Abstract
Many of the current antimycobacterial agents require some form of cellular activation unmasking reactive groups, which in turn will bind to their specific targets. Therefore, understanding the mechanisms of activation of current antimycobacterials not only helps to decipher mechanisms of drug resistance but may also facilitate the development of alternative activation strategies or of analogues that do not require such processes. Herein, through the use of genetically defined strains of Mycobacterium bovis BCG we provide evidence that EthA, previously shown to activate ethionamide, also converts isoxyl (ISO) and thiacetazone (TAC) into reactive species. These results were further supported by the development of an in vitro assay using purified recombinant EthA, which allowed direct assessment of the metabolism of ISO. Interestingly, biochemical analysis of [14C]acetate-labeled cultures suggested that all of these EthA-activated drugs inhibit mycolic acid biosynthesis via different mechanisms through binding to specific targets. This report is also the first description of the molecular mechanism of action of TAC, a thiosemicarbazone antimicrobial agent that is still used in the treatment of tuberculosis as a second-line drug in many developing countries. Altogether, the results suggest that EthA is a common activator of thiocarbamide-containing drugs. The broad specificity of EthA can now be used to improve the activation process of these drugs, which may help overcome the toxicity problems associated with clinical thiocarbamide use.
Despite the availability of effective therapies, tuberculosis (TB), caused by Mycobacterium tuberculosis, is still a leading cause of death (11). The human immunodeficiency virus pandemic, which contributes substantially to the morbidity and mortality from TB, and the emergence of multidrug-resistant strains of M. tuberculosis (23) have compounded the problem. Although infections by drug-sensitive strains can be successfully cured (7), the emergence of drug resistance has prompted new drug research, particularly the search for new drug targets and the definition of mechanisms of drug resistance (16). When TB cases cannot be treated by first-line protocols due to resistance issues, the last resort for combating multidrug-resistant infections relies on the action of second-line antitubercular drugs.
Work from the last decade has revealed M. tuberculosis to be unique among bacteria in that several drugs require activation in situ to become inhibitory. Drugs such as isoniazid (INH), ethionamide (ETH), and pyrazinamide (PZA) all require activation for activity against M. tuberculosis, and resistance can be mediated by mutations that eliminate the activation step. Such inactivation has been demonstrated for the catalase-peroxidase KatG in INH resistance (33), the nicotinamidase-peroxidase PncA in PZA resistance (24), and the flavin adenine dinucleotide (FAD)-containing Baeyer-Villiger monooxygenase EthA in ETH resistance (3, 9, 30). Interestingly, both activated forms of INH and ETH target the enoyl-acyl carrier protein reductase InhA of the type II fatty acid synthase (FAS-II) involved in the production of mycolic acids, despite their different activation processes (1, 31).
Initial studies demonstrated that overexpression of the M. tuberculosis gene Rv3855 generated ETH resistance (3, 9, 12) and that the corresponding protein shows homology with members of the TetR family of transcriptional repressors (3, 9, 12). The neighboring locus, Rv3854c, is transcribed divergently and encodes a protein with homology to known monooxygenases. Furthermore, its overproduction in mycobacteria resulted in substantially increased ETH sensitivity relative to parent strains (3, 9). Mycolic acid synthesis was also dramatically inhibited by ETH in the Rv3854c protein-overexpressing mycobacteria, suggesting that the Rv3854c protein (designated EthA) activates ETH and that this enzyme is under the regulatory control of the Rv3855 protein (designated EthR) (3, 9). In a manner similar to the KatG activation of INH, ETH undergoes activation via an EthA-mediated process. As expected, genetic alteration of ethA leads to increased ETH resistance, just as katG mutations confer INH resistance (9, 19).
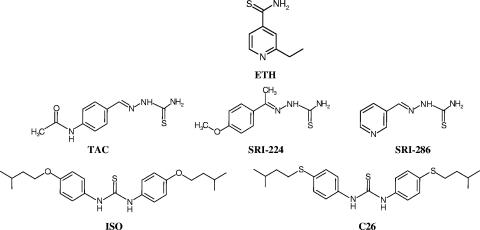
Analysis of ETH-resistant M. tuberculosis clinical isolates revealed cross-resistance to two other thiocarbamide-containing drugs (Fig. 1), thiacetazone (TAC) and isoxyl (ISO) (9). TAC is a thiosemicarbazone antimicrobial that has been widely used for TB treatment in Africa and South America as a cheap and effective substitute for p-aminosalicylic acid (8). This drug is bacteriostatic against M. tuberculosis but is useful in combination with INH (15). As TAC is often associated with dermatological side effects and Stevens-Johnson syndrome in AIDS patients, it is not available in the United States (15). Ethambutol replaced TAC in many low-income countries during the AIDS epidemic, when human immunodeficiency virus-infected patients were found to experience a high rate of serious and sometimes fatal skin reactions to TAC. TAC has also been shown to be very active against Mycobacterium avium infection in vitro (14) and in mice (5). However, the mechanism of action of TAC remains poorly understood.
FIG. 1.
Structures of the thiocarbamide-containing antitubercular drugs used in this study. SRI-224 and SRI-286 are TAC analogues, whereas C26 is related to ISO.
ISO was used for the clinical treatment of TB in the 1960s. ISO monotherapy demonstrated modest efficacy in cases of untreated pulmonary TB of various degrees of difficulty (27-29). However, a combination of ISO with INH was more effective than monotherapy with either drug. Like INH and ETH, ISO inhibits mycolic acid biosynthesis (32). Other studies extended this work by demonstrating that ISO, as well as ISO derivatives (6), inhibited the synthesis of both fatty acids and all subtypes of mycolic acids and shows promise in counteracting a wide variety of drug-sensitive and -resistant strains of M. tuberculosis (20). It was also established that, in addition to mycolic acid inhibition, ISO inhibits oleic acid biosynthesis by blocking the stearoyl coenzyme A (CoA) desaturase DesA3 (21).
