90°C 4 min, 85°C 4 min, 80°C 4 min, 75°C 4 min, 70°C 10 min and slowly cooled down to 10°C. The plasmid pCR3.1-GILZ was digested with HpaI and SalI to remove the GILZ LZ and the annealed reaction product was inserted into the digested pCR3.1-GILZ.
Myc-tagged proteins were generated by insertion of GILZ or GILZ mutants' cDNA into BamHI and XbaI sites of pcDNA3.1/Myc-His vector (Invitrogen). Xpress-tagged GILZ mutants were cloned into BamHI and XbaI sites of pcDNA6/His vector (Invitrogen), Xpress-tagged p65 was obtained by cloning in pcDNA6/His the p65 cDNA at BamHI and NotI sites. The mammalian expression constructs containing GILZ or its mutants ΔC-GILZ, ΔN-GILZ in N-terminal glutathione S-transferase (GST)-tagged vector pEBG (kindly provided by W. Kolch, Cancer Research, UK) were generated by subcloning into BamHI and NotI sites of the mammalian expressing vector pEBG.
In vitro binding assay
GILZ was in-frame cloned into the pGEX-4T2 plasmid (Pharmacia, Uppsala, Sweden) as described previously (16). GST fusion proteins were expressed in BL21 E.coli and purified with Glutathione Sepharose 4B beads following the manufacturer's instructions. GILZ, GILZ mutant and Fra-1 proteins were in vitro translated with [35S]methionine by using the rabbit reticulocyte-coupled in vitro transcription translation system (TNT Promega, Italy), under the T7 promoter according to the manufacturer's instruction. In vitro translated proteins were diluted with the binding buffer [final concentration: 25 mM HEPES (pH 7.5), 10% glycerol, 50 mM NaCl, 0.05% Nonidet P-40 and 1 mM DTT] and pre-cleared with glutathione beads for 45 min at 4°C. GST or GST–GILZ fusion protein were bound to glutathione beads. Protein extracts were then incubated with in vitro translated proteins for 20 min at 20°C. The beads were subsequently washed five times with 0.5 ml of phosphate-buffered saline (PBS) and bound proteins were recovered by boiling in SDS sample buffer and were resolved by SDS–PAGE.
Luciferase assay
Calcium phosphate-mediated transient DNA transfection and luciferase assays were performed as described (33). 293T cells were plated at 2 × 106/100 mm Petri dish 24 h before transfection. At 48 h after transfection, cells were harvested and transcription activation was assayed as luciferase activity. Different amounts of pCR3.1-GILZ (0.05, 0.5 and 5 μg) or its mutants, plus effector plasmids pCR3.1-p65 (1.5 μg) and pCR3.1-p52 (0.5 μg), were co-transfected with 15 μg reporter plasmid (pBIIXLUC) and 3 μg pEGFP-N1. The pEGFP-N1 plasmid was used to normalize the transfection efficiency of each sample. Cell lysis and luciferase quantification were performed using commercial reagents (Luciferase Reporter Gene Assay, Roche Diagnostics, Monza, Italy). The values are expressed as fold-increase above the level of luciferase activity of cells transfected with the reporter plasmids. The values marked endogenous control (EC) represent the values obtained by transfection of the reporter plasmids along with the control pCR-3.1 empty vector.
Immunoprecipitation (IP)
Myc-tagged GILZ or GILZ mutants, Xpress-tagged GILZ mutants and Xpress-tagged p65 were co-transfected in different combination, as described in the Figures, in COS-1 cells by using DEAE-dextran as described previously (34). After 48 h of transfection, whole-cell extracts were prepared and IP was performed in Co-IP buffer [50 mM Tris (pH 7.5), 100 mM NaCl, 0.1% Triton X-100 and 15 mM EGTA] supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF). Antigen–antibody complexes were precipitated with protein G bound to Sepharose beads (Santa Cruz Biotechnologies, Santa Cruz, CA). After being washed five times in Co-IP buffer, the immunoprecipitates were analyzed by immunoblotting.
GST-GILZ or its mutants GST-ΔC-GILZ, GST-ΔN-GILZ were separately co-transfected with Xpress-tagged p65 in 293T cells by using calcium phosphate method as reported previously. At 48 h after transfection, cells were harvested and incubated in Co-IP buffer with glutathione-agarose beads ON at 4°C. Then, beads were washed five times in Co-IP buffer; bound proteins were recovered by boiling in SDS sample buffer and were analyzed by SDS–PAGE.
Immunoblot analysis
Extracted or immunoprecipitated proteins and analyzed by western blot as described previously (35). Primary antibodies used in these experiments were specific for Xpress and Myc epitopes (Invitrogen) or GST (Calbiochem, Darmstadt, Germany). The antigen–antibody complexes were revealed by enhanced chemiluminescence according to the manufacturer's instructions (Super-Signal, Pierce, Rockford, IL).
Molecular dynamic simulation analysis
A virtual model of GILZ protein corresponding to the wild-type was constructed according to the computational protocol reported previously (36). The 3D models of mutants were obtained replacing the corresponding residues of proline and glutamate with residues of alanine according to the amino acid sequences of PER7-9. The above operations were carried out using Insight-II (Insight-II, Accelrys, San Diego, CA). Then, the resulting models were energy refined using 1000 steps of conjugate gradients minimization in order to remove bad contacts. At this purpose, Charmm22 force field was used (37). Each model was solvated with water molecules using Solvate v.1.0 and its default settings (Solvate v.1.0 is written by Helmut Grubmüller, Theoretical Biophysics Group, Institut für Medizinische Optik, Ludwig-Maximilians-Universität München, München, Germany). No counter-ions were added. All water molecules within 1.5 Å of any protein atom were then removed. After the construction of the solvent environment, we obtained four systems of ∼32 000 atoms.
All the models were energy minimized for 5000 steps using the method of conjugate gradients as implemented in NAMD v.2.5 (38). During the minimization protocol, the backbone of the enzyme was kept fixed. The water shell was then equilibrated for 500 ps at 300 K. The temperature of 300 K was gradually reached starting from 0 K in the first 10 ps of the simulation. The final coordinates and velocities of the resulting three systems were submitted to a final simulation of 3 ns where constrains on the backbone atoms were removed. The atomic coordinates of each system were saved every 10 ps. During the simulations, spherical harmonic boundary conditions (SBC) were applied. SBC consists in applying a potential with a constant force of 0.1 kcal/mol * Å2 and an exponent of two set to a distance of radius 45 Å centred on the centre of mass of the solute. A time step of 2 fs was used and the ShakeH algorithm was applied to all hydrogen atoms. Molecular dynamic calculations were performed using NAMD v.2.5 and the Charmm22 force field.
Enzyme-linked immunosorbent assay (ELISA) assay
3DO cells were transfected by electroporation (300 V and 960 μF) with 15 μg of linearized pCR3.1 vector (control) or linearized vector containing GILZ or GILZ mutants cDNA. At 24 h after transfection, cells where plated into 96-well plates (4-wells for each transfection), previously coated with anti-CD3 mAb (PharMingen, San Diego, CA, 1 μg/ml). Eighteen hours after treatment, the supernatants were collected and IL-2 production content was evaluated by sandwich ELISA. The specific anti-mouse antibody (Ab) used to capture and detect IL-2 was purchased from PharMingen, and the assays were performed following the manufacturer's instruction. The sensitivity limit was ∼3 U/ml for IL-2.
Statistical analysis
Each experiment was performed at least three times. Representative experiments are shown. Due to the non normal distribution of the data, nonparametric tests (Kruskall–Wallis' ANOVA) were adopted for statistical evaluation.
