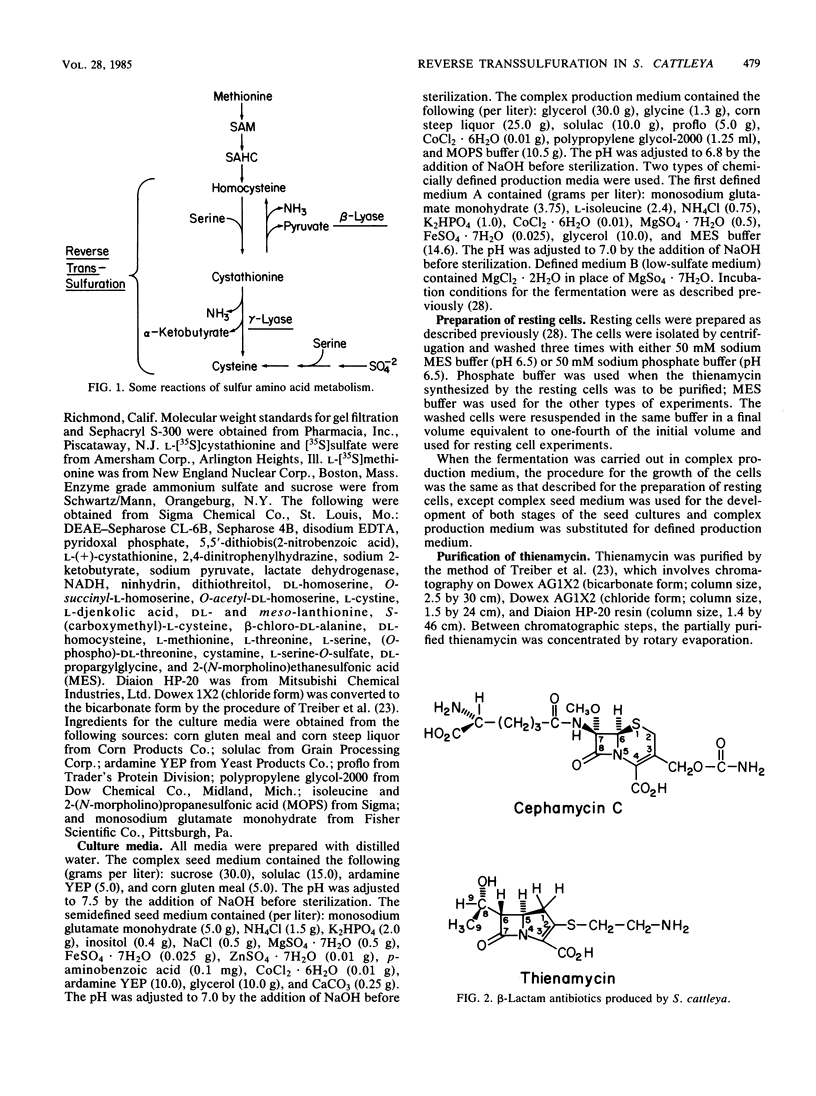
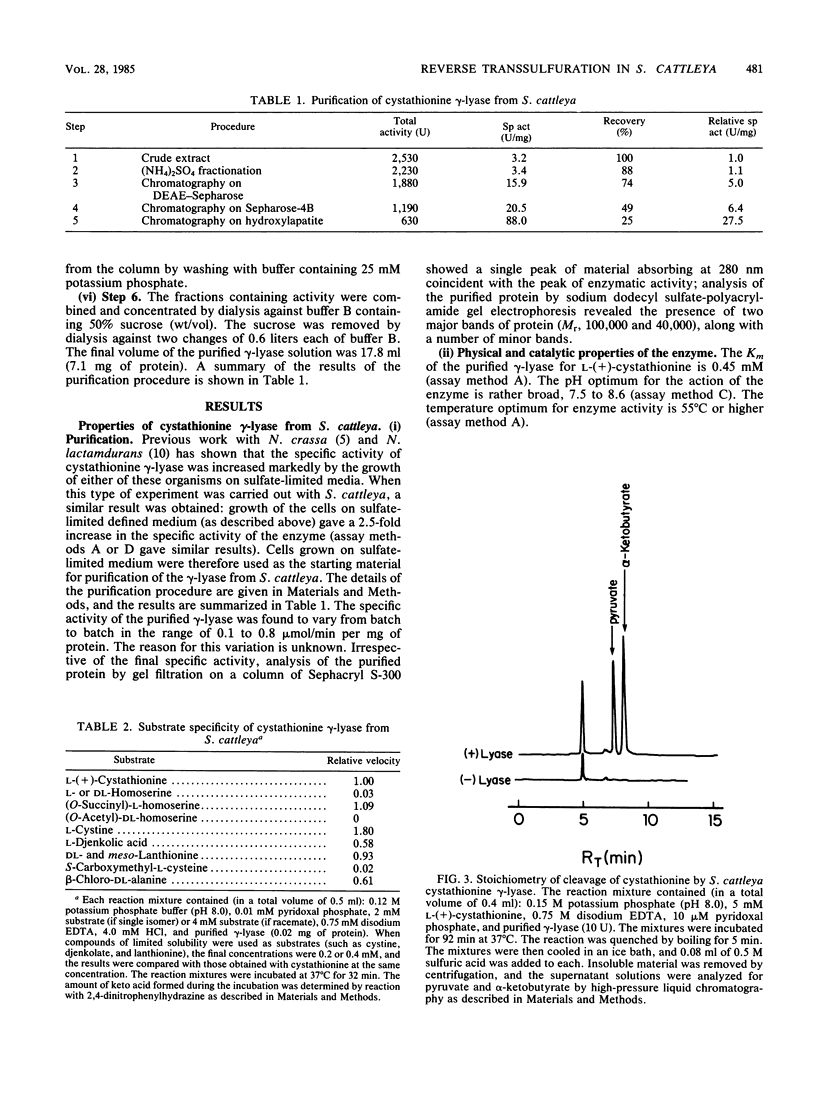
Abstract
Cystathionine gamma-lyase (EC 4.4.1.1) was purified from Streptomyces cattleya, an actinomycete which produces the unusual beta-lactam antibiotic thienamycin. The enzyme displays broad substrate specificity and is similar to gamma-lyases purified from other microorganisms. That the gamma-lyase functions in vivo to provide cysteine for antibiotic synthesis was shown by two types of experiments. First, cystathionine and methionine, as well as cysteine itself, are efficiently utilized by S. cattleya for thienamycin biosynthesis. Second, propargylglycine, a mechanism-based inactivator of cystathionine gamma-lyase in vitro, inhibits the synthesis of thienamycin in vivo. This inhibition can be substantially reversed by providing the cells with another source of cysteine, such as cystine.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles R. H., Walsh C. T. Acetylenic enzyme inactivators. Inactivation of gamma-cystathionase, in vitro and in vivo, by propargylglycine. J Am Chem Soc. 1973 Sep 5;95(18):6124–6125. doi: 10.1021/ja00799a053. [DOI] [PubMed] [Google Scholar]
- Caltrider P. G., Niss H. F. Role of methionine in cephalosporin synthesis. Appl Microbiol. 1966 Sep;14(5):746–753. doi: 10.1128/am.14.5.746-753.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DELAVIER-KLUTCHKO C., FLAVIN M. ENZYMATIC SYNTHESIS AND CLEAVAGE OF CYSTATHIONINE IN FUNGI AND BACTERIA. J Biol Chem. 1965 Jun;240:2537–2549. [PubMed] [Google Scholar]
- FLAVIN M., SEGAL A. PURIFICATION AND PROPERTIES OF THE CYSTATHIONINE GAMMA-CLEAVAGE ENZYME OF NEUROSPORA. J Biol Chem. 1964 Jul;239:2220–2227. [PubMed] [Google Scholar]