Whether EthA displays an exceptionally broad prodrug acceptance remains to be addressed and requires functional proof. Here, we examine in vitro, through the analyses of drug metabolism, and functionally, in genetically modified Mycobacterium bovis BCG strains, whether TAC and ISO and some of their structural analogues are activated by EthA in mycobacteria.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All cloning steps were performed in Escherichia coli XL1-Blue (Stratagene). Expression of ethR or ethA was conducted in the M. bovis BCG Pasteur strain 1173P2 (WHO, Stockholm, Sweden). M. bovis BCG was transformed, and recombinant M. bovis BCG clones were selected on Middlebrook 7H11 agar supplemented with oleic acid-albumin-dextrose-catalase enrichment (OADC; Difco) containing 25 μg/ml kanamycin. M. bovis BCG NCTC 5692 was used to generate an ethR-knockout mutant by insertion of a transposon carrying a hygromycin resistance cassette within the ethR gene (ethR::hyg) (3) and grown on Middlebrook 7H9 agar supplemented with OADC. Liquid cultures of M. bovis BCG strains were grown at 37°C in Sauton medium.
Plasmids and DNA manipulation.
The E. coli-mycobacterial shuttle vector pMV261 containing the hsp60 promoter was used as described previously (25). Restriction enzymes and T4 DNA ligase were purchased from Boehringer Mannheim, and Vent DNA polymerase was purchased from New England Biolabs. Both ethR and ethA were amplified from M. tuberculosis H37Rv genomic DNA using the following primer pairs: for ethA, pMVethAup (5′-CCA CCG AGC ACC TCG ACG-3′) and pMVethAdwn (5′-GAT CGA TCA AGC TTC TAA ACC CCC ACC GGG GCA-3′), and for ethR, pMVethRup (5′-GGA CCA CCT CCG CGG CCA GTC AGG-3′) and pMVethRdwn (5′-GAT CGA TCA AGC TTA GCG GTT CTC GCC G-3′). The respective PCR products were digested with HindIII (restriction site underlined in primer sequences), purified, and ligated with MscI- and HindIII-digested pMV261 to produce pMV261-ethR and pMV261-ethA. These cloning strategies introduced an alanine residue into the second position of the protein product, which was tolerated in both cases.
The ethA gene was also amplified using primers pETethAup (5′-GAT CGA TCC ATA TGA CCG AGC ACC TCG ACG TTG-3′) and pETethAdwn (5′-GAT CGA TCG CGG CCG CCT AAA CCC CCA CCG GGG CAG-3′) containing NdeI and NotI restriction sites (underlined), respectively, purified, digested with these enzymes, and ligated with similarly cut pET28b (Novagen) to generate pET28b-ethA. The integrity of all of these constructs was verified by nucleotide sequencing.
Drug susceptibility testing.
The susceptibility of M. bovis BCG strains to the various thiocarbamide-containing compounds was determined on Middlebrook 7H9 or 7H11 solid medium containing OADC enrichment with increasing drug concentrations. OADC was replaced with ADC when ISO or derivatives were used, as the oleic acid component of OADC enrichment relieves the DesA3 component of the target (21). Serial 10-fold dilutions of each actively growing culture were plated and incubated at 37°C for 10 to 14 days. The MIC was defined as the minimum concentration required to inhibit 99% of the growth.
Determination of the in vivo effects of thiocarbamide-containing drugs on fatty acid and mycolic acid synthesis in M. bovis BCG.
M. bovis BCG cultures (4 ml) were grown to mid-log phase in Sauton medium, and drugs were added at various concentrations followed by further incubation at 37°C for 16 h. At this point 1 μCi/ml of [2-14C]acetate (56 mCi/mmol; Amersham Biosciences) was added to the cultures followed by further incubation at 37°C for 8 h. The 14C-labeled cells were harvested by centrifugation, washed once with phosphate-buffered saline, and subjected to alkaline hydrolysis using 15% aqueous tetrabutylammonium hydroxide at 100°C overnight, followed by the addition of 4 ml of CH2Cl2, 300 μl of CH3I, and 2 ml of water. The entire reaction mixture was then mixed for 1 h. The upper aqueous phase was discarded, and the lower organic phase was washed twice with water and evaporated to dryness. Methyl esters of the fatty and mycolic acids thus formed were redissolved in diethyl ether, and the solution was again evaporated to dryness. The final residue was then dissolved in 200 μl of CH2Cl2. Equal counts of the resulting solution of fatty acid methyl esters (FAMEs) and mycolic acid methyl esters (MAMEs) were subjected to thin-layer chromatography (TLC), using silica gel one-dimensional plates (5735 silica gel 60 F254; Merck) impregnated with 10% silver nitrate. Lipids were resolved in petroleum ether-diethyl ether (17:3, vol/vol) three times. Autoradiograms were produced by overnight exposure to Kodak Biomax MR film to reveal 14C-labeled FAMEs and MAMEs.
Purification of His6-EthA.
The expression vector pET28b-ethA was used to transform Escherichia coli C41(DE3) cells (Imaxio, France). A single transformant colony was used to inoculate 50 ml LB broth containing 25 μg/ml kanamycin sulfate, which was grown to mid-exponential phase at 37°C. For expression studies, cultures were inoculated using 5 ml of the starter culture per liter of Terrific Broth at 37°C. Once the culture reached an A600 of 0.7 to 0.9, it was cooled to 22°C and ethA expression was induced with 1 mM isopropyl-1-thio-β-d-galactopyranoside. Growth was continued for a further 24 h, and cells were harvested by centrifugation and stored at −20°C.
The cells were resuspended in lysis buffer containing 50 mM Tris-HCl, 300 mM NaCl, 1% (vol/vol) Triton X-100, 6 mM imidazole, pH 7.9, and lysed using a French pressure cell at 3,000 lb/in2. The lysate was clarified by centrifugation at 27,000 × g, and the supernatant was loaded directly onto a Ni2+-loaded His-Trap affinity column (GE Healthcare) preequilibrated with the lysis buffer. After loading, the column was washed with 10 column volumes of 50 mM Tris-HCl, pH 7.9, 500 mM NaCl, and the protein was eluted with a gradient of increasing imidazole concentration in the same buffer. Elution was readily monitored via the yellow coloration imparted by the bound FAD prosthetic group. The fractions containing the pure protein, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, were pooled and dialyzed against 50 mM Tris-HCl, pH 7.9, containing 100 mM KCl and 10% glycerol.
In vitro drug metabolism.