RESULTS
LZ domain is necessary for GILZ homo-dimerization
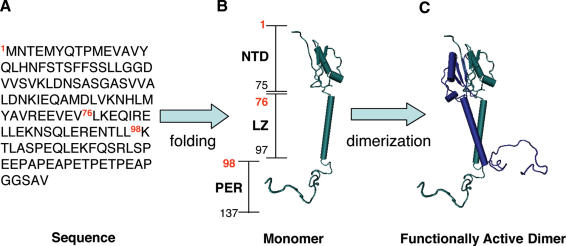
It has been previously suggested that GILZ could homo-dimerize (39). We have previously reported results describing the GILZ protein structure in a 3D model as evaluated by computer based homology modeling (36). Based on the 3D GILZ monomer model, we assembled a functionally active dimer computer analysis-based model showing that GILZ homo-dimerizes through the LZ domain (76–98 amino acids, Figure 1). In fact, it is known that LZ can favor protein dimerization and it has been shown that specific rules dictate dimerization specificity and stability (40–45). In particular, the presence of three attractive g–e interactions, isoleucine at the a position, two asparagines at a position of 3rd and 4th heptad, in addition to leucine in all four d positions, strongly suggest GILZ could form a stable homo-dimer.
Figure 1.
The sequence of 137 amino acid encoded by GILZ gene (A) is folded into a monomer (B), which is constituted by three domains: the N-terminal domain (NTD, 1–75 amino acids), the leucine zipper (LZ, 76–97 amino acids) and a C-terminal or PER domain (PER, 98–137 amino acids). Then, GILZ monomer is assembled into a functionally active dimer (C). The 3D model of the dimeric form of GILZ is obtained using the resolved structure of DIP as template [see Ref. (35)].
With the aim to analyze the possible requirement of GILZ homo-dimerization for GILZ interaction with NF-κB, we prepared a number of GILZ mutants at the LZ domain (Figure 2A) by amino acid replacement at positions d and a of the heptad repeats, reported previously to be important for optimal hydrophobic packing of this region and dimerization (40–44).
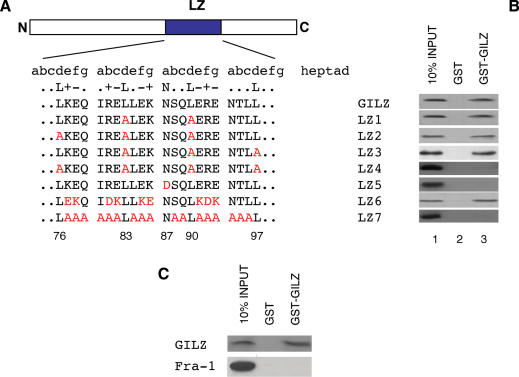
Figure 2.
GILZ LZ domain is necessary for homo-dimerization in vitro. (A) Amino acid sequence of the LZ region of GILZ protein. LZ heptads are grouped (abcdefg) to help visualize LZ structure and mutations in this region are represented by red colored letters. Numbers in the bottom show amino acid position of GILZ protein relative to N-terminal region. (B) The 35S-labeled in vitro transcription/translation product of full-length wild-type GILZ was incubated with GST (column 2) or GST-GILZ or GST-GILZ mutants (column 3) immobilized on glutathione sepharose beads. The protein bound to the resin was eluted, resolved by SDS–PAGE, and visualized by autoradiography (right panel). 10% input indicates 0.1 vol of the 35S-labeled product used in the pull-down assay (column 1). The results are representative of one of three independent experiments. (C) The 35S-labeled in vitro transcription/translation product of full-length wild-type Fra-1 was incubated with GST (column 2) or GST-GILZ (column 3) immobilized on glutathione sepharose beads. The protein bound to the resin was eluted, resolved by SDS–PAGE, and visualized by autoradiography (right panel). 10% input indicates 0.1 volumes of the 35S-labeled product used in the pull-down assay (column 1). The results are representative of one of three independent experiments.
Accordingly, we initially constructed mutants by leucine substitutions (Figure 2A: mutants LZ1, LZ2, LZ3 and LZ4) at position d (76, 83, 90, 97 amino acids). GST pull-down binding assay showed that GST–GILZ fusion protein was not able to bind in vitro translated LZ4 (all four leucines substituted at positions 76, 83, 90 and 97), whereas it was able to bind LZ1 (2 leucines substituted at positions 83 and 90), LZ2 (three leucines substituted at positions 76, 83 and 90) and LZ3 (three leucines substituted at position 83, 90 and 97) (Figure 2B, column 3). No binding was detected with GST alone (Figure 2B, column 2). These results indicate that GILZ can homo-dimerize and that all four leucines in the LZ domain are important for dimerization.
We then tested the possible relevance of another highly conserved residue in LZ domain, such as asparagine (87 amino acids), in the third heptad at position a (Figure 2A), that it is known to be important for homo-dimerization specificity (42–44). As shown in Figure 2, the mutant with asparagine residue mutated to aspartate (LZ5), did not dimerize (Figure 2B, column 3).
Furthermore, it is known that the coiled-coil is stabilized by polar amino acids usually present at e and g positions, adjacent to the hydrophobic interface (Figure 2A) (22,45). To investigate the contribution of these charged amino acids for GILZ homo-dimerization we generated two different mutants: a mutant where all charged amino acids, except the four leucines and the asparagine, were substituted with amino acids having an opposite charge (Figure 2A, LZ6), and a mutant where all amino acids, except the four leucines and the asparagine, were substituted with non-polar alanine residues (Figure 2A, LZ7). Results in Figure 2 show that LZ6 mutant bound GST-GILZ, whereas LZ7 did not (Figure 2B, column 3). These results indicate that the presence of charged amino acids within the LZ domain are necessary for homo-dimerization and that homo-dimerization can also occur when amino acids of opposite polarity are substituted in the molecule. Taken together, these results suggest that the LZ domain is necessary for GILZ homo-dimerization. As further specificity control, GILZ binding to another LZ protein, such as Fra-1, was tested. As shown in: Figure 2C, GILZ does not binds Fra-1, thus suggesting for a certain binding specificity.
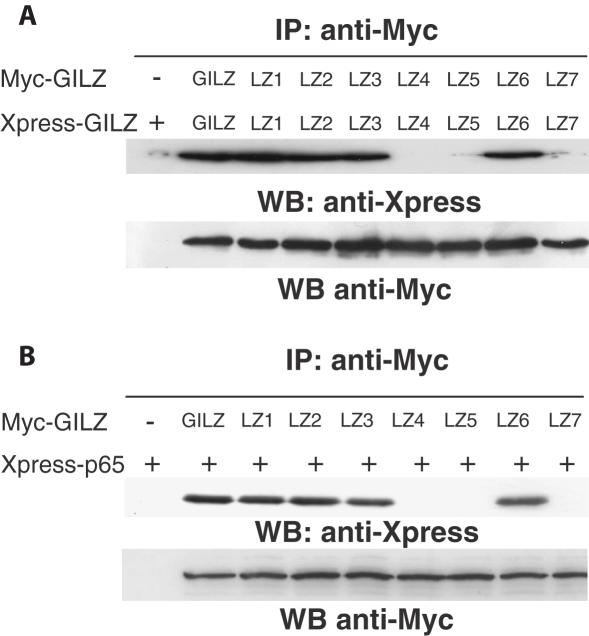
To confirm the results obtained by in vitro GST pull-down assay shown in Figure 2, we performed Co-IP experiments using cells co-transfected with GILZ and GILZ mutants. Cos-1 cells were transfected with expression vector containing Myc- and Xpress-tagged GILZ. GILZ-Xpress protein was detected in the immunocomplex formed with anti-Myc antibody from cell lysates co-transfected with Myc- and Xpress-tagged GILZ (Figure 3A, column 2), demonstrating that GILZ homo-dimerizes in vivo. As a control, GILZ-Xpress was not immunoprecipitated by anti-Myc Ab in cell transfected with GILZ-Xpress alone (Figure 3A, column 1).
