Abstract
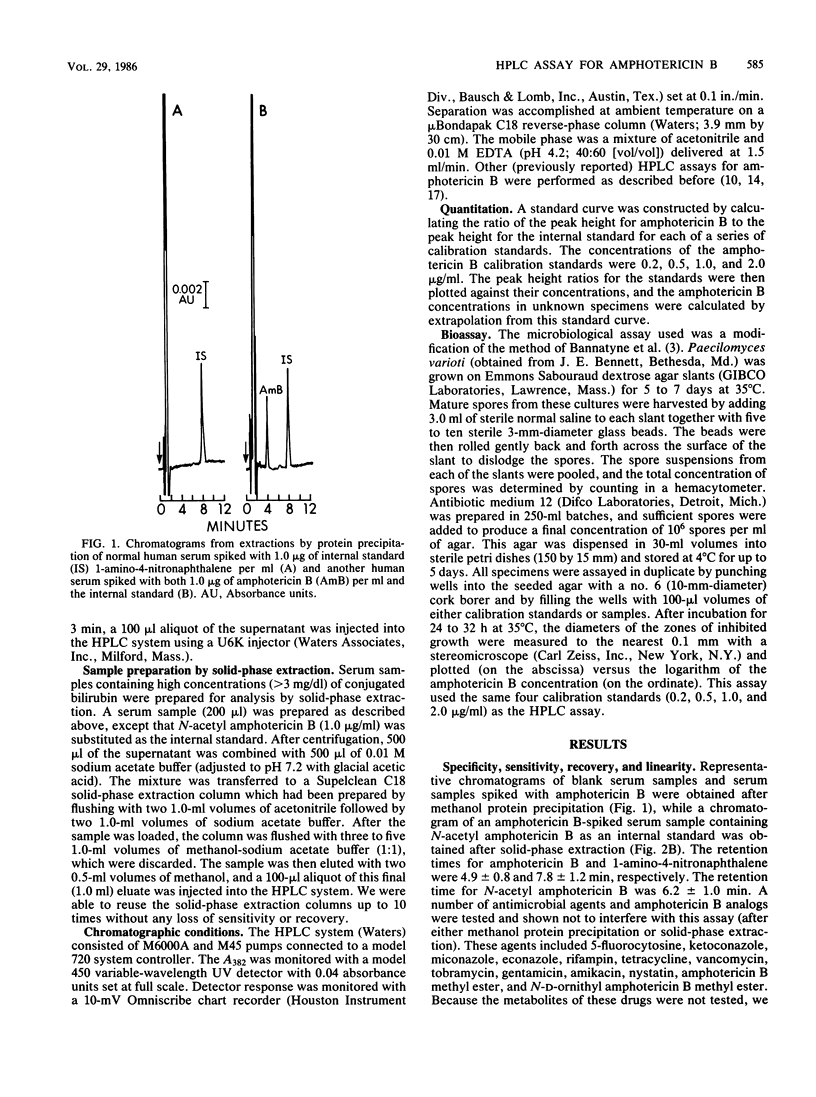
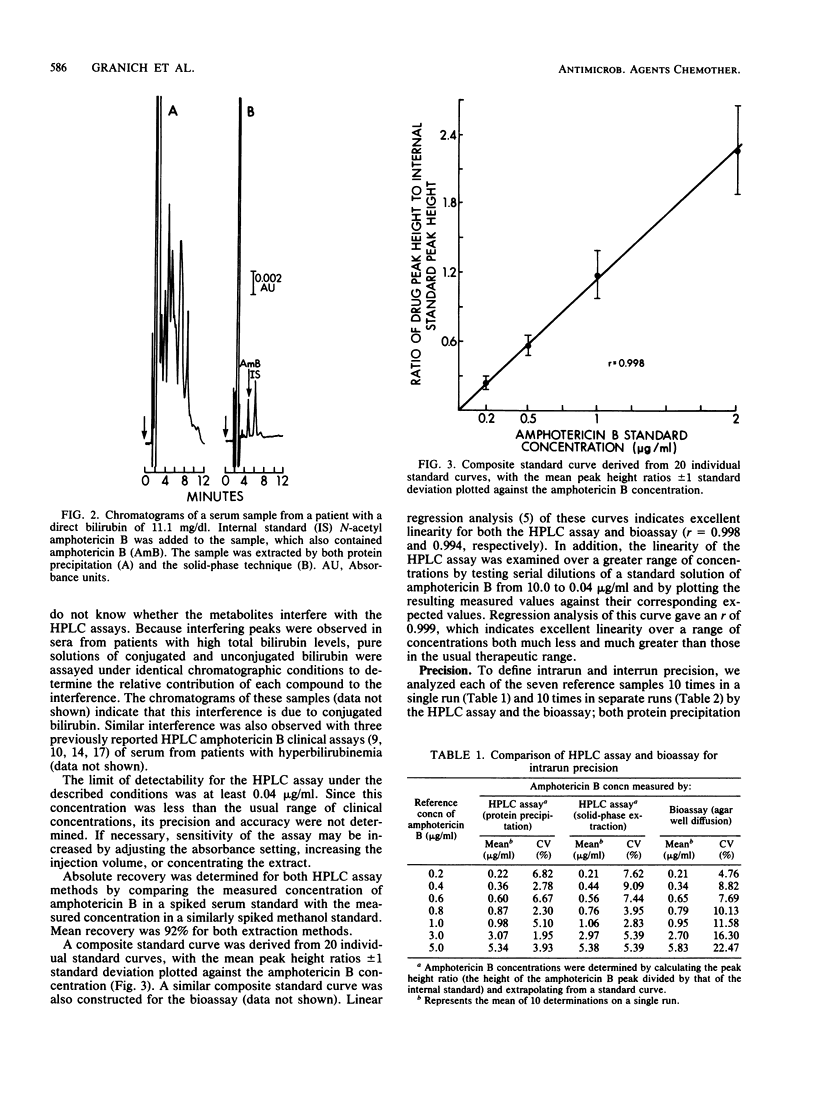
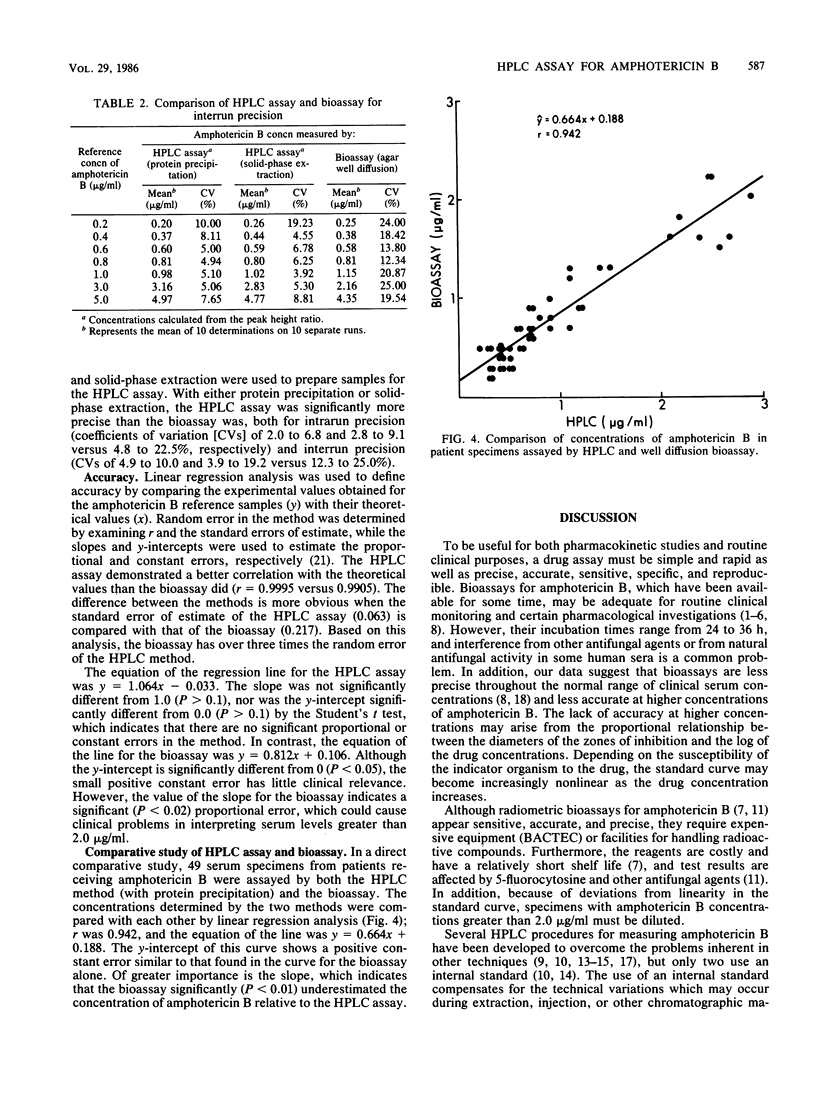
We developed a rapid, sensitive, high-pressure liquid chromatographic (HPLC) procedure which incorporates a commercially available internal standard, 1-amino-4-nitronaphthalene, to measure amphotericin B in serum. Recovery was quantitative (greater than or equal to 90%), and the standard curve was linear from 0.04 to at least 10.0 micrograms/ml. The reproducibility of the assay was good, with intrarun coefficients of variation from 2.0 to 6.8% and interrun coefficients of variation from 4.9 to 10.0%. Comparison by linear regression analysis of the HPLC assay with an agar well diffusion bioassay gave a correlation coefficient of 0.942, with the HPLC assay exhibiting greater precision and sensitivity. No interference was encountered from over 20 drugs and three amphotericin B analogs. However, serum specimens that contained high concentrations of conjugated bilirubin (greater than 3 mg/dl) produced interfering peaks in both this assay and other previously reported HPLC assays for amphotericin B. We also describe a solid-phase extraction procedure which effectively removes this interference and uses an alternative internal standard (N-acetyl amphotericin B).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson A. J., Jr, Bennett J. E. Amphotericin B pharmacokinetics in humans. Antimicrob Agents Chemother. 1978 Feb;13(2):271–276. doi: 10.1128/aac.13.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne R. M., Cheung R., Devlin H. R. Microassay foramphotericin B. Antimicrob Agents Chemother. 1977 Jan;11(1):44–46. doi: 10.1128/aac.11.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne R. M., Cheung R. Discrepant results of amphotericin B assays on fresh versus frozen serum samples. Antimicrob Agents Chemother. 