ISO, TAC, or ETH was incubated at 37°C in 500 μl of 50 mM Tris-HCl, pH 7.4, containing 100 mM KCl, 200 μg/ml bovine serum albumin, 4 mM NADPH, and 8 μg of EthA for 1 h. Samples were analyzed by high-pressure liquid chromatography (HPLC) on a Genesis reverse-phase column (C18-300, 250 mm × 7-mm internal diameter, 4-μm particle size). Reaction mixtures were diluted with an equal volume of water and filtered before loading. Elution was achieved using a linear gradient from 0.05% formic acid in water to 0.05% formic acid in acetonitrile and monitored by measuring absorbance at A254. The amount of residual drug was calculated through integration of peak areas.
Samples were also analyzed by TLC. Reaction mixtures as described above were thoroughly mixed with 600 μl ethyl acetate in microcentrifuge tubes and centrifuged at 18,000 × g for 1 min. The upper organic phase was removed, and the solvent was evaporated. The sample was resuspended in 20 μl of ethyl acetate, loaded onto aluminum-backed plates of silica gel 60 F254 (Merck), and developed using toluene-ethyl acetate (7:3, vol/vol). Drugs were visualized by careful charring with molybdophosphoric acid.
RESULTS
Susceptibility of M. bovis BCG strains to thiocarbamide-containing drugs.
EthR regulates production of EthA, which subsequently activates the prodrug ETH (3, 9). Chromosomal inactivation of ethR, whose translational product represses transcription of ethA, in M. bovis BCG NCTC 5692 led to ETH hypersensitivity (3). We therefore used the M. bovis BCG ethR::hyg strain to investigate the relationship between ethR inactivation and susceptibility to other thiocarbamide-containing drugs. As shown in Table 1, and in agreement with the previous report (3), inactivation of EthR led to ETH hypersensitivity. A striking effect was also found with regard to TAC inhibition. The M. bovis BCG ethR::hyg strain was found to be 10 times more sensitive to TAC with a MIC of 0.25 μg/ml. We next tested the antimycobacterial activity of two TAC-related compounds, SRI-224 and SRI-286 (Fig. 1), and found that SRI-224 was as active as the parental molecule against M. bovis BCG NCTC 5692, whereas SRI-286 displayed a 10- to 20-fold-reduced activity compared to TAC or SRI-224 (Table 1). Similarly, we found that SRI-224, but not SRI-286, exhibited very strong activity against M. tuberculosis H37Rv (data not shown). Interestingly, it was recently shown that although the MICs of TAC and SRI-224 are comparable for some M. avium strains in vitro, treatment with those compounds had no effect on M. avium-infected mice. However, the activity of SRI-286 was comparable to the activity of moxifloxacin, the most potent quinolone against M. avium in vitro (5). In contrast to ETH or TAC, we observed only a reproducible twofold hypersensitivity effect with ISO (also known as 4,4′-diisoamyloxydiphenylthiourea or thiocarlide) in the ethR::hyg strain compared with the wild-type strain (Table 1). One possible explanation is that in the parental strain sufficient EthA may be present to efficiently activate the ISO prodrug and that overproduction of EthA in the ethR::hyg strain does not result in significantly higher yields of activated drug. Therefore, the low-hypersensitivity effect of ISO may be linked to the intrinsic structure of ISO, which is very efficiently metabolized by endogenous EthA. We therefore tested the C26 analogue (Fig. 1), which has previously been shown to be less active than the parental molecule against various mycobacterial species (20). Consistently, our results show that C26 showed a 25-fold-reduced efficiency in inhibiting M. bovis BCG NCTC 5692 compared with that of ISO (Table 1). However, this ISO derivative was 10-fold more active in inhibiting growth of the ethR::hyg strain. It is possible that, in this case, the endogenous EthA is not sufficient to efficiently convert C26 into its active form but that the relatively high levels of EthA present in the ethR::hyg strain would increase the extent of C26 activation at any given drug concentration, rendering the mutant much more sensitive than the parent strain.
TABLE 1.
MICs of various thiocarbamide-containing drugs against M. bovis BCG NCTC 5692 and M. bovis BCG NCTC 5692 ethR::hyg in 7H9 agar supplemented with OADC or ADC
| Drug | MIC (μg/ml) for strain
|
|
|---|---|---|
| Wild type | ethR::hyg | |
| ETH | 5 | 0.5 |
| TAC | 2.5 | 0.25 |
| SRI-224 | 2.5 | 0.25 |
| SRI-286 | 25-50 | 5 |
| ISO | 1 | 0.5 |
| C26 | 25 | 2.5 |
Altogether, these results show that the ethR::hyg strain is more sensitive to all thiocarbamide-containing drugs tested and strongly suggest that EthA activates these drugs in vivo in mycobacteria.
Effect of thiocarbamide-containing drugs on fatty acid and mycolic acid biosynthesis.
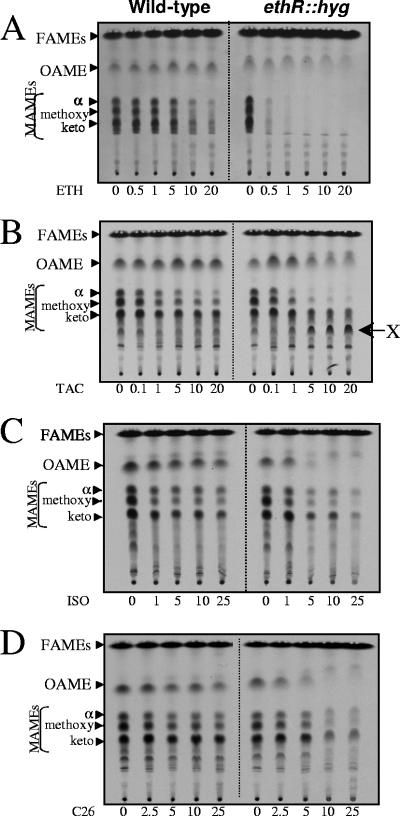
To test whether the increased susceptibility of the ethR::hyg strain was associated with inhibition of fatty acid/mycolic acid synthesis, M. bovis BCG cultures were grown in the presence of increasing concentrations of thiocarbamide-containing drugs and labeled with [2-14C]acetate. Following extraction, radiolabeled FAMEs and MAMEs were separated by one-dimensional argentation TLC in order to separate unsaturated from saturated fatty/mycolic acids. As reported earlier (3), ETH treatment of the ethR::hyg strain was accompanied with a rapid cessation of synthesis of all kinds of mycolates (α-, methoxy-, and keto-), in agreement with the MICs (Table 1), which supports the hypothesis that EthR represses the formation of the active ETH metabolite (Fig. 2A). Very little is known regarding the mode of action of TAC. We therefore examined whether administration of TAC might affect the fatty acid/mycolic acid profile of M. bovis BCG. TLC analyses of extracted [14C]FAMES/[14C]MAMES clearly showed that TAC inhibits mycolic acid biosynthesis (Fig. 2B), although the profile was different from that generated during ETH treatment (Fig. 2A). Albeit biogenesis of all mycolate subtypes was affected, synthesis of α- and methoxymycolates was inhibited more profoundly than that of ketomycolates. TAC did not inhibit oleic acid production. The ethR::hyg strain shows a more pronounced inhibition of mycolate biosynthesis than did the wild-type strain at equivalent doses. Furthermore, the accumulation of an unsaturated product (indicated by its retardation on argentation TLC [Fig. 2B]) designated lipid X was clearly evident, presumably corresponding to a mycolate precursor. This new product, which was barely detectable in the parental strain, accumulated in a dose-dependent manner in the TAC-hypersensitive ethR::hyg strain. Since similar metabolites are not evident on ETH treatment in this mutant, this suggests that TAC inhibits mycolic acid via a different mechanism. As activated ETH is known to inhibit mycolate synthesis by specifically targeting the enoyl-AcpM reductase InhA of FAS-II (1, 17), we tested whether TAC might alter FAS-II activity by determining the MICs of M. bovis BCG strains overexpressing various FAS-II components such as mtFabH, InhA, KasA, KasB, or MabA and found that the values were similar to those for the wild-type strain (data not shown). This suggests that none of these enzymes represents a cellular target of TAC in vivo. Together, these results allow us to propose, for the first time, that TAC exerts an antimycobacterial activity through inhibition of mycolic acid biogenesis and, furthermore, suggest that the target might participate in mycolate modification rather than meromycolate extension.