- Flavin M., Slaughter C. The derepression and function of enzymes of reverse trans-sulfuration in Neurospora. Biochim Biophys Acta. 1967 Mar 15;132(2):406–411. doi: 10.1016/0005-2744(67)90159-3. [DOI] [PubMed] [Google Scholar]
- Gaitonde M. K. A spectrophotometric method for the direct determination of cysteine in the presence of other naturally occurring amino acids. Biochem J. 1967 Aug;104(2):627–633. doi: 10.1042/bj1040627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan J. S., Kahan F. M., Goegelman R., Currie S. A., Jackson M., Stapley E. O., Miller T. W., Miller A. K., Hendlin D., Mochales S. Thienamycin, a new beta-lactam antibiotic. I. Discovery, taxonomy, isolation and physical properties. J Antibiot (Tokyo) 1979 Jan;32(1):1–12. doi: 10.7164/antibiotics.32.1. [DOI] [PubMed] [Google Scholar]
- Kern B. A., Inamine E. Cystathionine gamma-lyase activity in the cephamycin C producer Streptomyces lactamdurans. J Antibiot (Tokyo) 1981 May;34(5):583–589. doi: 10.7164/antibiotics.34.583. [DOI] [PubMed] [Google Scholar]
- Komatsu K. I., Kodaira R. Sulfur metabolism of a mutant of Cephalosporium acremonium with enhanced potential to utilize sulfate for cephalosporin C production. J Antibiot (Tokyo) 1977 Mar;30(3):226–233. doi: 10.7164/antibiotics.30.226. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MATSUO Y., GREENBERG D. M. A crystalline enzyme that cleaves homoserine and cystathionine. I. Isolation procedure and some physicochemical properties. J Biol Chem. 1958 Feb;230(2):545–560. [PubMed] [Google Scholar]
