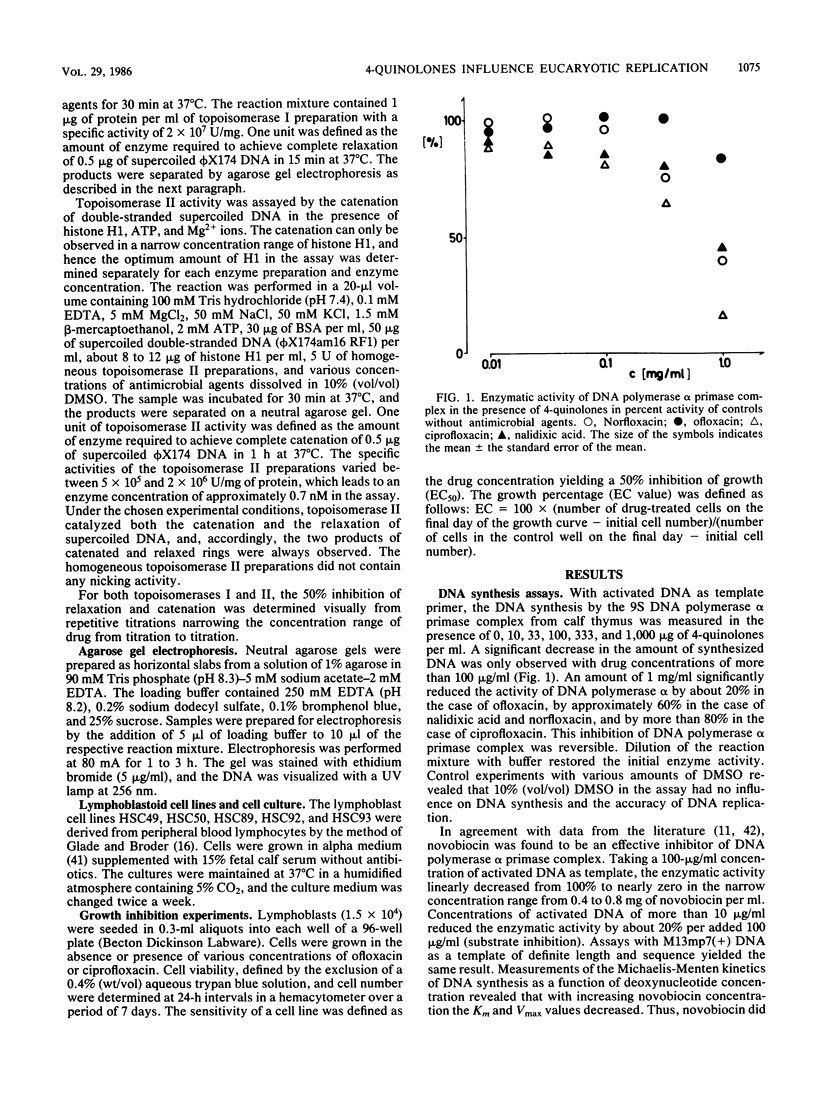
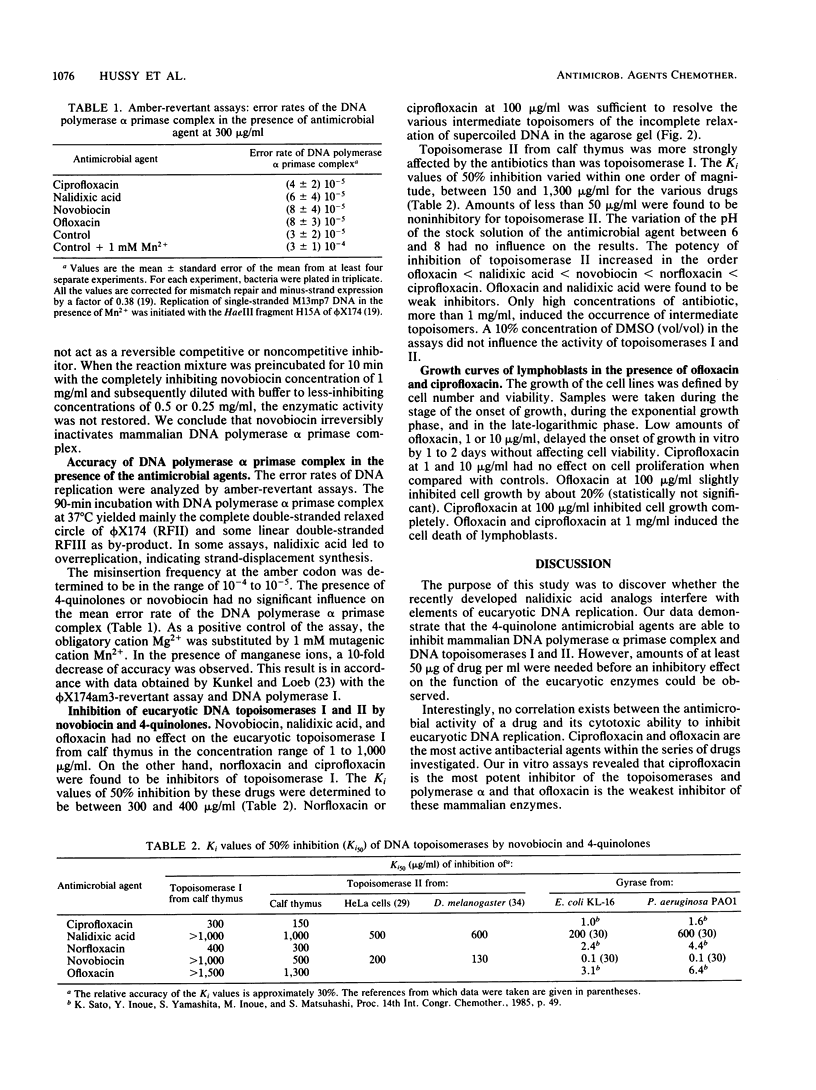
Abstract
The influence of ciprofloxacin, nalidixic acid, norfloxacin, novobiocin, and ofloxacin on elements of eucaryotic DNA replication was investigated in vitro. Each of the 4-quinolones, when present in amounts of more than 100 micrograms/ml, reversibly inhibited the DNA synthesis performed by the 95 DNA polymerase alpha primase complex from calf thymus. Novobiocin at 500 micrograms/ml or at higher concentrations irreversibly inactivated DNA polymerase alpha primase complex. The accuracy of in vitro DNA synthesis in the absence of repair mechanisms was determined from amber-revertant assays with phi X174am16(+) DNA as template. The antimicrobial agents did not significantly increase the frequencies of base pairing mismatches during the course of replication, indicating that the basal mutation rate is not affected by novobiocin and the 4-quinolones. The Ki values of 50% inhibition of DNA topoisomerases from calf thymus by ciprofloxacin, norfloxacin, novobiocin, nalidixic acid, and ofloxacin were 300, 400, 1,000 or more, 1,000 or more, and 1,500 or more micrograms/ml, respectively, in the case of topoisomerase I, and the Ki values were 150, 300, 500, 1,000, and 1,300 micrograms/ml, respectively, in the case of topoisomerase II. The procaryotic topoisomerase II is approximately 100-fold more sensitive to inhibition by ciprofloxacin, norfloxacin, and ofloxacin than is its eucaryotic counterpart. Growth curves of lymphoblasts were recorded in the presence of ofloxacin and ciprofloxacin. Neither 1 nor 10 micrograms of ciprofloxacin or of ofloxacin per ml affected cell proliferation. Ofloxacin and ciprofloxacin at 100 micrograms/ml inhibited cell growth; 1,000 micrograms/ml led to cell death. No correlation exists between the antimicrobial and cytotoxic activities of the 4-quinolones.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of Lambda Lysogenicity during Bacterial Recombination in Escherichia Coli K12. Genetics. 1954 Jul;39(4):429–439. doi: 10.1093/genetics/39.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind A., Petermüller C. In vitro activity of ciprofloxacin, norfloxacin and nalidixic acid. Eur J Clin Microbiol. 1983 Apr;2(2):111–115. doi: 10.1007/BF02001575. [DOI] [PubMed] [Google Scholar]
