Abstract
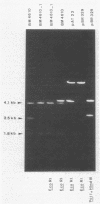
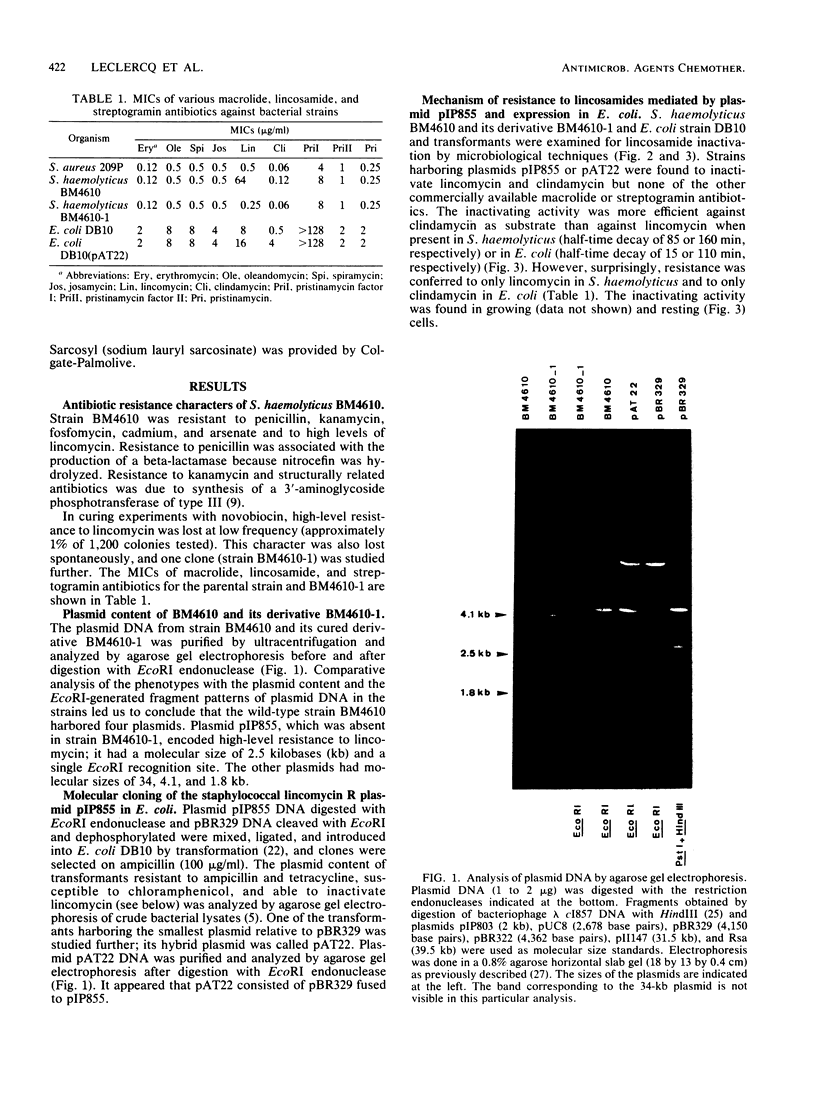
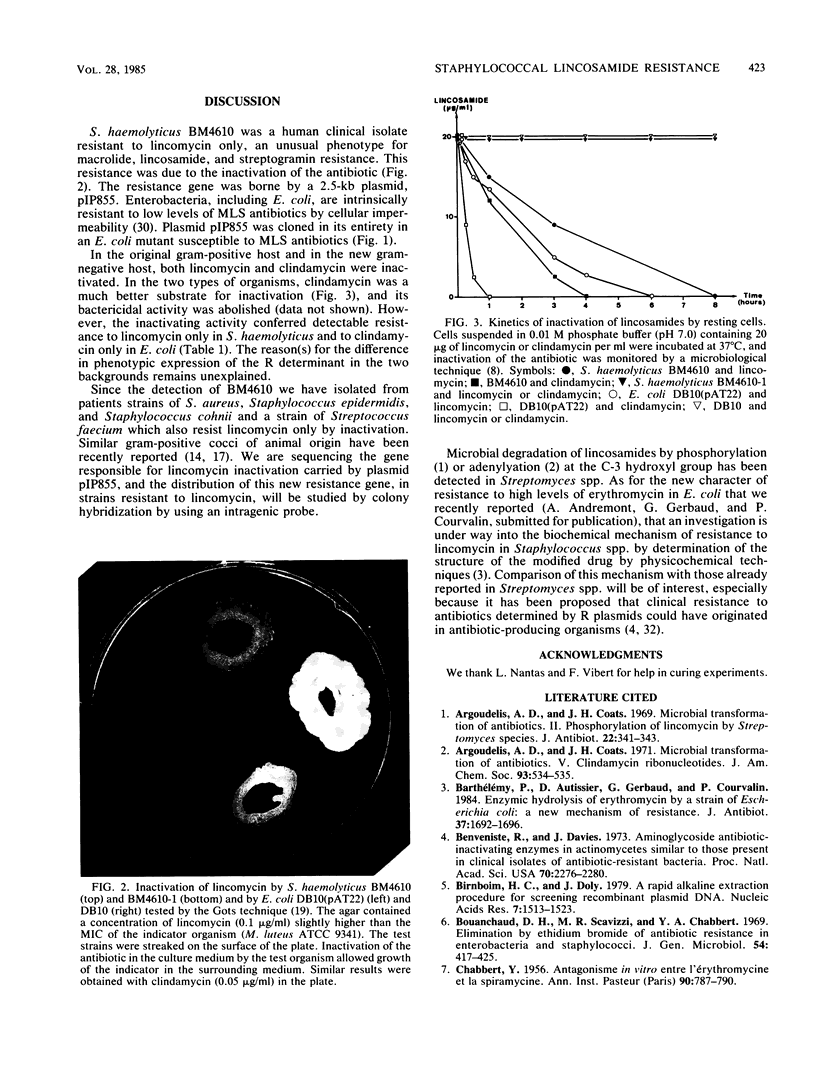
Staphylococcus haemolyticus BM4610 was resistant to high levels of lincomycin and susceptible to macrolides, clindamycin, and streptogramins. This resistance phenotype, not previously reported for a human clinical isolate, was due to inactivation of the antibiotic. The gene conferring resistance to lincomycin in strain BM4610 was carried by a 2.5-kilobase plasmid, pIP855, which was cloned in Escherichia coli. Plasmid pIP855 caused inactivation of both lincomycin and clindamycin in S. haemolyticus and in E. coli but conferred detectable resistance to lincomycin only in S. haemolyticus and to clindamycin only in E. coli.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argoudelis A. D., Coats J. H. Microbial transformation of antibiotics. II. Phosphorylation of lincomycin by Streptomyces species. J Antibiot (Tokyo) 1969 Jul;22(7):341–343. doi: 10.7164/antibiotics.22.341. [DOI] [PubMed] [Google Scholar]
- Argoudelis A. D., Coats J. H. Microbial transformation of antibiotics. V. Clindamycin ribonucleotides. J Am Chem Soc. 1971 Jan 27;93(2):534–535. doi: 10.1021/ja00731a047. [DOI] [PubMed] [Google Scholar]
- Barthélémy P., Autissier D., Gerbaud G., Courvalin P. Enzymic hydrolysis of erythromycin by a strain of Escherichia coli. A new mechanism of resistance. J Antibiot (Tokyo) 1984 Dec;37(12):1692–1696. doi: 10.7164/antibiotics.37.1692. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Aminoglycoside antibiotic-inactivating enzymes in actinomycetes similar to those present in clinical isolates of antibiotic-resistant bacteria. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2276–2280. doi: 10.1073/pnas.70.8.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- CHABBERT Y. Antagonisme in vitro entre l'érythromycine et la spiramycine. Ann Inst Pasteur (Paris) 1956 Jun;90(6):787–790. [PubMed] [Google Scholar]