1977 Oct;12(4):550–550. doi: 10.1128/aac.12.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindschadler D. D., Bennett J. E. A pharmacologic guide to the clinical use of amphotericin B. J Infect Dis. 1969 Oct;120(4):427–436. doi: 10.1093/infdis/120.4.427. [DOI] [PubMed] [Google Scholar]
- Craven P. C., Ludden T. M., Drutz D. J., Rogers W., Haegele K. A., Skrdlant H. B. Excretion pathways of amphotericin B. J Infect Dis. 1979 Sep;140(3):329–341. doi: 10.1093/infdis/140.3.329. [DOI] [PubMed] [Google Scholar]
- Drazin R. E., Lehrer R. I. Rubidium release: a rapid and sensitive assay for amphotericin B. J Infect Dis. 1976 Sep;134(3):238–244. doi: 10.1093/infdis/134.3.238. [DOI] [PubMed] [Google Scholar]
- Fields B. T., Jr, Bates J. H., Abernathy R. S. Amphotericin B serum concentrations during therapy. Appl Microbiol. 1970 Jun;19(6):955–959. doi: 10.1128/am.19.6.955-959.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golas C. L., Prober C. G., MacLeod S. M., Soldin S. J. Measurement of amphotericin B in serum or plasma by high-performance liquid chromatography. J Chromatogr. 1983 Dec 9;278(2):387–395. doi: 10.1016/s0378-4347(00)84798-2. [DOI] [PubMed] [Google Scholar]
- Hopfer R. L., Gröschel D. Radiometric determination of the concentration of amphotericin B in body fluids. Antimicrob Agents Chemother. 1977 Dec;12(6):733–735. doi: 10.1128/aac.12.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Lin C. High-pressure liquid chromatographic method for determination of Sch 28191 in biological fluids. Antimicrob Agents Chemother. 1984 Jan;25(1):45–48. doi: 10.1128/aac.25.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margosis M., Aszalos A. Quantitation of amphotericins by reverse-phase high-performance liquid chromatography. J Pharm Sci. 1984 Jun;73(6):835–838. doi: 10.1002/jps.2600730635. [DOI] [PubMed] [Google Scholar]