- Burke J. F., Duff P. M., Pearson C. K. Effect of drugs on deoxyribonucleic acid synthesis in isolated mammalian cell nuclei. Comparison with partially purified deoxyribonucleic acid polymerases. Biochem J. 1979 Mar 15;178(3):621–626. doi: 10.1042/bj1780621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E. Specificity and completeness of inhibition of DNA repair by novobiocin and aphidicolin. Carcinogenesis. 1982;3(10):1171–1174. doi: 10.1093/carcin/3.10.1171. [DOI] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M., Boulanger P. Effects of novobiocin on adenovirus DNA synthesis and encapsidation. Nucleic Acids Res. 1980 Apr 11;8(7):1625–1641. doi: 10.1093/nar/8.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detera S. D., Becerra S. P., Swack J. A., Wilson S. H. Studies on the mechanism of DNA polymerase alpha. Nascent chain elongation, steady state kinetics, and the initiation phase of DNA synthesis. J Biol Chem. 1981 Jul 10;256(13):6933–6943. [PubMed] [Google Scholar]
- Duguet M., Lavenot C., Harper F., Mirambeau G., De Recondo A. M. DNA topoisomerases from rat liver: physiological variations. Nucleic Acids Res. 1983 Feb 25;11(4):1059–1075. doi: 10.1093/nar/11.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Halligan B., Cooke C. A., Heck M. M., Liu L. F. Topoisomerase II is a structural component of mitotic chromosome scaffolds. J Cell Biol. 1985 May;100(5):1706–1715. doi: 10.1083/jcb.100.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Heck M. M. Localization of topoisomerase II in mitotic chromosomes. J Cell Biol. 1985 May;100(5):1716–1725. doi: 10.1083/jcb.100.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg H. J. Novobiocin inhibition of simian virus 40 DNA replication. Nature. 1980 Jul 31;286(5772):529–531. doi: 10.1038/286529a0. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Knill-Jones J. W. DNA polymerase accuracy and spontaneous mutation rates: frequencies of purine.purine, purine.pyrimidine, and pyrimidine.pyrimidine mismatches during DNA replication. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4251–4255. doi: 10.1073/pnas.78.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fersht A. R., Knill-Jones J. W. Fidelity of replication of bacteriophage phi X174 DNA in vitro and in vivo. J Mol Biol. 1983 Apr 25;165(4):633–654. doi: 10.1016/s0022-2836(83)80271-x. [DOI] [PubMed] [Google Scholar]
- Fleischmann G., Pflugfelder G., Steiner E. K., Javaherian K., Howard G. C., Wang J. C., Elgin S. C. Drosophila DNA topoisomerase I is associated with transcriptionally active regions of the genome. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6958–6962. doi: 10.1073/pnas.81.22.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Grosse F., Krauss G., Knill-Jones J. W., Fersht A. R. Accuracy of DNA polymerase-alpha in copying natural DNA. EMBO J. 1983;2(9):1515–1519. doi: 10.1002/j.1460-2075.1983.tb01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse F., Krauss G. Purification of a 9S DNA polymerase alpha species from calf thymus. Biochemistry. 1981 Sep 15;20(19):5470–5475. doi: 10.1021/bi00522a019. [DOI] [PubMed] [Google Scholar]