Figure 3.
GILZ co-immunoprecipitates with NF-κB in Cos-1 cells and its homo-dimerization is necessary for GILZ–NF-κB interaction. (A) Lysates from Cos-1 cells transfected with 10 μg of vector containing Myc-tagged GILZ or its mutants (LZ1-7) and 10 μg of Xpress-tagged GILZ or its mutants (LZ1-7), were used for the IP analysis performed using anti-Myc antibody (Ab). Xpress-tagged GILZ or its mutants in the immunoprecipitates were detected by western blotting (WB) using anti-Xpress Ab. WB with anti-Myc Ab was performed to check Myc-tagged GILZ or its mutant's expression levels in transfected cells. (B) GILZ–p65NF-κB interaction was assessed by co-transfection in Cos-1 cells with 10 μg of vector containing Myc-tagged GILZ or its mutants (LZ1-7) and 10 μg of Xpress-tagged p65 protein. Xpress-tagged p65 in the immunoprecipitates were detected by WB using anti-Xpress Ab. WB with anti-Myc Ab was performed to check Myc-tagged GILZ or its mutants expression level in transfected cells.
Moreover, the results of Co-IP assay performed with anti-Myc Ab in Cos-1 cells co-transfected with Myc- and Xpress-tagged GILZ mutants (Figure 3A), show that LZ1 (column 3), LZ2 (column 4), LZ3 (column 5), LZ6 (column 8) were able to dimerize in vivo, whereas LZ4 (column 6), LZ5 (column 7) and LZ7 (column 9) did not dimerize.
Together these results indicate that the LZ domain is essential for in vivo GILZ homo-dimerization.
GILZ homo-dimerization is required for GILZ/NF-κB interaction and for inhibition of NF-κB transcriptional activity
We have previously shown that GILZ and NF-κB-subunit p65 (p65NF-κB) can physically interact in vivo and in vitro (29) and Co-IP analysis in Cos-1 cells co-transfected with Xpress-p65 and Myc-GILZ confirm these data (Figure 3B, column 2). Moreover, we used GILZ mutants to evaluate the possible relevance of homo-dimerization for interaction with p65NF-κB. Results indicate that mutants which homo-dimerize (LZ1, LZ2, LZ3 and LZ6), as shown in Co-IP experiments (Figure 3A), are able to co-immunoprecipitate with p65 (Figure 3B, respectively columns 3, 4, 5 and 8). As a control, Xpress-p65 alone was not immunoprecipitated by anti-Myc Ab (Figure 3B, column 1). On the contrary, mutants, such as LZ4, LZ5 and LZ7 that do not dimerize (Figure 3A) did not co-immunoprecipitate with p65 (Figure 3B, respectively columns 6, 7 and 9). These results indicate that GILZ homo-dimerization is necessary for interaction with p65.
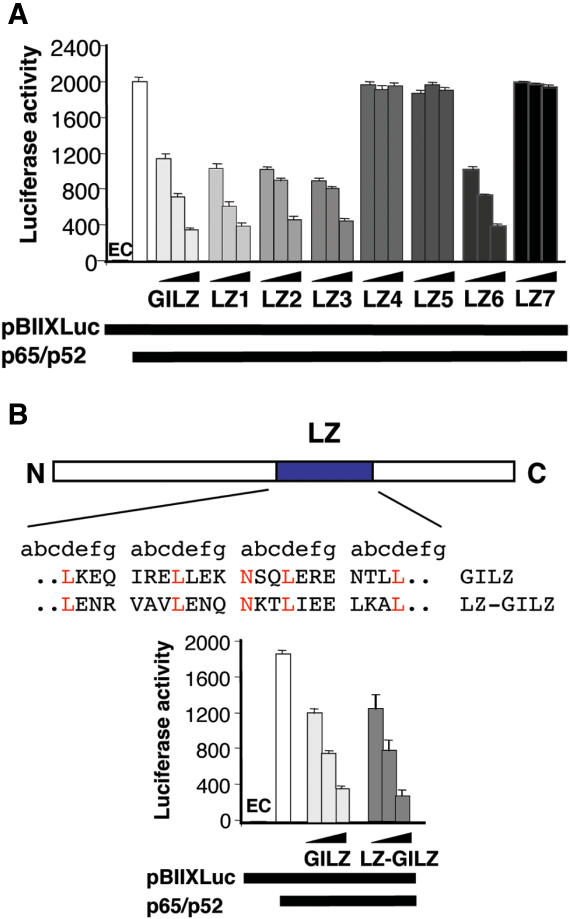
GILZ inhibits NF-κB transcription activity in vivo independently from other Rel-related or I-κB-related protein (29). To investigate the role of GILZ homo-dimerization in modulation of NF-κB transcription activity, we used 293T cells co-transfected with an NF-κB luciferase-reporter vector together with p65 and p52, in the presence or absence of GILZ or GILZ mutants' co-expression. Results in Figure 4 confirmed previous data indicating that GILZ inhibited NF-κB (p65/p52) transcriptional activity (29). However, this inhibitory effect was not observed when cells were co-transfected with LZ4, LZ5 or LZ7 mutants that do not homo-dimerize (Figure 4A). In contrast, LZ1, LZ2, LZ3 and LZ6 mutants that homo-dimerize, inhibited NF-κB transcriptional activity (Figure 4A). Together these results suggest that GILZ homo-dimerization is required for inhibition of NF-κB transcriptional activity. Moreover, by using a mutant in which we replaced the GILZ dimerization domain with CREB LZ domain (LZ-GILZ) we found that there was no difference between GILZ and LZ-GILZ inhibition of NF-κB transcriptional activity thus suggesting that the dimerization and inhibition are structurally separate (Figure 4B).
Figure 4.
(A) Homo-dimerization is important for GILZ inhibition of NF-κB transcriptional activity. Analysis of NF-κB transcriptional activity was performed in transiently co-transfected 293 cells. Different amounts of pCR3.1-GILZ (0.05, 0.5 and 5 μg) or its mutants, plus effector plasmids pCR3.1-p65 (1.5 μg) and pCR3.1-p52 (0.5 μg), were co-transfected with 15 μg of the reporter vector pBIIXLUC. Each transfection was performed in triplicate, and SD bars are shown. EC indicates endogenous control. (B) GILZ LZ domain was substituted with CREB LZ domain (LZ-GILZ, upper panel); NF-κB transcriptional activity was assessed by co-transfecting different amounts of pCR3.1-GILZ (0.05, 0.5 and 5 μg) or LZ-GILZ mutant, plus effector plasmids pCR3.1-p65 (1.5 μg) and pCR3.1-p52 (0.5 μg), together with 15 μg of the reporter vector pBIIXLUC. Each transfection was performed in triplicate, and SD bars are shown. EC indicates endogenous control.
