ETHYLENE RESPONSE 1 (ETR1) is one of five receptors for the plant hormone ethylene (Bleecker et al., 1988; Chang et al., 1993; for review, see Bleecker and Kende, 2000; Schaller and Kieber, 2002). ETR1 contains both an N-terminal ligand-binding hydrophobic domain and light signaling-implicated GAF domain (Fig. 1A; Chang et al., 1993). ETR1 also has a C-terminal His kinase (HK) domain fused with a response regulator motif.
Figure 1.
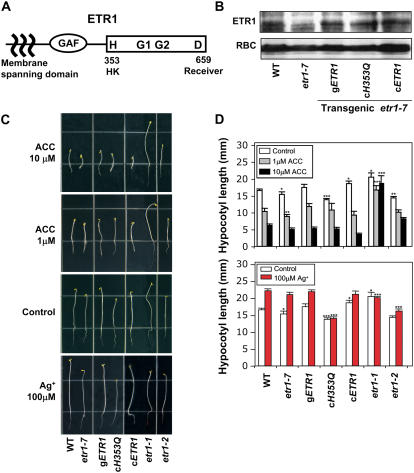
Structure and functions of ETR1 HK activity for plant growth promotion in the dark. A, The diagram depicts putative functional domains of ETR1. B, Expression of ETR1 proteins was detected by protein-blot analysis using anti-ETR1 antibody (Santa Cruz Biotech). Transgenic lines are etr1-7 plants transformed with the wild-type gETR1, the cDNA ETR1 gene containing a H353Q mutation (cH353Q), and the wild-type cETR1. For comparison, proteins of wild type (ecotype Columbia) and etr1-7 (etr1 null) were analyzed together. To detect the membrane protein ETR1, plants (14 d) were ground in 50 mm HEPES-KOH buffer, pH 7.6, containing 2 mm dithiothreitol, 2 mm EDTA, 10 mm β-glycerophosphate, 20% (v/v) glycerol, 1× complete protease inhibitor mix (Roche), and 1% (v/v) Triton X-100, and incubated on ice for 30 min. After removing tissue debris, protein loading buffer was added. The extracts were incubated at 65°C for 15 min and then 37°C for 30 min before running on 10% (w/v) SDS-polyacrylamide gels. The Rubisco (RBC) was shown as a protein loading control (Yanagisawa et al., 2003). C, Phenotypes of 4-d-old seedling growth in the dark. Seedlings were grown under saturating dosage of ACC (10 μm), subsaturating dosage of ACC (1 μm), ambient/endogenous ethylene (control), and ethylene-free (100 μm Ag+) conditions. For comparison, wild-type, etr1-7 (etr1 null), etr1-1 (strong ethylene-insensitive), and etr1-2 (weak ethylene-insensitive) seedlings were grown together with the transgenic etr1-7 lines. D, Quantitative analysis of hypocotyl length of 4-d-old etiolated seedlings. Values are means with sd, n = 30. Asterisks over bars indicate differences between wild type and mutant with statistical significance at *P < 0.05, **P < 0.01, and ***P < 0.001 (t test). [See online article for color version of this figure.]
Biochemical analyses have demonstrated functional HK activity (Gamble et al., 1998, 2002; Moussatche and Klee, 2004). Within the HK domain, both the catalytic G1 and ATP-binding G2 domains are required for the autophosphorylation of ETR1 at His-353. Protein-protein interaction between ETR1 and any of three Arabidopsis (Arabidopsis thaliana) His phosphotransfer proteins suggests that ETR1 HK activity may modulate Arabidopsis response regulators (ARRs) through two-component phosphor-relay system (Urao et al., 2000). Indeed, ETR1-dependent phosphorylation of the B-type ARR2 activates ethylene response transcription (Hass et al., 2004). Nevertheless, signaling through ARRs modulates ethylene signaling marginally, because combinations of B-type arr mutants are still sensitive to ethylene (Mason et al., 2005). Furthermore, ETR1 with inactive HK provides wild-type ethylene responsiveness in single etr1 or double etr1 ers1 null mutants (Gamble et al., 2002; Wang et al., 2003).
Thus far, the biological function or an in vivo signaling mechanism for ETR1 HK activity remains unknown. Only recently, the HK activity was shown to be important in the rate of growth recovery after ethylene removal (Binder et al., 2004). Otherwise, its role in ethylene or any other plant signaling is unclear.