- Mayhew J. W., Fiore C., Murray T., Barza M. An internally-standardized assay for amphotericin B in tissues and plasma. J Chromatogr. 1983 May 13;274:271–279. doi: 10.1016/s0378-4347(00)84430-8. [DOI] [PubMed] [Google Scholar]
- Mechlinski W., Schaffner C. P. Separation of polyene antifungal antibiotics by high-speed liquid chromatography. J Chromatogr. 1974 Nov 6;99(0):619–633. doi: 10.1016/s0021-9673(00)90890-2. [DOI] [PubMed] [Google Scholar]
- Medoff G., Kobayashi G. S. Strategies in the treatment of systemic fungal infections. N Engl J Med. 1980 Jan 17;302(3):145–155. doi: 10.1056/NEJM198001173020304. [DOI] [PubMed] [Google Scholar]
- Nilsson-Ehle I., Yoshikawa T. T., Edwards J. E., Schotz M. C., Guze L. B. Quantitation of amphotericin B with use of high-pressure liquid chromatography. J Infect Dis. 1977 Mar;135(3):414–422. doi: 10.1093/infdis/135.3.414. [DOI] [PubMed] [Google Scholar]
- Shadomy S., McCay J. A., Schwartz S. I. Bioassay for hamycin and amphotericin B in serum and other biological fluids. Appl Microbiol. 1969 Apr;17(4):497–503. doi: 10.1128/am.17.4.497-503.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A. H. Analysis and assay of polyene antifungal antibiotics. A review. Analyst. 1976 May;101(1202):321–340. doi: 10.1039/an9760100321. [DOI] [PubMed] [Google Scholar]
- Warnock D. W., Richardson M. D., Turner A. High performance liquid chromatographic (HPLC) and other non-biological methods for quantitation of antifungal drugs. J Antimicrob Chemother. 1982 Dec;10(6):467–478. doi: 10.1093/jac/10.6.467. [DOI] [PubMed] [Google Scholar]
- Westgard J. O., Hunt M. R. Use and interpretation of common statistical tests in method-comparison studies. Clin Chem. 1973 Jan;19(1):49–57. [PubMed] [Google Scholar]