GILZ C-terminal domain (PER region) is necessary for inhibition of NF-κB transcriptional activity and GILZ/p65NF-κB interaction
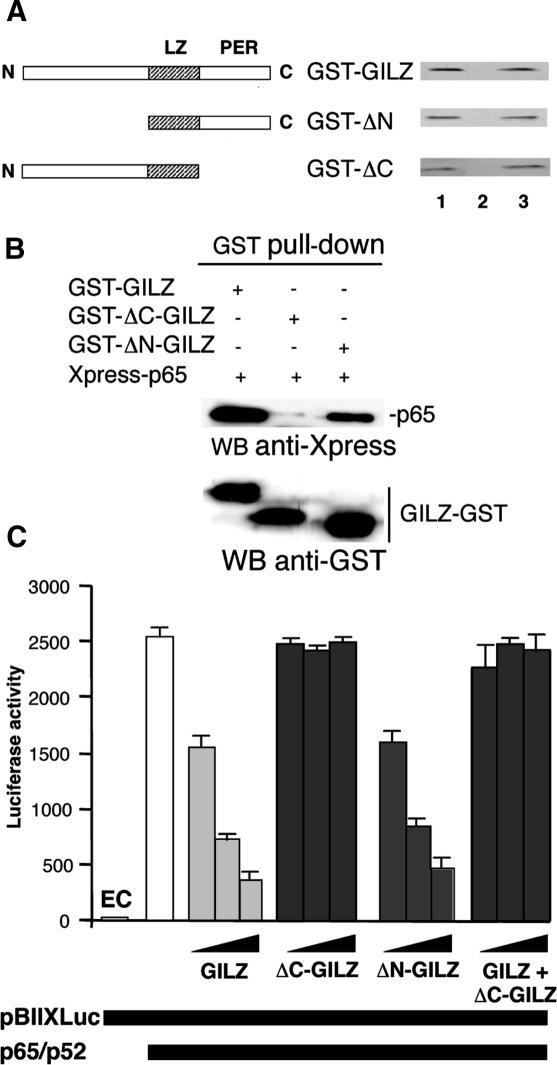
To determine whether GILZ homo-dimerization is the only feature required for inhibition of NF-κB transcriptional activity, we generated a GILZ truncated protein by eliminating flanking LZ domain N-terminal 1–75 amino acids (ΔN-GILZ) or C-terminal 98–137 amino acids (ΔC-GILZ) (Figure 5A, left part) and tested them in GST-pull-down experiments. Results indicate that both ΔN-GILZ and ΔC-GILZ were able to homo-dimerize (Figure 5A, right part, column 3) thus confirming the role of LZ for dimerization. We also performed Co-IP in Cos-1 cells transfected with eukaryotic GST–GILZ fusion proteins and Xpress-p65: interestingly, GST-ΔN-GILZ (column 3), but not GST-ΔC-GILZ (column 2) co-immunoprecipitated with p65NF-κB (Figure 5B), thus suggesting that C-terminal portion of GILZ protein is important for binding with p65NF-κB in vivo. Next, we tested the effect of ΔN-GILZ and ΔC-GILZ truncated mutants on in vivo NF-κB transcriptional activity. Results show that ΔN-GILZ inhibited NF-κB transcriptional activity whereas ΔC-GILZ did not.
Figure 5.
Homo-dimerization is necessary but not sufficient for GILZ inhibition of NF-κB transcriptional activity. (A) Left panel: schematic representation of GILZ and its deleted mutants. Right panel: The 35S-labeled in vitro transcription/translation product of full-length wild-type GILZ was incubated with GST (column 2) or GST-GILZ or GST-GILZ mutants (column 3) immobilized on glutathione sepharose beads. The protein bound to the resin were eluted, resolved by SDS–PAGE, and visualized by autoradiography (right panel). 10% input used in the pull-down assay is shown in column 1. The results are representative of one of three independent experiments. (B) Lysates containing Xpress-p65 and GST–GILZ or GST–ΔC–GILZ or GST–ΔN–GILZ fusions proteins were pulled-down by glutathione Sepharose beads. Presence of fusions proteins was detected by WB with anti-GST Ab. Xpress-p65 precipitated was detected by WB with anti-Xpress Ab. (C) Analysis of NF-κB transcriptional activity was performed on transiently co-transfected 293 cells. Different amounts of pCR3.1-GILZ (0.05, 0.5 and 5 μg) or its deleted mutants, plus effector plasmids pCR3.1-p65 (1.5 μg) and pCR3.1-p52 (0.5 μ), were co-transfected with 15 μg of the reporter vector pBIIXLUC. In columns 11–13 (GILZ + ΔC-GILZ) equimolar pCR3.1-GILZ (0.025, 0.25 and 2.5 μg) and pCR3.1-ΔC-GILZ (0.025, 0.25 and 2.5 μg) plus effector plasmids pCR3.1-p65 (1.5 μg) and pCR3.1-p52 (0.5 μg), were co-transfected with 15 μg of the reporter vector pBIIXLUC. Each transfection was performed in triplicate, and SD bars are shown. EC indicates endogenous control.
Together, above results indicate that: (i) C-terminal part of GILZ protein (PER region), although not necessary for homo-dimerization, is important for interaction with NF-κB and for inhibition of its transcriptional activity, (ii) GILZ homo-dimerization occurs in absence of PER region, but is not sufficient for NF-κB transcriptional activity inhibition (Figure 5C) and (iii) N-terminal part of GILZ is not necessary for homo-dimerization nor for NF-κB binding and inhibition.
Finally, we tested if both C-terminal domains are critical for function. To serve that purpose we made a system with a function dimer but only one C-terminal domain by transfecting equimolar GILZ and ΔC-GILZ plasmids. Results in Figure 5C indicated that GILZ plus ΔC-GILZ-transfected cells are not able to inhibit NF-κB transcriptional activity suggesting that both C-terminal domains are important for function.
Analysis of PER region domains involved in GILZ/p65NF-κB interaction
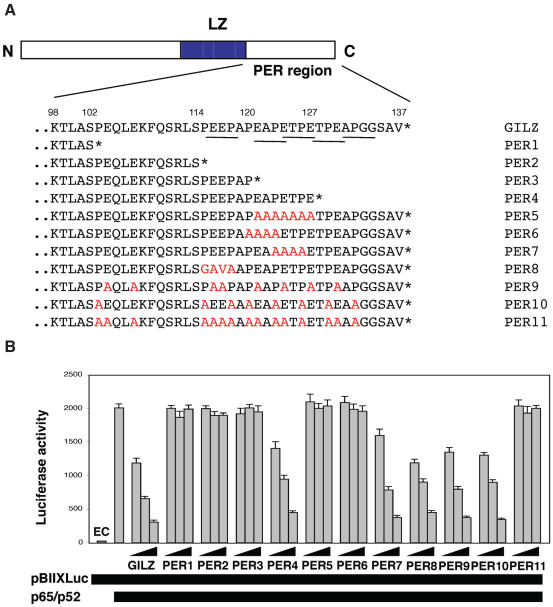
Despite the fact that C-terminal part (98–137 amino acids) of GILZ does not have obvious homologies to any known functional domain, it shows some interesting features. This 40 amino acid region contains eight prolines (P), eight glutamic acid (E) residues and five PxxP sequences where x denotes any amino acid (Figure 6A, underscored sequences). Such motives have been previously shown to mediate protein–to–protein interaction in eukaryotic cells (20).
Figure 6.
