Abstract
Forty-one isolates of multiply resistant gram-negative bacteria causing infection in a urological unit of a Dublin hospital were collected during a 6-month period. Twenty-one isolates transferred multiple resistance to an Escherichia coli K-12 recipient in liquid matings. Serratia marcescens, Proteus morganii, Proteus vulgaris, and E. coli isolates harbored similar 120-megadalton IncC plasmids, whereas Enterobacter cloacae strains transferred a 160-megadalton plasmid of a different Inc group. Southern hybridization experiments were performed with purified fragments cloned from one IncC plasmid as probes. They were hybridized to plasmid sequences in total cellular DNA extracts, showing that the IncC plasmids were very closely related. This suggests that the same plasmid has transferred to different bacterial species in the hospital environment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkharn J. I., Hughes V. M. Suction apparatus and hospital infection due to multiply-resistant Klebsiella aerogenes. J Hosp Infect. 1982 Jun;3(2):173–178. doi: 10.1016/0195-6701(82)90010-x. [DOI] [PubMed] [Google Scholar]
- Bullock D. W., Bidwell J. L., Reeves D. S., White L. O., Turner A., Speller D. C., Wilkinson P. J. Outbreaks of hospital infection in southwest England caused by gentamicin-resistant Serratia marcescens. J Hosp Infect. 1982 Sep;3(3):263–273. doi: 10.1016/0195-6701(82)90045-7. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Effect of growth conditions on the formation of the relaxation complex of supercoiled ColE1 deoxyribonucleic acid and protein in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1135–1146. doi: 10.1128/jb.110.3.1135-1146.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Coleman D., Falkiner F. R., Carr M. E., Dowd G., Dougan G., Keane C. T. Simultaneous outbreaks of infection due to Serratia marcescens in a general hospital. J Hosp Infect. 1984 Sep;5(3):270–282. doi: 10.1016/0195-6701(84)90076-8. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman C. J., Foster T. J. Posttranscriptional regulation of the inducible nonenzymatic chloramphenicol resistance determinant of IncP plasmid R26. J Bacteriol. 1985 Jan;161(1):147–152. doi: 10.1128/jb.161.1.147-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Inamine J. M., Minshew B. H. Common plasmid specifying tobramycin resistance found in two enteric bacteria isolated from burn patients. Antimicrob Agents Chemother. 1978 Feb;13(2):312–317. doi: 10.1128/aac.13.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkiner F. R., Keane C. T., Dalton M., Clancy M. T., Jacoby G. A. Cross infection in a surgical ward caused by Pseudomonas aeruginosa with transferable resistance to gentamicin and tobramycin. J Clin Pathol. 