FIG. 2.
Dose-response effects of thiocarbamide-containing drugs on fatty acid and mycolic acid biosynthesis in M. bovis BCG NCTC 5692 and M. bovis BCG NCTC 5692 ethR::hyg. The inhibitory effect on the incorporation of [2-14C]acetate was assayed by labeling in the presence of increasing concentrations (μg/ml) of ETH (A), TAC (B), ISO (C), or C26 (D). The corresponding FAMEs, oleic acid methyl esters (OAMEs), and MAMEs were extracted, and equal counts were loaded onto an argentation-impregnated TLC plate (20,000 cpm [A], 50,000 cpm [B], 30,000 cpm [C], and 15,000 cpm [D]). Lipids were developed three times with petroleum ether-diethyl ether (17:3, vol/vol) and exposed overnight to a film. α, α-mycolates; keto, ketomycolates; methoxy, methoxymycolates. The arrow shown in panel B indicates the position of the lipid X that accumulates following treatment with TAC.
Like ETH, ISO is known to inhibit mycolic acid biosynthesis (20, 32). In addition, ISO has been shown to block the synthesis of oleic acid by targeting the stearoyl-CoA desaturase DesA3 (21). However, the specific target of the mycolate pathway has not been identified yet. In agreement with these studies, our results show that ISO inhibits both mycolic acid and oleic acid synthesis in the wild-type strain. This effect was exaggerated in the ethR::hyg strain (Fig. 2C), suggesting that inactivation of EthR leads to a more pronounced inhibition of mycolic acid and oleic acid synthesis by ISO. A similar effect was observed using the C26 analogue, which led to a more potent inhibition of mycolate and oleate synthesis in the ethR::hyg strain (Fig. 2D). Neither ISO nor C26 treatment led to the accumulation of the novel product observed during TAC treatment, suggesting that these drugs do not share the same target with TAC. Moreover, overexpression of InhA does not increase resistance to ISO, indicating that InhA is not the target of ISO in mycobacteria (data not shown).
Overall, these results suggest that ETH, TAC, and ISO are prodrugs that are all activated by EthA in vivo and that each activated drug acts on different targets that ultimately lead to mycolic acid biosynthesis inhibition.
Inhibition of mycolic acid synthesis in M. bovis BCG overexpressing ethR or ethA.
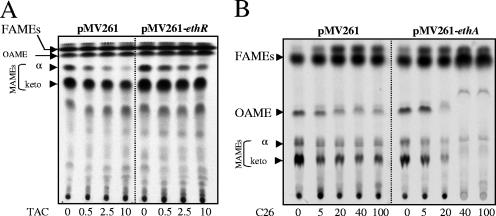
The coding sequences of ethR or ethA were amplified by PCR and cloned downstream of the hsp60 promoter in the pMV261 vector. The resulting plasmids, pMV261-ethR and pMV261-ethA, respectively, were used to transform M. bovis BCG Pasteur. The MICs for these recombinant strains were compared with those of a BCG strain harboring pMV261 and are summarized in Table 2. As expected for ETH, the ethR-overexpressing strain exhibited a high level of resistance to ETH, whereas the ethA-overexpressing strain demonstrated hypersensitivity to ETH (3). Assays of susceptibility to TAC, SRI-224, and SRI-286 showed that BCG(pMV261-ethR) was more resistant to these different drugs, suggesting a direct correlation between the level of ethR expression and resistance. As anticipated, this TAC resistance phenotype of M. bovis BCG Pasteur(pMV261-ethR) correlated with a decreased inhibition of mycolic acid synthesis in comparison to wild-type M. bovis BCG (Fig. 3A) treated with identical drug doses. Similarly, the dramatic inhibition of mycolic acid synthesis observed following SRI-224 treatment could be partially reversed in the EthR-overproducing strain (data not shown). However, and in contrast to ETH, only a modest effect of increase in sensitivity was observed in the strain overproducing EthA. The M. bovis BCG Pasteur strain is very sensitive to TAC and SRI-224 (approximately 10 times more than M. bovis BCG NCTC 5692), and the endogenous level of EthA in this strain may be sufficient to efficiently activate these drugs, which may explain why overexpression of the activator does not increase TAC or SRI-224 susceptibility (Table 2). The basis of the discrepancies between the two M. bovis BCG strains is currently unknown. Although they differ in their mycolic acid profile (M. bovis BCG Pasteur lacks methoxymycolates due to a point mutation in the mma3 gene [4]), these strains may also present differential endogenous EthR/EthA expression levels and/or cell wall permeabilities to TAC and SRI-224.