- CHABBERT Y., BOULINGRE H. Modifications pratiques concernant le dosage des antibiotiques en clinique. Rev Fr Etud Clin Biol. 1957 Jun;2(6):636–640. [PubMed] [Google Scholar]
- Courvalin P., Davies J. Plasmid-medicated aminoglycoside phosphotransferase of broad substrate range that phosphorylates amikacin. Antimicrob Agents Chemother. 1977 Apr;11(4):619–624. doi: 10.1128/aac.11.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Fiandt M. Aminoglycoside-modifying enzymes of Staphylococcus aureus; expression in Escherichia coli. Gene. 1980 May;9(3-4):247–269. doi: 10.1016/0378-1119(90)90326-m. [DOI] [PubMed] [Google Scholar]
- Courvalin P., Ounissi H., Arthur M. Multiplicity of macrolide-lincosamide-streptogramin antibiotic resistance determinants. J Antimicrob Chemother. 1985 Jul;16 (Suppl A):91–100. doi: 10.1093/jac/16.suppl_a.91. [DOI] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Becker D., Davies J. Plasmid-determined fusidic acid resistance in the Enterobacteriaceae. J Gen Microbiol. 1974 Jul;83(0):191–196. doi: 10.1099/00221287-83-1-191. [DOI] [PubMed] [Google Scholar]
- Devriese L. A. Two new types of resistance to lincomycin in pathogenic staphylococci from animals. Ann Microbiol (Paris) 1980 Nov-Dec;131B(3):261–266. [PubMed] [Google Scholar]
- Dublanchet A., Soussy C. J., Squinazi F., Duval J. Résistance de Staphylococcus aureus aux streptogramines. Ann Microbiol (Paris) 1977 Apr;128A(3):277–287. [PubMed] [Google Scholar]
- Dutta G. N., Devriese L. A. Resistance to macrolide, lincosamide and streptogramin antibiotics and degradation of lincosamide antibiotics in streptococci from bovine mastitis. J Antimicrob Chemother. 1982 Nov;10(5):403–408. doi: 10.1093/jac/10.5.403. [DOI] [PubMed] [Google Scholar]