1977 Aug;30(8):731–737. doi: 10.1136/jcp.30.8.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E., Jr Molecular analysis of plasmids in epidemiologic investigation. J Infect Dis. 1983 Jul;148(1):1–6. doi: 10.1093/infdis/148.1.1. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Howe T. G., Richmond K. M. Translocation of the tetracycline resistance determinant from R100-1 to the Escherichia coli K-12 chromosome. J Bacteriol. 1975 Dec;124(3):1153–1158. doi: 10.1128/jb.124.3.1153-1158.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster T. J. Plasmid-determined resistance to antimicrobial drugs and toxic metal ions in bacteria. Microbiol Rev. 1983 Sep;47(3):361–409. doi: 10.1128/mr.47.3.361-409.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French G. L., Casewell M. W., Roncoroni A. J., Knight S., Phillips I. A hospital outbreak of antibiotic-resistant Acinetobacter anitratus: epidemiology and control. J Hosp Infect. 1980 Jun;1(2):125–131. doi: 10.1016/0195-6701(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Gaffney D. F., Cundliffe E., Foster T. J. Chloramphenicol resistance that does not involve chloramphenicol acetyltransferase encoded by plasmids from gram-negative bacteria. J Gen Microbiol. 1981 Jul;125(1):113–121. doi: 10.1099/00221287-125-1-113. [DOI] [PubMed] [Google Scholar]
- Goldstein F. W., Labigne-Roussel A., Gerbaud G., Carlier C., Collatz E., Courvalin P. Transferable plasmid-mediated antibiotic resistance in Acinetobacter. Plasmid. 1983 Sep;10(2):138–147. doi: 10.1016/0147-619x(83)90066-5. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. A., Gibson M. F. Comparative epidemiology of gentamicin-resistant enterobacteria: persistence of carriage and infection. J Clin Pathol. 1982 Apr;35(4):452–457. doi: 10.1136/jcp.35.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. A. Nosocomial gentamicin- and multiply-resistant enterobacteria at one hospital. 1. Description of an outbreak. J Hosp Infect. 1982 Mar;3(1):15–28. doi: 10.1016/0195-6701(82)90027-5. [DOI] [PubMed] [Google Scholar]
- Hart C. A. Nosocomial gentamicin- and multiply-resistant enterobacteria at one hospital. Factors associated with carriage. J Hosp Infect. 1982 Jun;3(2):165–172. doi: 10.1016/0195-6701(82)90009-3. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Rodriguez-Lemoine V., Datta N. R factors from Serratia marcescens. J Gen Microbiol. 1975 Jan;86(1):88–92. doi: 10.1099/00221287-86-1-88. [DOI] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
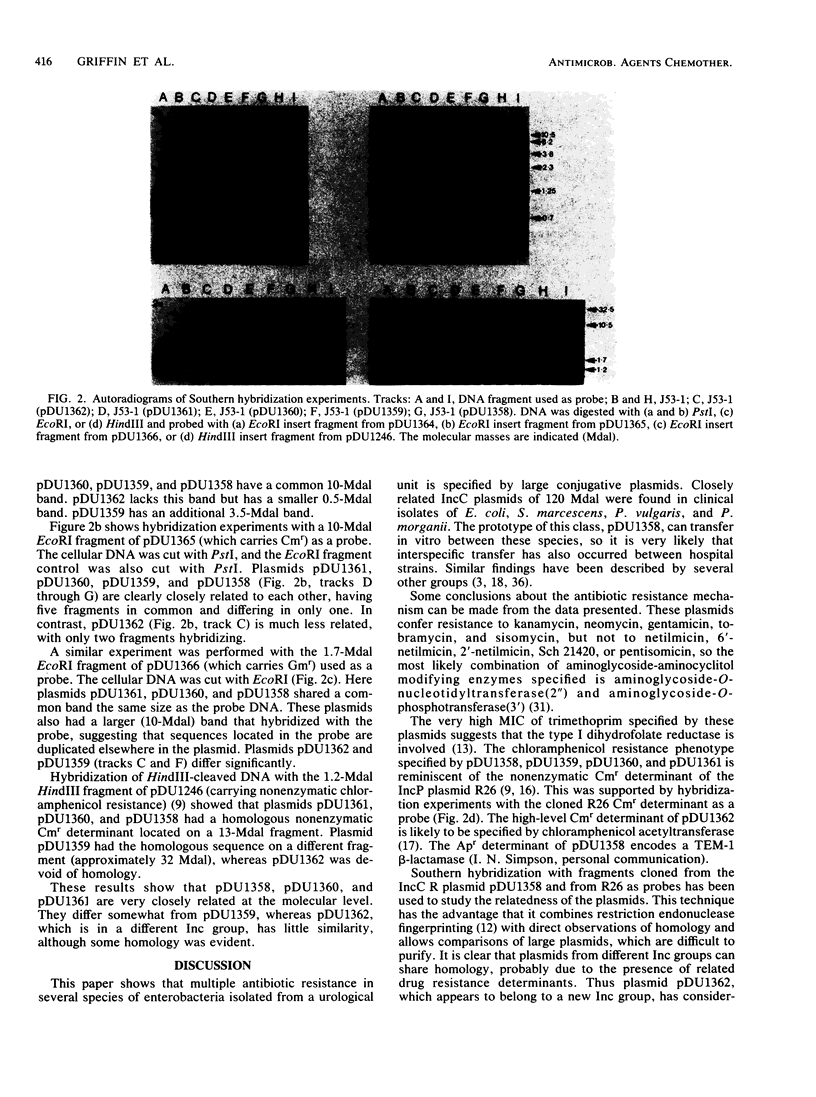
- Lee S. C., Gerding D. N., Cleary P. P. Plasmid macroevolution in a nosocomial environment: demonstration of a persistent molecular polymorphism and construction of a cladistic phylogeny on the basis of restriction data. Mol Gen Genet. 1984;194(1-2):173–178. doi: 10.1007/BF00383513. [DOI] [PubMed] [Google Scholar]