TABLE 2.
MICs of thiocarbamide-containing drugs against M. bovis BCG Pasteur overexpressing either EthR or EthA in 7H11 supplemented with OADC
| Drug | MIC (μg/ml) for strain carrying:
|
||
|---|---|---|---|
| pMV261 | pMV261-ethR | pMV261-ethA | |
| ETH | 2-5 | 10-25 | 0.25 |
| TAC | 0.5 | 2.5 | 0.25 |
| SRI-224 | 0.25 | 0.5-1 | 0.25 |
| SRI-286 | 10-25 | 75 | 10 |
| ISO | 0.25 | 0.25 | 0.1 |
| C26 | 40 | 40 | 5 |
FIG. 3.
Dose-response effects of thiocarbamide-containing drugs on fatty acid and mycolic acid biosynthesis in M. bovis BCG Pasteur overexpressing EthR or EthA. The inhibitory effect on the incorporation of [2-14C]acetate was assayed by labeling either M. bovis BCG(pMV261), M. bovis BCG(pMV261-ethR), or M. bovis BCG(pMV261-ethA) in the presence of increasing concentrations (μg/ml) of either TAC (A) or C26 (B). The corresponding FAMEs, oleic acid methyl esters (OAMEs), and MAMEs were extracted, and equal counts were loaded onto an argentation-impregnated TLC plate (150,000 cpm [A] and 10,000 cpm [B]). Lipids were developed three times with petroleum ether-diethyl ether (17:3, vol/vol) and exposed to a film. α, α-mycolates; keto, ketomycolates.
Table 2 indicates that the EthA-overproducing strain was more susceptible to C26 inhibition than was the control strain. In vivo analysis of the FAME/MAME profile following C26 treatment shows that the M. bovis BCG strain carrying pMV261-ethA is hypersensitive compared to the control strain with regard to oleic acid and mycolic acid inhibition (Fig. 3B).
In vitro analyses of EthA-mediated drug metabolism.
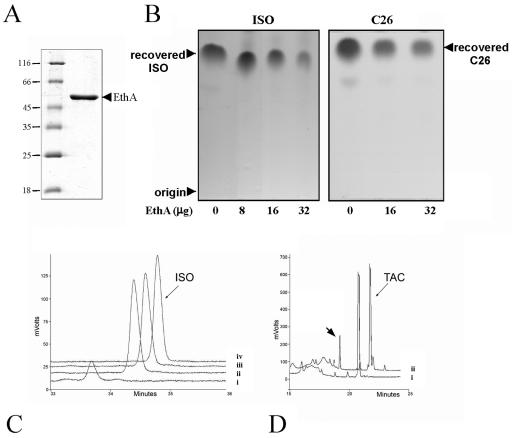
In order to establish a direct link between thiocarbamide activation and EthA activity, we cloned and overexpressed ethA to produce an N-terminal His6-tagged fusion protein (Fig. 4A). We found that the enzyme tolerated this N-terminal fusion, but tagging at the C terminus compromised the ability of the protein to retain its FAD prosthetic group (data not shown). We next developed a relatively simple in vitro assay to follow ISO metabolization. The drug is readily extracted from aqueous solution into ethyl acetate, and after incubation with pure recombinant EthA, a clear reduction in the recovery of ISO was evident with increasing enzyme concentration when analyzed by TLC (Fig. 4B). This metabolism of ISO was also confirmed by reverse-phase HPLC analysis and was found to be strictly dependent on the supply of NADPH (Fig. 4C). However, no products were evident in the ethyl acetate extract or in material retained from identical reactions analyzed by reverse-phase HPLC (data not shown), strongly suggesting the generation of a relatively polar product(s). To determine whether the C26 analogue was also metabolized by EthA, we compared drug recovery levels after 120 min of incubation with EthA. We found that the C26 analogue was metabolized as readily as the parent ISO molecule (Fig. 4B) with specific activities of 7.37 μmol/min/mg and 7.56 μmol/min/mg, respectively.
FIG. 4.
In vitro metabolization of thiocarbamide-containing drugs by EthA. (A) Coomassie blue-stained polyacrylamide gel showing the purity of recombinant EthA produced in E. coli. Numbers at left are molecular masses in kilodaltons. (B) TLC analysis illustrating the degradation of ISO (left panel) and C26 (right panel) to relatively polar products in the presence of increasing amounts of EthA. Samples were incubated with the enzyme and NADPH for 2 h, extracted with ethyl acetate, concentrated, and analyzed by TLC as described in Materials and Methods. Recovered drugs were visualized by charring with molybdophosphoric acid. (C) Chromatograms corresponding to the metabolization of ISO. Enzyme and NADPH dependence of the reaction was investigated using reverse-phase HPLC as described in Materials and Methods: trace i, complete reaction time of 90 min; trace ii, as trace i but time of 0 min; trace iii, as trace i but without EthA; trace iv, as trace i but without NADPH. (D) In vitro metabolism of TAC monitored using the same HPLC system: trace i, complete reaction time of 0 min; trace ii, as trace i but time of 120 min. The arrowhead in trace ii indicates the presence of a novel peak corresponding to a TAC metabolite.
A similar approach was undertaken to analyze the metabolism of TAC, but little activity could be detected using the TLC method presumably because highly polar products were not generated in the reaction. However, analyses of reaction products using reverse-phase HPLC revealed the generation of a novel product eluting from the C18 column earlier than TAC (Fig. 4D). Qian and Ortiz de Montellano recently described the oxidative metabolism of TAC by human and mycobacterial flavin-containing monooxygenases, including EthA (22). The product that we report here may correspond to the TAC-sulfinic acid product described by these authors.
Thus, we show here that both TAC and ISO can be metabolized by EthA in vitro.
DISCUSSION
For decades, thioamides have been used to treat TB infections, but only recently has it been demonstrated that, in M. tuberculosis, the antimycobacterial activity of the most widely used thioamide, ETH, requires activation by a specific prodrug activator (3, 9). This activator, EthA, is an NADPH-specific FAD-containing monooxygenase (30) capable of performing Baeyer-Villiger oxidation reactions (13). DeBarber et al. (9) reported a series of mutations in the ethA gene conferring resistance to ETH. Interestingly, some of these isolates also showed cross-resistance to two other thiocarbamide-containing drugs, ISO and TAC. This prompted the authors to propose that EthA also converts ISO and TAC into active metabolites. However, conclusive proof of this statement awaited experimental work. Given the high structural similarities between thiocarbamide-containing drugs (Fig. 1), we conducted a study aimed at investigating whether all these drugs are activated by EthA, based on the use of genetically modified M. bovis BCG strains.