Role of Pro/Glu rich (PER) region of GILZ in homo-dimerization and NF-κB transcriptional activity. (A) Amino acid sequence of the PER region of GILZ protein. The C-terminus is denoted with an asterisk. Point mutation is colored in red. PxxP domains are underscored. Numbers in the bottom show amino acid position of GILZ protein relative to the N-terminus. (B) Different amounts of pCR3.1-GILZ or GILZ mutants (0.05, 0.5 and 5 μg), plus effector plasmids pCR3.1-p65 (1.5 μg) and pCR3.1-p52 (0.5 μg), were co-transfected with 15 μg of the reporter vector pBIIXLUC. Each transfection was performed in triplicate, and SD bars are shown. EC indicates endogenous control.
To identify the PER region domains involved in GILZ/NF-κB interaction we generated a number of mutants for this region that are represented in Figure 6A. First we tested truncated mutants, such as PER1 (truncated at 102 amino acid), PER2 (truncated at 114 amino acid), PER3 (truncated at 120 amino acid) and PER4 (truncated at 127 amino acid). Results indicate that PER1, PER2 and PER3 did not inhibit NF-κB transcriptional activity (Figure 6B). On the contrary, PER4 inhibited NF-κB activity at the same level as the entire wild-type GILZ (Figure 6B) suggesting that amino acids between 120 and 127 are important. Moreover, substitution of 121–127 amino acids with alanines, completely abrogates GILZ inhibitory effect (PER5, Figure 6B), suggesting that this domain is important for GILZ–NF-κB interaction. The PER region comprising 120–127 amino acids was further analyzed by mutating PxxP domains. PER6 (120–123 amino acids) and PER5 (121–127 amino acids) completely lost capability to inhibit NF-κB activity (Figure 6B), whereas PER7 (with alanine substitution in 123–126 amino acids) inhibited as wild-type GILZ did, thus indicating that mutation at the 121–123 within PxxP domain was sufficient to abrogate the GILZ inhibitory effect on NF-κB transcriptional activity. Of note, PER8 (115–118 amino acids) also trans-repressed as GILZ did (Figure 6B), thus indicating that mutants at other PxxP domains are irrelevant.
Finally, we performed experiments to evaluate the possible effect of post-translational GILZ modifications. In particular, we generated mutants to remove potential phopshorylation sites at positions 8, 88 and 114. All these mutants did not lose the ability to dimerize and to trans-repress NF-κB (data not shown), thus suggesting that the potential phopshorylation sites at position 8, 88 and 114 are not important for GILZ–NF-κB interaction.
Molecular dynamic simulation analysis
Results indicate that the three 121–123 amino acids, within the PxxP domain of the PER region are important for interaction with NF-κB. Moreover, PER7 with substituted proline at 123 tends to exclude a role of such residue. To address the role of proline and glutamic acid residues of PER domain in GILZ–p65NF-κB interaction, we generated an additional panel of mutants and tested for ability to dimerize and inhibit NF-κB transcriptional activity (PER9-11, Figure 6A). PER9, with all eight glutamic acid residues substituted with alanine, and PER10, with all 8 proline residues substituted with alanine retained GILZ ability to trans-repress NF-κB transcriptional activity. On the contrary, PER11, which contains alanine residues in place of all prolines and all glutamic acids, was not able to inhibit NF-κB transcriptional activity (Figure 6B).
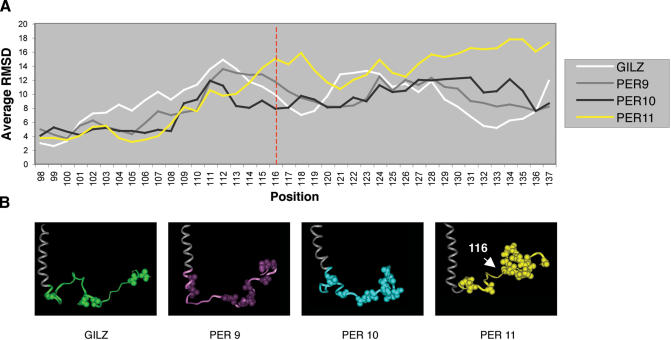
In order to gain insight into the effect that such a double mutation could produce in the PER region, we carried out four molecular dynamic simulations with GILZ and its mutants PER9, PER10 and PER11 to analyze their conformational space. In particular, we investigated how the proline and glutamate replacements would affect the conformation of the PER region. Indeed, the functional activity of protein domains is often linked to the stabilization/destabilization of active/inactive conformations. Although the active conformation of the PER region that binds to and inhibits NF-κB is not known, some information can be inferred from the comparative inspection of the conformational profiles displayed by active and inactive GILZ mutants along molecular dynamic simulations. Thus, we calculated the fluctuation of each residue belonging to the different domains of GILZ in the four systems. The residue fluctuation is an index of the stability of a given conformation in proteins and was calculated averaging the root mean square deviation for each atom position of the backbone during the whole time of simulation. In all four systems, the PER region is endowed with the highest average fluctuation compared to both the LZ and the N-terminal domain (Table 1). In particular, the mutant PER11 displays a higher fluctuation in the PER region than the wild-type GILZ and PER9-10 mutants (Figure 7A). This means that the original extended conformation of the PER region observed in GILZ is destabilized upon proline and glutamate replacements with alanine residues and the observed conformational movement is a bending of the PER region which reduces its overall extension (Figure 7B).
Table 1.
Averages and SDs of backbone fluctuation of residues in different domains of GILZ
| NTD region RMSD (Å) | LZ domain RMSD (Å) | PER region RMSD (Å) | |
|---|---|---|---|
| WT | 4.51 ± 1.02 | 5.47 ± 0.88 | 8.63 ± 2.34 |
| PER9 | 4.41 ± 0.96 | 5.62 ± 0.58 | 8.45 ± 2.43 |
| PER10 | 4.22 ± 1.14 | 5.15 ± 0.60 | 8.30 ± 2.41 |
| PER11 | 4.28 ± 1.35 | 5.46 ± 0.84 | 9.58 ± 3.54 |
Figure 7.
(A) Backbone fluctuation of residues of PER region in the GILZ and PER9-11 mutants. Position 116, where the conformational bending of PER11 mutant starts, is marked with a red line. (B) Comparison of GILZ, PER9, PER10 and PER11 mutant conformations obtained at the end of the simulation. Alanine residues are shown in CPK style.
Two events contribute to the driving force of such a movement: (i) the replacement of torsional constrained residues with alanines which reduces the rigidity of the PER region and (ii) the substitution of polar charged residues with hydrophobic ones that decreases the number of hydrogen bonds in water and, in turn, the stability of the extended PER chain. The simultaneous presence of both events is required to promote the above conformational shift. Indeed, the bending of the PER region is not observed in either GILZ or PER9-10 mutants, which in these events are respectively absent or partly present.
Since PER9-10 mutants maintain the ability to bind and inhibit NF-κB, the loss of NF-κB transcriptional inhibition on part of mutant PER11 is ascribed to the bending conformation adopted by the terminal PER region that hampers the interaction with NF-κB. Noteworthy, the bending occurs at position 116 of the PER region (Figure 7A). Thus, upon the conformational change, there is a shortening of the extension of the C-terminal region. We have shown that truncated proteins at positions 102 (PER1), 114 (PER2) and 120 (PER3), display an impaired ability to inhibit NF-κB. In agreement with these data, the results of molecular dynamic simulations confirm that an extended conformation of the region encompassing amino acid residues 116–137, comprising the 120–123 domains, is critical to the inhibitor activity of GILZ on NF-κB.