To examine ETR1 HK function in vivo, we generated transgenic lines of etr1-7, an ETR1 null mutant, that contained either a genomic transgene of ETR1 (gETR1) or a cDNA of ETR1 (cETR1) under the control of native ETR1 promoter (2.2 kb). We also generated etr1-7 lines that were transformed with the cDNA of ETR1H353Q (cETR1H353Q), in which Gln (Q) replaces the only phosphorylatable His (H) 353 (Moussatche and Klee, 2004). For our analysis, each of several transgenic lines was selected both for a homozygous single transgene insertion and for similar transgenic protein expression at T3 generation (Fig. 1B; see Supplemental Fig. S1).
First, we reassessed whether ETR1 HK activity is involved in ethylene signaling by observing etiolated seedlings growing on the Murashige and Skoog media containing 1% (w/v) Suc and 10 μm of 1-aminocylopropane-1-carboxylic acid (ACC), which is the immediate precursor of ethylene. Wild-type, etr1-7, and transgenic gETR1, cETR1H353Q, and cETR1 seedlings displayed a typical triple response: inhibition of hypocotyl and root growth, exaggeration of apical hook formation, and hypocotyl thickening (Fig. 1, C and D). As expected, the etr1-1 and etr1-2 alleles showed strong and weak insensitivity to ACC, respectively (Bleecker et al., 1988; Hall et al., 1999).
An indifferent response among the transgenic lines at a saturating dose of ACC (10 μm) could be caused by the functional redundancy of receptors in ethylene signaling (Hua and Meyerowitz, 1998). To uncover ETR1 function among other redundant members, we observed etiolated seedlings growing on 1 μm of ACC (Alonso et al., 2003). Again, hypocotyls and roots of wild-type, etr1-7, and transgenic seedlings displayed similar growth inhibition (Fig. 1, C and D). Thus, our results with quantitative triple response assays support the notion that ETR1 HK activity is dispensable in ethylene signaling (Gamble et al., 2002; Wang et al., 2003; Binder et al., 2004; Qu and Schaller, 2004).
Next, we evaluated the role of ETR1 HK activity in response to ambient and/or endogenous ethylene. Wild-type, transgenic gETR1, and cETR1 etiolated seedlings growing without ACC addition displayed normal hypocotyl elongation (Fig. 1, C and D). However, dark-grown seedlings appear to be sensitive to the ambient/endogenous ethylene, because seedlings exhibited enhanced hypocotyl elongation in the presence of silver. Silver has been shown to competitively inhibit ethylene receptor function (Rodríguez et al., 1999). Indeed, etr1-1 seedlings, in which ethylene binding is abolished, displayed enhanced hypocotyl elongation without silver but did not show further elongation with silver, demonstrating its strong ethylene insensitivity. The etr1-2 displayed slow hypocotyl elongation without silver and enhanced hypocotyl elongation with silver, which is consistent with its weak ethylene insensitivity.
We note that etr1-7, in comparison to wild type, displayed relatively slow hypocotyl elongation in the absence of silver with statistic confidence (*P < 0.05) and fully enhanced hypocotyl elongation in the presence of silver (Fig. 1, C and D). This observation may implicate a role for ETR1 in plant growth that is independent of ethylene signaling (Hua and Meyerowitz, 1998), or the seedlings without ETR1 may be hypersensitive to ethylene (Cancel and Larsen, 2002).
Interestingly, transgenic cETR1H353Q seedlings exhibited even slower hypocotyl elongation than etr1-7 without silver and lacked further hypocotyl elongation with silver. This indicates that the growth defect caused by the loss of ETR1 HK activity is not because of the increased ethylene production or ethylene responsiveness.
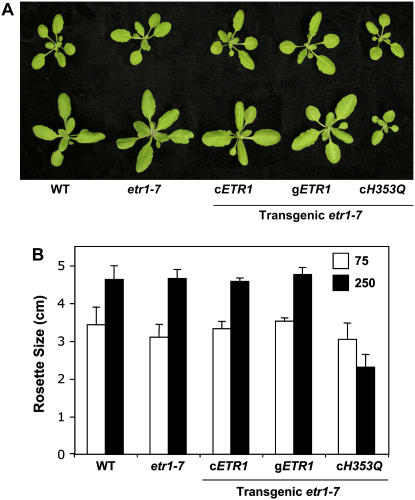
Because plant growth in either the light or darkness is mechanically distinct, we decided to also observe growth of transgenic plants under light. With the exception of cETR1H353Q, all other lines were indistinguishable from wild type under normal growth conditions (the cycle of 16-h light and 8-h dark with the light intensity of 75 μE m−2 s−1). The cETR1H353Q lines showed a minor reduction in rosette size (Fig. 2, A [top] and B). In growth-promoting light conditions (the light intensity of 250 μE m−2 s−1), wild-type, etr1-7, and transgenic cETR1 and gETR1 plants exhibited relatively bigger rosette size (Fig. 2, A [bottom] and B). However, transgenic cETR1H353Q plants failed to promote such enhanced rosette growth, indicating that ETR1 HK activity is also involved in growth promotion under light. It will be interesting to further test if the light signaling-implicated GAF domain of ETR1 plays an additional role in the high light-driven growth promotion.