When we tested the susceptibility of the M. bovis BCG ethR::hyg strain against thiocarbamides, we observed a striking effect using TAC with a 10-fold increase in sensitivity over the parent strain, presumably correlating with the maximal ethA transcription in this mutant. Similarly, the mutant strain was around 10-fold more sensitive to the TAC analogues SRI-286 and SRI-224 than the parent strain was. In contrast to ETH or TAC, only a twofold increase in sensitivity to ISO was observed in the ethR::hyg strain. This decrease in the magnitude of the sensitization effect of the ethR disruption might reflect the highly efficient activation of the ISO prodrug by the endogenous ethA expression in the parental strain and the fact that overproduction of EthA in the ethR::hyg strain does not result in functionally significant higher yields of activated drug. The C26 analogue, which was 25-fold less active than ISO, was 10-fold more active in inhibiting growth of the ethR::hyg strain. Herein, we suggest that higher levels of EthA present in the ethR::hyg strain allow the drug to be activated more readily, rendering it much more sensitive than the wild-type strain. Interestingly, the in vitro assays revealed that C26 was metabolized at the same rate as ISO. The relative insensitivity of the parent organism to C26 might reflect its poor penetration within the cell, but more likely, the electronegativity of the side chain oxygen atoms, both replaced here by sulfur (Fig. 1), could be of importance to the binding of the active metabolite to one of its cellular targets. We are currently seeking to identify the mycolate-related cellular target(s) and analyzing a library of ISO derivatives in order to clarify these issues. In general, the ethR::hyg strain was found to be more sensitive to all thiocarbamide-containing drugs, suggesting that EthA activates these drugs in mycobacteria.
Like that of ETH, administration of TAC affected the biosynthesis of all mycolate subtypes, especially α- and methoxymycolates. In the ethR::hyg strain this inhibition was more acute at equivalent concentrations of the drug with a dose-dependent accumulation of a new product, presumably corresponding to a mycolate unsaturated precursor. Since this product did not accumulate following ETH treatment, we propose that TAC inhibits mycolic acid synthesis without binding to the enoyl-AcpM reductase InhA like ETH (1, 17) but via a different mechanism. Overexpression of various FAS-II components such as mtFabH, InhA, KasA, KasB, or MabA did not alleviate the toxicity of TAC, suggesting that none of these enzymes is a target of TAC in vivo (data not shown). Studies are currently in progress to determine the cellular target(s) of the active TAC metabolite(s).
ISO is known to inhibit mycolic acid biosynthesis (20, 32). In addition, it has been shown to block the synthesis of oleic acid by targeting the stearoyl-CoA desaturase DesA3 (21). However, the specific target in the mycolate pathway has not been identified yet. Consistently, ISO inhibited both mycolic acid and oleic acid synthesis in the wild-type strain with both effects being exaggerated in the ethR::hyg strain. Neither ISO nor the less active C26 analogue showed accumulation of the mycolate precursor observed during TAC treatment, suggesting that ISO does not share the same target as TAC. Moreover, overexpression of InhA does not increase resistance to ISO, suggesting that InhA is not the target of ISO in mycobacteria (data not shown). Overall, these results suggest that ETH, TAC, and ISO are prodrugs that all require activation by EthA in vivo and that each activated drug acts on different targets, although all ultimately inhibit mycolic acid biogenesis.
In order to establish a direct link between thiocarbamide activation and EthA activity, we conducted in vitro analyses of drug metabolism using purified recombinant EthA. Following incubation with EthA, a clear reduction of ISO recovery from reaction mixtures was evident. Consistent with a specific EthA-dependent reaction, this metabolism of ISO was strictly dependent on NADPH. The mechanism of ISO activation probably includes relatively polar reaction products, which are not detected in reverse-phase HPLC analyses and cannot be extracted from the reaction mixtures by using ethyl acetate. Our efforts to isolate and identify these activated products by using a library of analogues are ongoing.
A recent study described the oxidative metabolism of TAC by human and mycobacterial flavin-containing monooxygenases, including EthA (22). Our analyses of TAC-derived reaction products using reverse-phase HPLC revealed the generation of a novel product eluting from the octadecylsilane column earlier than TAC, which may correspond to the TAC-sulfinic acid product described by these authors, which is prevalent at neutral pH.
We present here for the first time the modulation of EthA concentration, either by inactivation or by overexpression of its transcriptional repressor and also by overexpression of the structural gene itself in M. bovis BCG, which can be linked to improvement in MICs of ISO, TAC, and analogues thereof. This confirms their metabolism by EthA in vivo.
Many current antimycobacterial agents require some form of cellular activation corresponding to the oxidative, reductive, or hydrolytic unmasking of reactive groups. Understanding the mechanism participating in activation of current antimycobacterials may also facilitate the development of alternative activation strategies or of analogues that do not require such processes. This study adds TAC and ISO to the growing list of prodrugs that are converted by mycobacterial enzymes to exert their killing effects. So far, EthA appears to be the only activator which is common to different prodrugs (Fig. 5). But why are so many drugs metabolically activated by M. tuberculosis? As suggested by Barry et al. (2), oxidative activation is intimately associated with the extraordinary capacity of M. tuberculosis to adapt and survive within an environment that is continually bombarded by oxidative species produced by host cells. Because M. tuberculosis is a natural mutant of OxyR (10), a transcriptional regulator that serves to up-regulate genes involved in defense against oxidative stress, including katG, it has a constitutively expressed KatG protein as a means to compensate for a defective oxidative stress regulon. To circumvent the oxidative species destruction, one may speculate that the bacilli have developed and expressed a large battery of oxidative enzymes, which also render the organism sensitive to oxidatively activated prodrugs.