Mutants able to trans-repress NF-κB also inhibit anti-CD3-induced IL-2 production
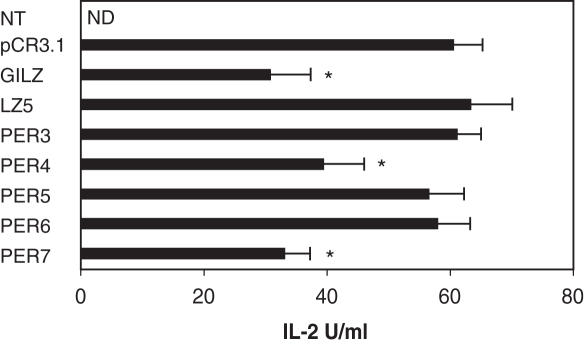
We have previously shown that GILZ inhibits TCR-induced IL-2 production in T cells (29). To evaluate the role of the specific GILZ/NF-κB interaction here described, we tested the effect of over-expression of GILZ and some of the above described mutants on IL-2 production in T cells. Results in Figure 8 show that all the mutants that did not inhibit NF-κB (LZ5, PER3, PER5 and PER6) did not counter IL-2 production in anti-CD3 stimulated 3DO T cells, whereas those that did inhibit NF-κB (PER4 and PER7) were also able to counter IL-2 production at levels comparable to GILZ wild-type.
Figure 8.
GILZ inhibits anti-CD3 IL-2 production. IL-2 production (U/ml) in untreated (NT) and anti-CD3 treated (1 μg/ml) for 18 h, empty vector transfected (pCR3.1) or GILZ or GILZ mutants (LZ5, PER3, PER4, PER5, PER6 and PER7) transfected 3DO cells as evaluated by ELISA assay. ND: not detected. Each transfection was performed in triplicate, and SD bars are shown. *P < 0.05.
These results indicate that mutations that impair GILZ–NF-κB interaction and inhibition of NF-κB transcriptional activity also impair the GILZ capability of inhibiting IL-2production.
DISCUSSION
GC are used in treatment of many human diseases but induce a number of different pharmacological effects which can cause adverse reactions that limit the drug use in patients (46,47). The deeper study of molecular mechanisms responsible for GC actions could suggest new therapeutic approaches aimed at avoiding unwanted adverse effects and increasing drug treatment efficacy.
Most of GC actions relate to their capability of antagonizing NF-κB activity through different molecular mechanisms (8,14,15) including induction of expression of GILZ, a transcription regulator able to bind to and inhibit NF-κB activity (29).
The aim of our work was to analyze the molecular features of GILZ underlying its ability to inhibit NF-κB activity. For this purpose we have generated a panel of GILZ mutants and tested their ability to bind and inhibit p65NF-κB. Results indicate that GILZ homo-dimerization is necessary for NF-κB inhibition and C-terminal PER region is also important, in particular the amino acids of the PxxP domain at position 120–123. Moreover, we demonstrated that the dimerization and inhibition are structurally separate and that both C-terminal domains are critical for function.
Among several GILZ mutants, we have identified several critical features for homo-dimerization. First, it is clear that the LZ (76–97 amino acids) domain, but not the C-terminal (98–137 amino acids) or the N-terminal (1–75 amino acids) domains, is responsible for homo-dimerization. In addition, characteristics of GILZ LZ are compatible with previously refined rules that dictate dimerization specificity and stability (40–45). For example, the amino acids sequence of the GILZ LZ it is four heptads long, it forms three attractive g–e interactions in 1st, 3rd and 4th heptad. Futhermore, it has isoleucin at a position, 2 asparagine at a position of 3rd and 4th heptad in addition to leucine in all four d positions. All these determinants strongly suggest that GILZ would form a stable homo-dimer and are compatible with our results. It is noteworthy that LZ6, which contains charged amino acids of the opposite polarity is still able to homo-dimerize, indicating that switching to opposite charges does not affect homo-dimer stability and further suggesting that it is the presence of positive and negative charges at specific positions rather than the amino acid identity that is important for stabilization of GILZ homo-dimer. Interestingly, all the mutants that do not homo-dimerize are not able to interact with p65NF-κB. Moreover, these mutants have lost the ability to inhibit the transactivating function of NF-κB. These data clearly indicate that GILZ homo-dimerization is essential for both binding to NF-κB and functional trans-repression of NF-κB transcriptional activity.
Previous studies suggest that GILZ is a molecule able to interact with different proteins (29,36). In particular, we have shown that GILZ can bind to Raf and that this interaction occurs in reason of a GILZ/Raf ratio of 1:1 (36). In fact, GILZ mutants unable to homo-dimerize interact with and inhibit Raf (36). Results here described indicate that GILZ homo-dimerization is necessary for GILZ–p65NF-κB interaction suggesting that GILZ can preferentially interact with Raf, and inhibit MAPK pathway acting as monomer, while interacting with and inhibiting NF-κB as a homo-dimer. Levels of GILZ expression during the time and/or intracellular distribution could allow the monomer or dimer formation to gain specificity for Raf or NF-κB and this hypothesis warrants further investigation.
To investigate the structural requirements of GILZ–NF-κB interaction, we have generated and analyzed N- and C-terminal deletion mutants of GILZ and we have found that neither C-terminal nor N-terminal parts of the protein are necessary for homo-dimerization. Nevertheless, mutant lacking C-terminal region (ΔC-GILZ), although able to homo-dimerize was not able to bind p65NF-κB nor to inhibit NF-κB transcriptional activity suggesting that, in addition to homo-dimerization, C-terminal part of GILZ is important for interaction with NF-κB and for its functional trans-repression. These results exclude a role for N-terminal domain that has been previously shown to be necessary for GILZ–Raf interaction (36). Thus while the C-terminal domain (PER region) is necessary for GILZ–NF-κB interaction, the N-terminal domain is necessary for GILZ–Raf interaction (36), further suggesting that, depending on the monomeric or homo-dimeric conformation and through different domains, GILZ can selectively interact with NF-κB and/or Raf.
Proline-rich proteins have been implicated in the regulation of many cell functions (20). Protein–to–protein interactions are often mediated by the recognition of proline-rich domains, such as the PxxP motif, by specific amino acid sequences including the SH3 and WW modules (20,48,49). Of note, GILZ C-terminal PER domain contains eight prolines as well as eight glutamic acids within the 40 amino acids of the C-terminal portion. Moreover, proline residues are organized in a way to form five PxxP domains. Although SH3 and WW domains are not present in NF-κB complex, we performed experiments to analyze the possible role of the prolines of the PER region. We have generated mutants of the GILZ PER region targeting proline and glutamate residues as well as altering the length of the region. First, we confirmed that alterations in the PER region do not affect the ability of GILZ to homo-dimerize, since neither truncations nor proline and/or glutamic acid substitutions affected the stability of GILZ homo-dimer (data not shown). Results indicated that GILZ–NF-κB interaction was abrogated by truncation of PER region at position 120, while it was still evident with mutant truncated at 127. Moreover, PER6, with mutated PxxP at 120–123 amino acids lost inhibitory effect in NF-κB activity, whereas PER7 and PER8, mutated at other PxxP domains, did maintain inhibitory activity comparable to that of GILZ wild-type thus suggesting that the PxxP at positions 120–123 or part of it, is important for GILZ/NF-κB interaction. PER5, with substituted amino acids at positions 121–127, did not inhibit NF-κB activity thus indicating that 121–123 amino acids, but not the canonical PxxP motif, are necessary for GILZ–NF-κB interaction.
Consistent with these results, substitution of all prolines (PER10) did not affect GILZ inhibitory effect on NF-κB transcriptional activity, thus indicating that proline residues are not necessary for GILZ–NF-κB interaction and further suggesting that the 121–122 amino acids, within the PxxP domain, are relevant for this effect.