- MATSUO Y., GREENBERG D. M. A crystalline enzyme that cleaves homoserine and cystathionine. IV. Mechanism of action, reversibility, and substrate specificity. J Biol Chem. 1959 Mar;234(3):516–519. [PubMed] [Google Scholar]
- Nagasawa T., Kanzaki H., Yamada H. Cystathionine gamma-lyase of Streptomyces phaeochromogenes. The occurrence of cystathionine gamma-lyase in filamentous bacteria and its purification and characterization. J Biol Chem. 1984 Aug 25;259(16):10393–10403. [PubMed] [Google Scholar]
- SEGEL I. H., JOHNSON M. J. Accumulation of intracellular inorganic sulfate by Penicillium chrysogenum. J Bacteriol. 1961 Jan;81:91–98. doi: 10.1128/jb.81.1.91-98.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scannell J. P., Pruess D. L., Demny T. C., Weiss F., Williams T. Antimetabolites produced by microorganisms. II. L-2-amino-4-pentynoic acid. J Antibiot (Tokyo) 1971 Apr;24(4):239–244. doi: 10.7164/antibiotics.24.239. [DOI] [PubMed] [Google Scholar]
- TARDREW P. L., JOHNSON M. J. Sulfate utilization by penicillin-producing mutants of Penicillium chrysogenum. J Bacteriol. 1958 Oct;76(4):400–405. doi: 10.1128/jb.76.4.400-405.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney J. G., Brannon D. R., Mabe J. A., Wicker K. J. Incorporation of labeled precursors into A16886B, a novel -lactam antibiotic produced by Streptomyces clavuligerus. Antimicrob Agents Chemother. 1972 Mar;1(3):247–251. doi: 10.1128/aac.1.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. M., Inamine E., Wilson K. E., Douglas A. W., Liesch J. M., Albers-Schönberg G. Biosynthesis of the beta-lactam antibiotic, thienamycin, by Streptomyces cattleya. J Biol Chem. 1985 Apr 25;260(8):4637–4647. [PubMed] [Google Scholar]


