Abstract
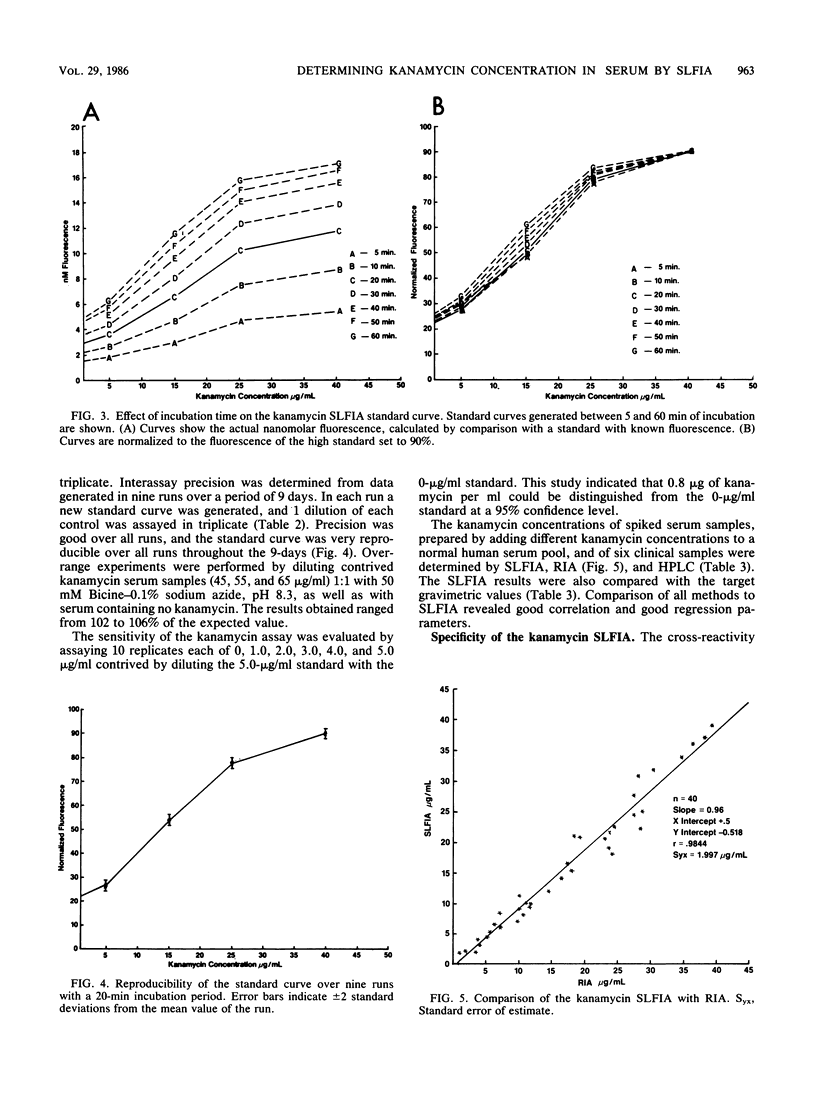
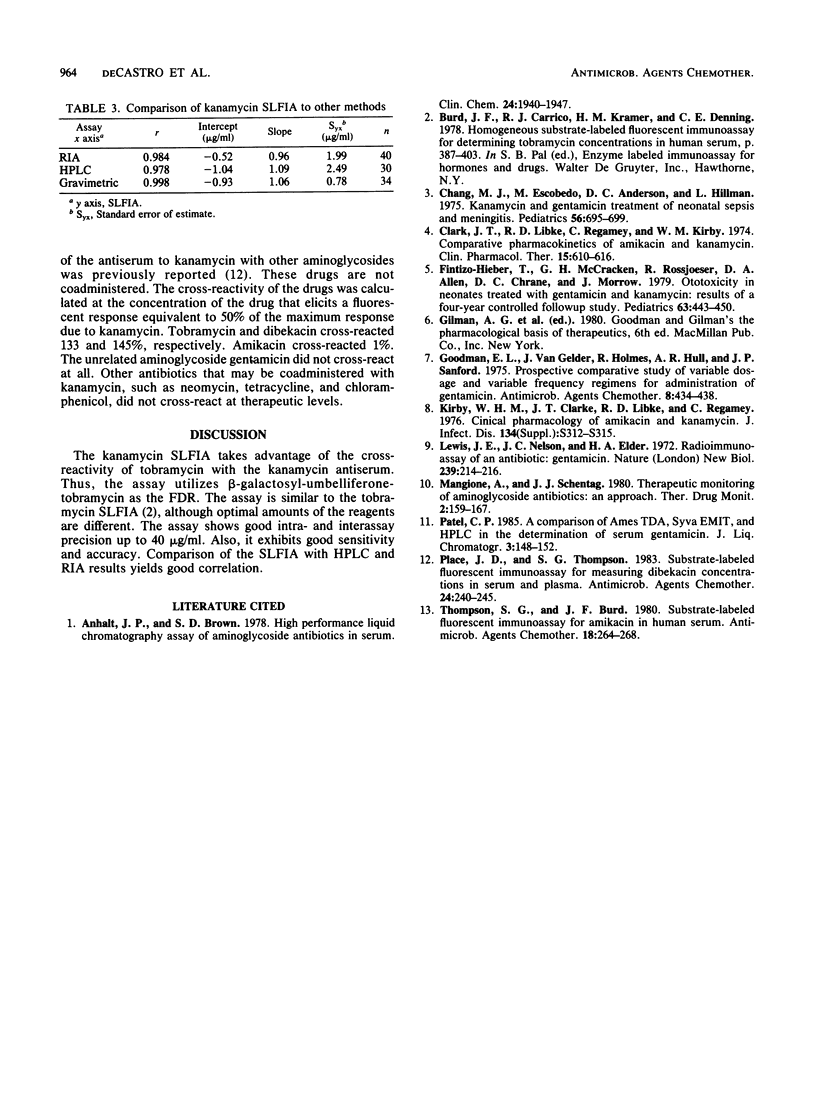
A homogeneous substrate-labeled fluorescent immunoassay was developed for the measurement of kanamycin concentrations in serum. A fluorogenic drug reagent (FDR) (beta-galactosyl-umbelliferone-tobramycin) was prepared that is nonfluorescent under the conditions of the assay but is hydrolyzed upon catalysis by beta-galactosidase to yield a fluorescent product. Binding of the FDR to the antiserum to kanamycin prevented enzyme hydrolysis. The fixed level of FDR in the assay competed with kanamycin in the sample for a limited number of antibody-binding sites. Unbound FDR was hydrolyzed by beta-galactosidase to release a fluorescent product that is proportional to the kanamycin concentration in the sample. The assay exhibited good sensitivity, precision, and accuracy and correlated well with other methods.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anhalt J. P., Brown S. D. High-performance liquid-chromatographic assay of aminoglycoside antibiotics in serum. Clin Chem. 1978 Nov;24(11):1940–1947. [PubMed] [Google Scholar]
- Chang M. J., Escobedo M., Anderson D. C., Hillman L., Feigin R. D. Kanamycin and gentamicin treatment of neonatal sepsis and meningitis. Pediatrics. 1975 Nov;56(5):695–699. [PubMed] [Google Scholar]
- Clarke J. T., Libke R. D., Regamey C., Kirby W. M. Comparative pharmacokinetics of amikacin and kanamycin. Clin Pharmacol Ther. 1974 Jun;15(6):610–616. doi: 10.1002/cpt1974156610. [DOI] [PubMed] [Google Scholar]