- El Solh N., Bismuth R., Allignet J., Fouace J. M. Résistance à la pristinamycine (ou virginiamycine) des souches de Staphylococcus aureus. Pathol Biol (Paris) 1984 May;32(5):362–368. [PubMed] [Google Scholar]
- Gots J. S. THE DETECTION OF PENICILLINASE-PRODUCING PROPERTIES OF MICROORGANISMS. Science. 1945 Sep 21;102(2647):309–309. doi: 10.1126/science.102.2647.309. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- McHugh G. L., Swartz M. N. Elimination of plasmids from several bacterial species by novobiocin. Antimicrob Agents Chemother. 1977 Sep;12(3):423–426. doi: 10.1128/aac.12.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Hong G. F., Hill D. F., Petersen G. B. Nucleotide sequence of bacteriophage lambda DNA. J Mol Biol. 1982 Dec 25;162(4):729–773. doi: 10.1016/0022-2836(82)90546-0. [DOI] [PubMed] [Google Scholar]
- Shinnick T. M., Lund E., Smithies O., Blattner F. R. Hybridization of labeled RNA to DNA in agarose gels. Nucleic Acids Res. 1975 Oct;2(10):1911–1929. doi: 10.1093/nar/2.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUBENECK U. Susceptibility of Proteus mirabilis and its stable L-forms to erythromycin and other macrolides. Nature. 1962 Oct 13;196:195–196. doi: 10.1038/196195b0. [DOI] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. S., Walker J. B. Streptomycin biosynthesis and metabolism. Enzymatic phosphorylation of dihydrostreptobiosamine moieties of dihydro-streptomycin-(streptidino) phosphate and dihydrostreptomycin by Streptomyces extracts. J Biol Chem. 1970 Dec 25;245(24):6683–6689. [PubMed] [Google Scholar]
- Weisblum B. Inducible erythromycin resistance in bacteria. Br Med Bull. 1984 Jan;40(1):47–53. doi: 10.1093/oxfordjournals.bmb.a071947. [DOI] [PubMed] [Google Scholar]