Figure 2.
Functions of ETR1 HK activity for plant growth promotion under light. A, Phenotypes of the wild-type, etr1-7, and transgenic etr1-7 plants grown in the cycle of 16-h light and 8-h dark with light intensity of 75 μE m−2 s−1 (top) or 250 μE m−2 s−1 (bottom) for 21 d. B, Quantitative analysis (rosette diameter) of plant growth phenotypes under normal (75) and high (250) light intensity conditions. Values are means with sd, n = 20.
Because ethylene receptors have at least partial functional redundancy, the growth defects of cETR1H353Q suggest that ETR1H353Q has a dominant negative effect on the other ethylene receptors. This may indicate the existence of heteromeric receptor complexes. Currently, a receptor complex containing an ETR1 homodimer and CONSTITUTIVE TRIPLE RESPONSE 1 (CTR1) has been biochemically characterized; however, other partners remain to be identified (Schaller et al., 1995; Chen et al., 2002; Gao et al., 2003). Recently, a genetic screen for suppressors of etr1-2 identified the recessive mutant reversion to ethylene sensitivity 1, which acts upstream of ETR1 (Resnick et al., 2006) and is a potential component of the ETR1 receptor complex.
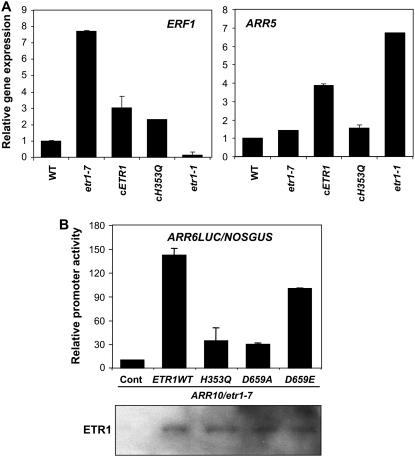
To gain insight into the molecular mechanism(s) underlying the growth defects associated with the loss of ETR1 HK activity, we monitored gene expression in etiolated seedlings using quantitative reverse transcription-PCR. Typically, a receptor HK is a signal input module of a two-component system (Hwang et al., 2002). Thus, we examined marker gene expression that is induced by the two-component system as well as by ethylene signaling. The gene expression of ERF1 is a diagnostic feature of ethylene signaling (Solano et al., 1998) and was significantly higher in etr1-7 but abolished in the dominant ethylene-insensitive etr1-1 compared with wild type (Fig. 3A). The ERF1 gene expression was similarly suppressed in both transgenic cETR1 and cETR1H353Q, supporting the contention that the growth defect in the transgenic cETR1H353Q is not caused by ethylene responsiveness but by the lack of growth promotion resulting from the loss of HK activity.
Figure 3.
Functions of ETR1 HK activity in the activation of two-component system. A, Relative expressions of marker genes (ERF1, At3G23240; ARR5, At3g48100) in the 4-d-old etiolated seedlings growing at the ambient/endogenous ethylene condition. Values are means with sd (n = 3) obtained by using quantitative reverse transcription-PCR and calculated relatively to those of wild type. Expression level of eIF4a (At3g13920) gene was served as a control. B, The ARR6-LUC reporter activity induced by various ETR1 effecters. Values are means with sd (n = 5). Effecter constructs including ETR1WT, inactive form ETR1 genes containing H353Q mutation and D659A mutation, and an activated form ETR1 gene containing D659E mutation were generated by PCR and site-specific mutagenesis in plant expression vectors. The designated constructs were cotransfected to leaf mesophyll protoplasts generated from etr1-7 leaf tissues, and the LUC reporter assay was performed as before (Hwang and Sheen, 2001; Sheen Web site, http://genetics.mgh.harvard.edu/sheenweb). The effecter (ETR1 variants), mediator (ARR10, At4g31920), reporter (ARR6LUC), and internal control reporter (NOSGUS) constructs were transfected in the ratio of 4:1:4:1. Expressions of ETR1 proteins were shown by protein-blot analysis using anti-ETR1 antibody.