- Lowes J. A., Smith J., Tabaqchali S., Shaw E. J. Outbreak of infection in a urological ward. Br Med J. 1980 Mar 8;280(6215):722–722. [PMC free article] [PubMed] [Google Scholar]
- McHale P. J., Walker F., Scully B., English L., Keane C. T. Providencia stuartii infections: a review of 117 cases over an eight year period. J Hosp Infect. 1981 Jun;2(2):155–165. doi: 10.1016/0195-6701(81)90024-4. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983 Nov;35(1):253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- Mendez F. J., Mendoza M. C., Llaneza J. J., Hardisson C. Transfer of drug-resistance plasmids by conjugation from nosocomial strains of Serratia marcescens to Escherichia coli in biological fluids of human origin. J Hosp Infect. 1982 Sep;3(3):285–292. doi: 10.1016/0195-6701(82)90047-0. [DOI] [PubMed] [Google Scholar]
- Ni'Bhriain N. N., Silver S., Foster T. J. Tn5 insertion mutations in the mercuric ion resistance genes derived from plasmid R100. J Bacteriol. 1983 Aug;155(2):690–703. doi: 10.1128/jb.155.2.690-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. F., Ross D. G., Guzman M. A., Medeiros A. A., Hedges R. W., Botstein D. Dissemination of an antibiotic resistance plasmid in hospital patient flora. Antimicrob Agents Chemother. 1980 Apr;17(4):537–543. doi: 10.1128/aac.17.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rubens C. E., Farrar W. E., Jr, McGee Z. A., Schaffner W. Evolution of a plasmid mediating resistance to multiple antimicrobial agents during a prolonged epidemic of nosocomial infections. J Infect Dis. 1981 Feb;143(2):170–181. doi: 10.1093/infdis/143.2.170. [DOI] [PubMed] [Google Scholar]
- Schaberg D. R., Haley R. W., Highsmith A. K., Anderson R. L., McGowan J. E., Jr Nosocomial bacteriuria: a prospective study of case clustering and antimicrobial resistance. Ann Intern Med. 1980 Sep;93(3):420–424. doi: 10.7326/0003-4819-93-3-420. [DOI] [PubMed] [Google Scholar]
- Shlaes D. M., Vartian C., Currie C. A. Variability in DNA sequence of closely related nosocomial gentamicin-resistant plasmids. J Infect Dis. 1983 Dec;148(6):1013–1018. doi: 10.1093/infdis/148.6.1013. [DOI] [PubMed] [Google Scholar]
- Smith P. W., Rusnak P. G. Aminoglycoside-resistant Pseudomonas aeruginosa urinary tract infection: study of an outbreak. J Hosp Infect. 1981 Mar;2(1):71–75. doi: 10.1016/0195-6701(81)90008-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tompkins L. S., Plorde J. J., Falkow S. Molecular analysis of R-factors from multiresistant nosocomial isolates. J Infect Dis. 1980 May;141(5):625–636. doi: 10.1093/infdis/141.5.625. [DOI] [PubMed] [Google Scholar]