Similar results were obtained when all glutamic acids were substituted. As an apparent exception, GILZ inhibitory activity was lost when all the proline and glutamic acid residues of PER region were substituted in the same mutant. Noteworthy, the comparison of GILZ wild-type, PER9, PER10 and PER11 mutant structures, obtained by molecular dynamic simulation analysis, showed that PER11 mutant adopted a bending conformation of the C-terminal region. In particular, the bending occurred at position 116, before 121 amino acid, and can be explained by the concomitant presence of two events: the replacement of all prolines that reduces the conformational rigidity of the PER region and the insertion of alanines in place of glutamates that decreases the stability of the C-terminal region in water. Thus, the overall effect of the removal of all prolines and glutamates is a conformational change that leads the PER region to shorten its extension at position 116. This is in agreement with the lack of activity observed in C-domain truncated mutants, further suggesting that PER region length, particularly the 121–123 domains is important in GILZ–p65NF-κB interaction.
These results are reinforced by investigation with GILZ and GILZ mutants, on inhibition of IL-2 production. As reported previously, GILZ is able to inhibit IL-2 production in anti-CD3-activated 3DO T cells (29). GILZ mutants that lack inhibitory activity on NF-κB failed to inhibit IL-2 production thus suggesting that molecular structural characteristics responsible for inhibition of NF-κB transcriptional activity have a biological relevance.
In conclusion, we have shown here results aimed at identifying the molecular requirements of GILZ–p65NF-κB interaction and evidencing the importance of homo-dimerization and of the C-terminal PER region, particularly the 121–123 amino acids. Results here reported and previous studies show that GILZ can bind NF-κB but not other LZ proteins such as Fra-1, thus suggesting a certain binding specificity (29). However, the NF-κB family is composed of a number of molecules that constitute a complex system of different homo- and hetero-dimers participating to transcription regulation (50) and future studies will be devoted to analyze GILZ interaction with other molecules of the NF-κB family. Identifying the molecular mechanisms of GILZ interaction with NF-κB family molecules may be an attractive possibility for future studies aimed at designing new molecules and therapeutic approaches able to mimic or antagonize GILZ–NF-κB interaction, thus modulating immune/inflammatory response.
Acknowledgments
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC), Milan, Italy. Funding to pay the Open Access publication charges for this article was provided by AIRC.
Conflict of interest statement. None declared.
REFERENCES
- 1.Iwata M., Hanaoka S., Sato K. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: a possible in vitro model for positive selection of the T cell repertoire. Eur. J. Immunol. 1991;21:643–648. doi: 10.1002/eji.1830210316. [DOI] [PubMed] [Google Scholar]
- 2.Kwak L.W., Longo D.L. Lymphomas. Cancer Chemother. Biol. Response Modif. 1996;16:376–440. [PubMed] [Google Scholar]
- 3.Raff M.C. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 4.Frankfurt O., Rosen S.T. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr. Opin. Oncol. 2004;16:553–563. doi: 10.1097/01.cco.0000142072.22226.09. [DOI] [PubMed] [Google Scholar]
- 5.Barnes P.J., Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol. Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- 6.Cupps T.R., Fauci A.S. Corticosteroid-mediated immunoregulation in man. Immunol. Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman G.S. Immunosuppressive therapy for autoimmune diseases. Ann. Allergy. 1993;70:263–274. [PubMed] [Google Scholar]
- 8.Auphan N., DiDonato J.A., Rosette C., Helmberg A., Karin M. Immunosuppression by glucocorticoids: inhibition of NF-kappa B activity through induction of I kappa B synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 9.Beato M. Transcriptional control by nuclear receptors. FASEB J. 1991;5:2044–2051. doi: 10.1096/fasebj.5.7.2010057. [DOI] [PubMed] [Google Scholar]
- 10.Diamond M.I., Miner J.N., Yoshinaga S.K., Yamamoto K.R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990;249:1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- 11.Riccardi C., Cifone M.G., Migliorati G. Glucocorticoid hormone-induced modulation of gene expression and regulation of T-cell death: role of GITR and GILZ, two dexamethasone-induced genes. Cell Death Differ. 1999;6:1182–1189. doi: 10.1038/sj.cdd.4400609. [DOI] [PubMed] [Google Scholar]
- 12.Ray A., Prefontaine K.E. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proc. Natl Acad. Sci. USA. 1994;91:752–756. doi: 10.1073/pnas.91.2.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schule R., Rangarajan P., Kliewer S., Ransone L.J., Bolado .J., Yang N., Verma I.M., Evans R.M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990;62:1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- 14.Scheinman R.I., Cogswell P.C., Lofquist A.K., Baldwin A.S., Jr Role of transcriptional activation of I kappa B alpha in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 15.De Bosscher K., Schmitz M.L., Vanden Berghe W., Plaisance S., Fiers W., Haegeman G. Glucocorticoid-mediated repression of nuclear factor-kappaB-dependent transcription involves direct interference with transactivation. Proc. Natl Acad. Sci. USA. 1997;94:13504–13509. doi: 10.1073/pnas.94.25.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D'Adamio F., Zollo O., Moraca R., Ayroldi E., Bruscoli S., Bartoli A., Cannarile L., Migliorati G., Riccardi C. A new dexamethasone-induced gene of the leucine zipper family protects T lymphocytes from TCR/CD3-activated cell death. Immunity. 1997;7:803–812. doi: 10.1016/s1074-7613(00)80398-2. [DOI] [PubMed] [Google Scholar]
- 17.Kester H.A., Blanchetot C., den Hertog J., van der Saag P.T., van der Burg B. Transforming growth factor-beta-stimulated clone-22 is a member of a family of leucine zipper proteins that can homo- and heterodimerize and has transcriptional repressor activity. J. Biol. Chem. 1999;274:27439–27447. doi: 10.1074/jbc.274.39.27439. [DOI] [PubMed] [Google Scholar]
- 18.Busch S.J., Sassone-Corsi P. Dimers, leucine zippers and DNA-binding domains. Trends Genet. 1990;6:36–40. doi: 10.1016/0168-9525(90)90071-d. [DOI] [PubMed] [Google Scholar]
- 19.Vinson C.R., Sigler P.B., McKnight S.L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989;246:911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- 20.Holt M.R., Koffer A. Cell motility: proline-rich proteins promote protrusions. Trends Cell. Biol. 2001;11:38–46. doi: 10.1016/s0962-8924(00)01876-6. [DOI] [PubMed] [Google Scholar]