The gene expression of the A-type ARR5 is a hallmark of two-component system activity (D'Agostino et al., 2000) and was relatively higher in transgenic cETR1 in comparison to etr1-7, cETR1H353Q, and wild type (Fig. 3A). The ARR5 gene expression was significantly higher in etr1-1. Mature plant growth of etr1-1 is also greatly enhanced with a higher level of the ETR1 mutant protein (etr1-1: ETR1C65Y) accumulation, which is incompetent in ethylene signaling (Zhao et al., 2002). Most likely, the induced ARR5 gene expression in etr1-1 resulted from the two-component system activated by the etr1-1.
We also detected a high level of the B-type ARR1 expression in etr1-1 (see Supplemental Fig. S2). Currently, the function of increased ARR1 gene expression is unknown; however, it may balance the constitutive A-type ARR activity, which plays a repressor role in the two-component system (Hwang et al., 2002).
To substantiate the downstream molecular mechanism of ETR1 HK activity, we examined whether the kinase activity could modulate two-component system by taking advantage of the well-established ARR6 promoter fused with luciferase reporter (ARR6-LUC; Hwang and Sheen, 2001). We generated constructs of an ETR1 wild type (ETR1WT), inactive forms (H353Q and D659A), and a constitutive active form (D659E). Individual construct was cotransfected with ARR6-LUC to the mesophyll protoplasts generated from etr1-7 leaf tissues. A minimum level of the B-type ARR positive mediator (ARR10) was also cotransfected to sensitize the ethylene-sensitive etr1-7 cells to the two-component system activity. Both ETR1WT and D659E induced the ARR6-LUC reporter activity in a similar fashion, whereas such reporter induction was greatly lowered with H353Q and D659A (Fig. 3B). In summary, ETR1 appears to activate two-component system through the conserved HK (H353) and response regulator (D659) residues.
Because ETR1WT was as potent as D659E for reporter induction, the ETR1WT appeared to be activated upon its expression. This is consistent with the previous observation that ETR1 is autophosphorylated without its ligand binding in vitro (Gamble et al., 1998, 2002; Moussatche and Klee, 2004). Therefore, the activation mechanism of ETR1 HK appears distinct from other Arabidopsis HKs functioning in cytokinin signaling, of which activities are largely dependent on the ligand binding (Hwang and Sheen, 2001).
Here we have shown that ETR1 HK activity triggers the two-component system and promotes plant growth in Arabidopsis. It remains to be determined if the two-component system activated by ETR1 HK activity contributes to the plant growth promotion. It would also be interesting to know if ETR1 HK activity switches on and off in the absence and presence of ethylene, respectively, to coordinate plant growth promotion and inhibition. Further elucidation of molecular mechanisms underlying ETR1 action will improve our understanding of ethylene functions in plant growth and developments.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Consistent phenotypes of independent transgenic lines.
Supplemental Figure S2. Relative expressions of ARR1.
Supplementary Material
Acknowledgments
We thank the late Dr. Tony Bleecker for sharing etr1-7 seeds and his excellent contribution in the ethylene-signaling field. We appreciate Dr. Meyerowitz for generating the etr1-7 line, Ms. Mandy Reading and Dr. Caren Chang for sharing etr1-2 seeds, Dr. Bruno Mueller for sharing the ARR10 construct and helping with the manuscript preparation, and Drs. Michael McManus and Andrew Diener for providing many comments on the manuscript preparation. We also express our sincere gratitude to Dr. Jen Sheen for her support during our research tenure as postdoctoral fellows in Massachusetts General Hospital/Harvard Medical School.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sang-Dong Yoo (yoo@molbio.mgh.harvard.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
References
- Alonso JM, Stepano AN, Solano R, Wisman E, Ferrari S, Ausbel FM, Ecker JR (2003) Five components of the ethylene-response pathway identified in a screen for weak ethylene-insensitive mutants in Arabidopsis. Proc Natl Acad Sci USA 100 2992–2997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder BM, O'Malley RC, Wang W, Moore JM, Parks BM, Spalding EP, Bleecker AB (2004) Arabidopsis seedling growth response and recovery to ethylene: a kinetic analysis. Plant Physiol 136 2913–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 241 1086–1089 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16 1–18 [DOI] [PubMed] [Google Scholar]