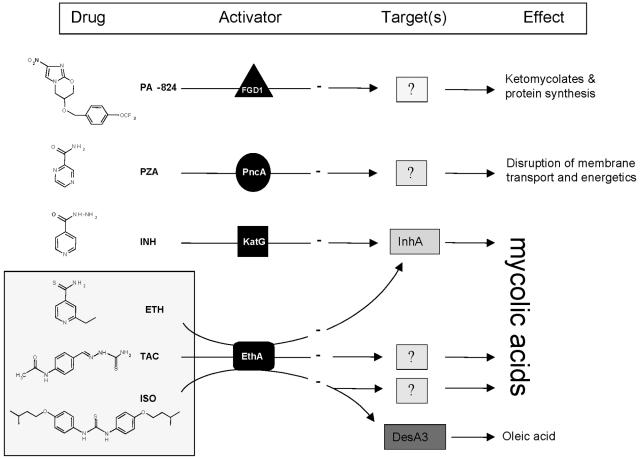
FIG. 5.
Summary of the activation mechanisms of major antitubercular drugs. Prodrugs get into tubercle bacilli by passive diffusion, where they are activated by specific proteins to a range of reactive radicals or species. PZA is activated by the nicotinamidase-peroxidase PncA, and the nitroimidazooxazine PA-824 is activated by an F420-dependent, glucose-6-phosphate dehydrogenase (FGD1) (18, 26), whereas INH is activated by the catalase-peroxidase KatG. These radicals then attack multiple targets and specific targets in the cell. The present study indicates that EthR is a common activator for at least three other antitubercular drugs, ETH, TAC, and ISO. Once activated, ETH inhibits mycolic acid biosynthesis by targeting InhA, which is also the primary target of activated INH. In contrast, activated TAC or ISO inhibits mycolic acid biosynthesis through InhA-independent mechanisms, which remain to be determined. Recent work demonstrated that pyrazinoic acid (the active form of PZA) targets the membrane and interferes with the energetics and function of the membrane (34), although the type I fatty acid synthase had been proposed as a target of PZA in a study using Mycobacterium smegmatis and 5-Cl-PZA (35). Oleic acid synthesis appears to be specifically inhibited through inhibition of the stearoyl-CoA desaturase DesA3 by activated ISO (21).
The broad specificity of EthA can now be utilized to improve these drugs in terms of their activation. Probing the structure-activity relationships of libraries of TAC and ISO analogues in parallel with EthA structural studies should lead to the determination of the characteristics pertinent to efficient thiocarbamide activation. In addition, improvements in activation may facilitate reduced dosage and hopefully will alleviate the toxicity problems associated with clinical use of thiocarbamide drugs.
Acknowledgments
We thank Ken Duncan for the generous gift of the M. bovis BCG ethR::hyg strain.
G.S.B. acknowledges support from James Bardrick in the form of a Personal Chair, the Lister Institute as a former Jenner Research Fellow, the Medical Research Council (United Kingdom), and the Wellcome Trust. L.K. is supported by a grant from the Centre National de la Recherche Scientifique (CNRS) (Action Thématique Incitative sur Programme “Microbiologie Fondamentale”).
Footnotes
Published ahead of print on 12 January 2007.
REFERENCES
- 1.Banerjee, A., E. Dubnau, A. Quemard, V. Balasubramanian, K. S. Um, T. Wilson, D. Collins, G. de Lisle, and W. R. Jacobs, Jr. 1994. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 263:227-230. [DOI] [PubMed] [Google Scholar]
- 2.Barry, C. E., III, R. A. Slayden, A. E. Sampson, and R. E. Lee. 2000. Use of genomics and combinatorial chemistry in the development of new antimycobacterial drugs. Biochem. Pharmacol. 59:221-231. [DOI] [PubMed] [Google Scholar]
- 3.Baulard, A. R., J. C. Betts, J. Engohang-Ndong, S. Quan, R. A. McAdam, P. J. Brennan, C. Locht, and G. S. Besra. 2000. Activation of the pro-drug ethionamide is regulated in mycobacteria. J. Biol. Chem. 275:28326-28331. [DOI] [PubMed] [Google Scholar]
- 4.Behr, M. A., B. G. Schroeder, J. N. Brinkman, R. A. Slayden, and C. E. Barry III. 2000. A point mutation in the mma3 gene is responsible for impaired methoxymycolic acid production in Mycobacterium bovis BCG strains obtained after 1927. J. Bacteriol. 182:3394-3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez, L. E., R. Reynolds, P. Kolonoski, P. Aralar, C. B. Inderlied, and L. S. Young. 2003. Thiosemicarbazole (thiacetazone-like) compound with activity against Mycobacterium avium in mice. Antimicrob. Agents Chemother. 47:2685-2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhowruth, V., A. K. Brown, R. C. Reynolds, G. D. Coxon, S. P. Mackay, D. E. Minnikin, and G. S. Besra. 2006. Symmetrical and unsymmetrical analogues of isoxyl; active agents against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 16:4743-4747. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg, H. M., W. J. Burman, R. E. Chaisson, C. L. Daley, S. C. Etkind, L. N. Friedman, P. Fujiwara, M. Grzemska, P. C. Hopewell, M. D. Iseman, R. M. Jasmer, V. Koppaka, R. I. Menzies, R. J. O'Brien, R. R. Reves, L. B. Reichman, P. M. Simone, J. R. Starke, and A. A. Vernon. 2003. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. [DOI] [PubMed] [Google Scholar]
- 8.Davidson, P. T., and H. Q. Le. 1992. Drug treatment of tuberculosis—1992. Drugs 43:651-673. [DOI] [PubMed] [Google Scholar]
- 9.DeBarber, A. E., K. Mdluli, M. Bosman, L. G. Bekker, and C. E. Barry III. 2000. Ethionamide activation and sensitivity in multidrug-resistant Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 97:9677-9682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deretic, V., W. Philipp, S. Dhandayuthapani, M. H. Mudd, R. Curcic, T. Garbe, B. Heym, L. E. Via, and S. T. Cole. 1995. Mycobacterium tuberculosis is a natural mutant with an inactivated oxidative-stress regulatory gene: implications for sensitivity to isoniazid. Mol. Microbiol. 17:889-900. [DOI] [PubMed] [Google Scholar]
- 11.Dye, C. 2006. Global epidemiology of tuberculosis. Lancet 367:938-940. [DOI] [PubMed] [Google Scholar]