- 21.Landschulz W.H., Johnson P.F., McKnight S.L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988;240:1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- 22.Alber T. Structure of the leucine zipper. Curr. Opin. Genet. Dev. 1992;2:205–210. doi: 10.1016/s0959-437x(05)80275-8. [DOI] [PubMed] [Google Scholar]
- 23.Hurst H.C. Transcription factors. 1: bZIP proteins. Protein Profile. 1994;1:123–168. [PubMed] [Google Scholar]
- 24.Nomura T., Sakai N., Sarai A., Sudo T., Kanei-Ishii C., Ramsay R.G., Favier D., Gonda T.J., Ishii S. Negative autoregulation of c-Myb activity by homodimer formation through the leucine zipper. J. Biol. Chem. 1993;268:21914–21923. [PubMed] [Google Scholar]
- 25.Sassone-Corsi P., Ransone L.J., Lamph W.W., Verma I.M. Direct interaction between fos and jun nuclear oncoproteins: role of the ‘leucine zipper’ domain. Nature. 1988;336:692–695. doi: 10.1038/336692a0. [DOI] [PubMed] [Google Scholar]
- 26.Podust L.M., Krezel A.M., Kim Y. Crystal structure of the CCAAT box/enhancer-binding protein beta activating transcription factor-4 basic leucine zipper heterodimer in the absence of DNA. J. Biol. Chem. 2001;276:505–513. doi: 10.1074/jbc.M005594200. [DOI] [PubMed] [Google Scholar]
- 27.O'Shea E.K., Klemm J.D., Kim P.S., Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 28.Cannarile L., Zollo O., D'Adamio F., Ayroldi E., Marchetti C., Tabilio A., Bruscoli S., Riccardi C. Cloning, chromosomal assignment and tissue distribution of human GILZ, a glucocorticoid hormone-induced gene. Cell Death Differ. 2001;8:201–203. doi: 10.1038/sj.cdd.4400798. [DOI] [PubMed] [Google Scholar]
- 29.Ayroldi E., Migliorati G., Bruscoli S., Marchetti C., Zollo O., Cannarile L., D'Adamio F., Riccardi C. Modulation of T-cell activation by the glucocorticoid-induced leucine zipper factor via inhibition of nuclear factor kappaB. Blood. 2001;98:743–753. doi: 10.1182/blood.v98.3.743. [DOI] [PubMed] [Google Scholar]
- 30.Riccardi C., Zollo O., Nocentini G., Bruscoli S., Bartoli A., D'Adamio F., Cannarile L., Delfino D., Ayroldi E., Migliorati G. Glucocorticoid hormones in the regulation of cell death. Therapie. 2000;55:165–169. [PubMed] [Google Scholar]
- 31.Berrebi D., Bruscoli S., Cohen N., Foussat A., Migliorati G., Bouchet-Delbos L., Maillot M.C., Portier A., Couderc J., Galanaud P., et al. Synthesis of glucocorticoid-induced leucine zipper (GILZ) by macrophages: an anti-inflammatory and immunosuppressive mechanism shared by glucocorticoids and IL-10. Blood. 2003;101:729–738. doi: 10.1182/blood-2002-02-0538. [DOI] [PubMed] [Google Scholar]
- 32.Cannarile L., Fallarino F., Agostini M., Cuzzocrea S., Mazzon E., Vacca C., Genovese T., Migliorati G., Ayroldi E., Riccardi C. Increased GILZ expression in transgenic mice up-regulates Th-2 lymphokines. Blood. 2006;107:1039–1047. doi: 10.1182/blood-2005-05-2183. [DOI] [PubMed] [Google Scholar]
- 33.Chang C.C., Zhang J., Lombardi L., Neri A., Dalla-Favera R. Mechanism of expression and role in transcriptional control of the proto-oncogene NFKB-2/LYT-10. Oncogene. 1994;9:923–933. [PubMed] [Google Scholar]
- 34.Luo Z.J., Zhang X.F., Rapp U., Avruch J. Identification of the 14.3.3 zeta domains important for self-association and Raf binding. J. Biol. Chem. 1995;270:23681–23687. doi: 10.1074/jbc.270.40.23681. [DOI] [PubMed] [Google Scholar]
- 35.Ayroldi E., Migliorati G., Cannarile L., Moraca R., Delfino D.V., Riccardi C. CD2 rescues T cells from T-cell receptor/CD3 apoptosis: a role for the Fas/Fas-L system. Blood. 1997;89:3717–3726. [PubMed] [Google Scholar]
- 36.Ayroldi E., Zollo O., Macchiarulo A., Di Marco B., Marchetti C., Riccardi C. Glucocorticoid-induced leucine zipper inhibits the Raf-extracellular signal-regulated kinase pathway by binding to Raf-1. Mol. Cell. Biol. 2002;22:7929–7941. doi: 10.1128/MCB.22.22.7929-7941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bernhard R.B., Bruccoleri R.E., Olafson B.D., States D.J., Swaminathan S., Karplus M. CHARMM: a program for macromolecular energy, minimization, and dynamics calculations. J. Comp. Chem. 1983;4:187–217. [Google Scholar]
- 38.Laxmikant K., Skeel R., Bhanbarkar M., Brunner R., Gursoy A., Krawetz N., Phillips J., Shinozaki A., Varadarajan K., Schulten K. NAMD2: greater scalability for parallel molecular dynamics. J. Comp. Phys. 1999;151:283–312. [Google Scholar]
- 39.Mittelstadt P.R., Ashwell J.D. Inhibition of AP-1 by the glucocorticoid-inducible protein GILZ. J. Biol. Chem. 2001;276:29603–29610. doi: 10.1074/jbc.M101522200. [DOI] [PubMed] [Google Scholar]
- 40.Landschulz W.H., Johnson P.F., McKnight S.L. The DNA binding domain of the rat liver nuclear protein C/EBP is bipartite. Science. 1989;243:1681–1688. doi: 10.1126/science.2494700. [DOI] [PubMed] [Google Scholar]
- 41.Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988;336:646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- 42.Acharya A., Ruvinov S.B., Gal J., Moll J.R., Vinson C. A heterodimerizing leucine zipper coiled coil system for examining the specificity of a position interactions: amino acids I, V, L, N, A, and K. Biochemistry. 2002;41:14122–14131. doi: 10.1021/bi020486r. [DOI] [PubMed] [Google Scholar]
- 43.Zeng X., Herndon A.M., Hu J.C. Buried asparagines determine the dimerization specificities of leucine zipper mutants. Proc. Natl Acad. Sci. USA. 1997;94:3673–3678. doi: 10.1073/pnas.94.8.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tchan M.C., Choy K.J., Mackay J.P., Lyons A.T., Bains N.P., Weiss A.S. Interfacial asparagine residues within an amide tetrad contribute to Max helix-loop-helix leucine zipper homodimer stability. J. Biol. Chem. 2000;275:37454–37461. doi: 10.1074/jbc.M004264200. [DOI] [PubMed] [Google Scholar]
- 45.Zeng X., Zhu H., Lashuel H.A., Hu J.C. Oligomerization properties of GCN4 leucine zipper e and g position mutants. Protein Sci. 1997;6:2218–2226. doi: 10.1002/pro.5560061016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bijlsma J.W., Van Everdingen A.A., Huisman M., De Nijs R.N., Jacobs J.W. Glucocorticoids in rheumatoid arthritis: effects on erosions and bone. Ann. N. Y. Acad. Sci. 2002;966:82–90. doi: 10.1111/j.1749-6632.2002.tb04205.x. [DOI] [PubMed] [Google Scholar]
- 47.Adcock I.M. Glucocorticoid-regulated transcription factors. Pulm. Pharmacol. Ther. 2001;14:211–219. doi: 10.1006/pupt.2001.0283. [DOI] [PubMed] [Google Scholar]
- 48.Chen H.I., Einbond A., Kwak S.J., Linn H., Koepf E., Peterson S., Kelly J.W., Sudol M. Characterization of the WW domain of human yes-associated protein and its polyproline-containing ligands. J. Biol. Chem. 1997;272:17070–17077. doi: 10.1074/jbc.272.27.17070. [DOI] [PubMed] [Google Scholar]
- 49.Weng Z., Rickles R.J., Feng S., Richard S., Shaw A.S., Schreiber S.L., Brugge J.S. Structure-function analysis of SH3 domains: SH3 binding specificity altered by single amino acid substitutions. Mol. Cell. Biol. 1995;15:5627–5634. doi: 10.1128/mcb.15.10.5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosh S., May M.J., Kopp E.B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]