- Finitzo-Hieber T., McCracken G. H., Jr, Roeser R. J., Allen D. A., Chrane D. F., Morrow J. Ototoxicity in neonates treated with gentamicin and kanamycin: results of a four-year controlled follow-up study. Pediatrics. 1979 Mar;63(3):443–450. [PubMed] [Google Scholar]
- Goodman E. L., Van Gelder J., Holmes R., Hull A. R., Sanford J. P. Prospective comparative study of variable dosage and variable frequency regimens for administration of gentamicin. Antimicrob Agents Chemother. 1975 Oct;8(4):434–438. doi: 10.1128/aac.8.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby W. M., Clarke J. T., Libke R. D., Regamey C. Clinical pharmacology of amikacin and kanamycin. J Infect Dis. 1976 Nov;134(Suppl):S312–S315. doi: 10.1093/infdis/135.supplement_2.s312. [DOI] [PubMed] [Google Scholar]
- Lewis J. E., Nelson J. C., Elder H. A. Radioimmunoassay of an antibiotic: gentamicin. Nat New Biol. 1972 Oct 18;239(94):214–216. doi: 10.1038/newbio239214a0. [DOI] [PubMed] [Google Scholar]
- Mangione A., Schentag J. J. Therapeutic monitoring of aminoglycoside antibiotics: an approach. Ther Drug Monit. 1980;2(2):159–167. doi: 10.1097/00007691-198004000-00010. [DOI] [PubMed] [Google Scholar]
- Place J. D., Thompson S. G. Substrate-labeled fluorescent immunoassay for measuring dibekacin concentrations in serum and plasma. Antimicrob Agents Chemother. 1983 Aug;24(2):240–245. doi: 10.1128/aac.24.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. G., Burd J. F. Substrate-labeled fluorescent immunoassay for amikacin in human serum. Antimicrob Agents Chemother. 1980 Aug;18(2):264–268. doi: 10.1128/aac.18.2.264. [DOI] [PMC free article] [PubMed] [Google Scholar]