- Cancel JD, Larsen PB (2002) Loss-of-function mutations in the ethylene receptor ETR1 cause enhanced sensitivity and exaggerated response to ethylene in Arabidopsis. Plant Physiol 129 1557–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM (1993) Arabidopsis ethylene response gene ETR1: similarity of product to two component regulators. Science 262 539–544 [DOI] [PubMed] [Google Scholar]
- Chen Y-F, Randlett MD, Findell JL, Schaller GE (2002) Localization of the ethylene receptor ETR1 to the endoplasmic reticulum of Arabidopsis. J Biol Chem 277 19861–19866 [DOI] [PubMed] [Google Scholar]
- D'Agostino IB, Deruere J, Kieber JJ (2000) Characterization of the response of the Arabidopsis response regulator gene family to cytokinin. Plant Physiol 124 1706–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Coonfield ML, Schaller GE (1998) Histidine kinase activity of the ETR1 ethylene receptor from Arabidopsis. Proc Natl Acad Sci USA 95 7825–7829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble RL, Qu X, Schaller GE (2002) Mutational analysis of the ethylene receptor ETR1: role of the histidine kinase domain in dominant ethylene insensitivity. Plant Physiol 128 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Chen Y-F, Randlett MD, Zhao X-C, Findell JL, Schaller GE, Kieber JJ, Schaller GE (2003) Localization of the Raf-like kinase CTR1 to the endoplasmic reticulum of Arabidopsis through participation in ethylene receptor signaling complexes. J Biol Chem 278 34725–34732 [DOI] [PubMed] [Google Scholar]
- Hall AE, Chen QG, Findell JL, Schaller GE, Bleecker AB (1999) The relationship between ethylene binding and dominant insensitivity conferred by mutant forms of the ETR1 ethylene receptor. Plant Physiol 121 291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass C, Lohrmann J, Albrecht V, Sweere U, Hummel F, Yoo SD, Hwang I, Zhu T, Schafer E, Kudla J, et al (2004) The response regulator 2 mediates ethylene signalling and hormone signal integration in Arabidopsis. EMBO J 23 3290–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM (1998) Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana. Cell 94 261–271 [DOI] [PubMed] [Google Scholar]
- Hwang I, Chen H-C, Sheen J (2002) Two-component signal transduction pathways in Arabidopsis. Plant Physiol 129 500–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang I, Sheen J (2001) Two-component circuitry in Arabidopsis cytokinin signal transduction. Nature 413 383–389 [DOI] [PubMed] [Google Scholar]
- Mason M, Mathews DE, Argyros DA, Maxwell BB, Kieber JJ, Alonso JM, Ecker JR, Schaller GE (2005) Multiple type-B response regulators mediate cytokinin signal transduction in Arabidopsis. Plant Cell 17 3007–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussatche P, Klee HJ (2004) Autophosphorylation activity of the Arabidopsis ethylene receptor multigene family. J Biol Chem 279 48734–48741 [DOI] [PubMed] [Google Scholar]
- Qu X, Schaller GE (2004) Requirement of the histidine kinase domain for signal transduction by the ethylene receptor ETR1. Plant Physiol 136 2961–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick JS, Wen C-K, Shockey JA, Chang C (2006) Reversion-to-ethylene sensitivity1, a conserved gene that regulates ethylene receptor function in Arabidopsis. Proc Natl Acad Sci USA 103 7917–7922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB (1999) A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283 996–998 [DOI] [PubMed] [Google Scholar]
- Schaller GE, Kieber JJ (2002) Ethylene. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD
- Schaller GE, Ladd AN, Lanahan MB, Spanbauer JM, Bleecker AB (1995) The ethylene response mediator ETR1 from Arabidopsis forms a disulfide-linked dimer. J Biol Chem 270 12526–12530 [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urao T, Miyata S, Yamaguchi-Shinozaki K, Shinozaki K (2000) Possible His to Asp phosphorelay signaling in an Arabidopsis two-component system. FEBS Lett 478 227–232 [DOI] [PubMed] [Google Scholar]
- Wang W, Hall AE, O'Malley R, Bleecker AB (2003) Canonical histidine kinase activity of the transmitter domain of the ETR1 ethylene receptor from Arabidopsis is not required for signal transmission. Proc Natl Acad Sci USA 100 352–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa S, Yoo SD, Sheen J (2003) Differential regulation of EIN3 stability by glucose and ethylene signalling in plants. Nature 425 521–525 [DOI] [PubMed] [Google Scholar]
- Zhao X, Qu X, Mattews DE, Schaller GE (2002) Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiol 130 1983–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.