- 12.Engohang-Ndong, J., D. Baillat, M. Aumercier, F. Bellefontaine, G. S. Besra, C. Locht, and A. R. Baulard. 2004. EthR, a repressor of the TetR/CamR family implicated in ethionamide resistance in mycobacteria, octamerizes cooperatively on its operator. Mol. Microbiol. 51:175-188. [DOI] [PubMed] [Google Scholar]
- 13.Fraaije, M. W., N. M. Kamerbeek, A. J. Heidekamp, R. Fortin, and D. B. Janssen. 2004. The prodrug activator EtaA from Mycobacterium tuberculosis is a Baeyer-Villiger monooxygenase. J. Biol. Chem. 279:3354-3360. [DOI] [PubMed] [Google Scholar]
- 14.Heifets, L. B., P. J. Lindholm-Levy, and M. Flory. 1990. Thiacetazone: in vitro activity against Mycobacterium avium and M. tuberculosis. Tubercle 71:287-291. [DOI] [PubMed] [Google Scholar]
- 15.Houston, S., and A. Fanning. 1994. Current and potential treatment of tuberculosis. Drugs 48:689-708. [DOI] [PubMed] [Google Scholar]
- 16.Kremer, L. S., and G. S. Besra. 2002. Current status and future development of antitubercular chemotherapy. Expert Opin. Investig. Drugs 11:1033-1049. [DOI] [PubMed] [Google Scholar]
- 17.Larsen, M. H., C. Vilcheze, L. Kremer, G. S. Besra, L. Parsons, M. Salfinger, L. Heifets, M. H. Hazbon, D. Alland, J. C. Sacchettini, and W. R. Jacobs, Jr. 2002. Overexpression of inhA, but not kasA, confers resistance to isoniazid and ethionamide in Mycobacterium smegmatis, M. bovis BCG and M. tuberculosis. Mol. Microbiol. 46:453-466. [DOI] [PubMed] [Google Scholar]
- 18.Manjunatha, U. H., H. Boshoff, C. S. Dowd, L. Zhang, T. J. Albert, J. E. Norton, L. Daniels, T. Dick, S. S. Pang, and C. E. Barry III. 2006. Identification of a nitroimidazo-oxazine-specific protein involved in PA-824 resistance in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 103:431-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morlock, G. P., B. Metchock, D. Sikes, J. T. Crawford, and R. C. Cooksey. 2003. ethA, inhA, and katG loci of ethionamide-resistant clinical Mycobacterium tuberculosis isolates. Antimicrob. Agents Chemother. 47:3799-3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phetsuksiri, B., A. R. Baulard, A. M. Cooper, D. E. Minnikin, J. D. Douglas, G. S. Besra, and P. J. Brennan. 1999. Antimycobacterial activities of isoxyl and new derivatives through the inhibition of mycolic acid synthesis. Antimicrob. Agents Chemother. 43:1042-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phetsuksiri, B., M. Jackson, H. Scherman, M. McNeil, G. S. Besra, A. R. Baulard, R. A. Slayden, A. E. DeBarber, C. E. Barry III, M. S. Baird, D. C. Crick, and P. J. Brennan. 2003. Unique mechanism of action of the thiourea drug isoxyl on Mycobacterium tuberculosis. J. Biol. Chem. 278:53123-53130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qian, L., and P. R. Ortiz de Montellano. 2006. Oxidative activation of thiacetazone by the Mycobacterium tuberculosis flavin monooxygenase EtaA and human FMO1 and FMO3. Chem. Res. Toxicol. 19:443-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raviglione, M. 2006. XDR-TB: entering the post-antibiotic era? Int. J. Tuberc. Lung Dis. 10:1185-1187. [PubMed] [Google Scholar]
- 24.Scorpio, A., and Y. Zhang. 1996. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat. Med. 2:662-667. [DOI] [PubMed] [Google Scholar]
- 25.Stover, C. K., V. F. de la Cruz, G. P. Bansal, M. S. Hanson, T. R. Fuerst, W. R. Jacobs, Jr., and B. R. Bloom. 1992. Use of recombinant BCG as a vaccine delivery vehicle. Adv. Exp. Med. Biol. 327:175-182. [DOI] [PubMed] [Google Scholar]
- 26.Stover, C. K., P. Warrener, D. R. VanDevanter, D. R. Sherman, T. M. Arain, M. H. Langhorne, S. W. Anderson, J. A. Towell, Y. Yuan, D. N. McMurray, B. N. Kreiswirth, C. E. Barry, and W. R. Baker. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962-966. [DOI] [PubMed] [Google Scholar]
- 27.Titscher, R. 1966. Monotherapy with isoxyl-DAT in cases of tuberculosis under hospital care. Prax. Pneumol. 20:202-206. (In German.) [PubMed] [Google Scholar]
- 28.Urbancik, B. 1970. Clinical experience with thiocarlide (isoxyl). Antibiot. Chemother. 16:117-123. [DOI] [PubMed] [Google Scholar]
- 29.Urbancik, B. 1966. A clinical trial of thiocarlide (4-4′ diisoamyloxythiocarbanilide). Tubercle 47:283-288. [DOI] [PubMed] [Google Scholar]
- 30.Vannelli, T. A., A. Dykman, and P. R. Ortiz de Montellano. 2002. The antituberculosis drug ethionamide is activated by a flavoprotein monooxygenase. J. Biol. Chem. 277:12824-12829. [DOI] [PubMed] [Google Scholar]
- 31.Vilcheze, C., F. Wang, M. Arai, M. H. Hazbon, R. Colangeli, L. Kremer, T. R. Weisbrod, D. Alland, J. C. Sacchettini, and W. R. Jacobs, Jr. 2006. Transfer of a point mutation in Mycobacterium tuberculosis inhA resolves the target of isoniazid. Nat. Med. 12:1027-1029. [DOI] [PubMed] [Google Scholar]
- 32.Winder, F. G., P. B. Collins, and D. Whelan. 1971. Effects of ethionamide and isoxyl on mycolic acid synthesis in Mycobacterium tuberculosis BCG. J. Gen. Microbiol. 66:379-380. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, Y., B. Heym, B. Allen, D. Young, and S. Cole. 1992. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 358:591-593. [DOI] [PubMed] [Google Scholar]
- 34.Zhang, Y., M. M. Wade, A. Scorpio, H. Zhang, and Z. Sun. 2003. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J. Antimicrob. Chemother. 52:790-795. [DOI] [PubMed] [Google Scholar]
- 35.Zimhony, O., J. S. Cox, J. T. Welch, C. Vilcheze, and W. R. Jacobs, Jr. 2000. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat. Med. 6:1043-1047. [DOI] [PubMed] [Google Scholar]