- Gudas L. J. The induction of protein X in DNA repair and cell division mutants of Escherichia coli. J Mol Biol. 1976 Jul 5;104(3):567–587. doi: 10.1016/0022-2836(76)90121-2. [DOI] [PubMed] [Google Scholar]
- Ishida R., Buchwald M. Susceptibility of Fanconi's anemia lymphoblasts to DNA-cross-linking and alkylating agents. Cancer Res. 1982 Oct;42(10):4000–4006. [PubMed] [Google Scholar]
- King A., Shannon K., Phillips I. The in-vitro activity of ciprofloxacin compared with that of norfloxacin and nalidixic acid. J Antimicrob Chemother. 1984 Apr;13(4):325–331. doi: 10.1093/jac/13.4.325. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. Effect of divalent metal ion activators and deoxyrionucleoside triphosphate pools on in vitro mutagenesis. J Biol Chem. 1979 Jul 10;254(13):5718–5725. [PubMed] [Google Scholar]
- Lavin M. F. Effect of novobiocin on DNA synthesis and structure in human lymphoblastoid cells. Biochem Biophys Res Commun. 1981 May 15;100(1):328–335. doi: 10.1016/s0006-291x(81)80100-3. [DOI] [PubMed] [Google Scholar]
- Lynch W. E., Short J., Lieberman I. The 7.1 S nuclear DNA polymerase and DNA replication in intact liver. Cancer Res. 1976 Mar;36(3):901–904. [PubMed] [Google Scholar]
- Mattern M. R., Scudiero D. A. Dependence of mammalian DNA synthesis on DNA supercoiling. III. Characterization of the inhibition of replicative and repair-type DNA synthesis by novobiocin and nalidixic acid. Biochim Biophys Acta. 1981 Apr 27;653(2):248–258. doi: 10.1016/0005-2787(81)90160-x. [DOI] [PubMed] [Google Scholar]
- Meechan P. J., Killpack S., Cleaver J. E. Novobiocin-mediated inhibition of polymerization and ligation of DNA in vitro. Mutat Res. 1984 Oct;141(2):69–73. doi: 10.1016/0165-7992(84)90013-7. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. G., Liu L. F., Englund P. T. A homogeneous type II DNA topoisomerase from HeLa cell nuclei. J Biol Chem. 1981 Sep 10;256(17):9334–9339. [PubMed] [Google Scholar]
- Miller R. V., Scurlock T. R. DNA gyrase (Topoisomerase II) from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1983 Jan 27;110(2):694–700. doi: 10.1016/0006-291x(83)91205-6. [DOI] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J., van Veldhuizen G. Comparative activities of ciprofloxacin (Bay o 9867), norfloxacin, pipemidic acid, and nalidixic acid. Antimicrob Agents Chemother. 1983 Aug;24(2):302–304. doi: 10.1128/aac.24.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Sugino A. Novobiocin and nalidixic acid target proteins in yeast. Biochem Biophys Res Commun. 1980 Sep 16;96(1):306–312. doi: 10.1016/0006-291x(80)91215-2. [DOI] [PubMed] [Google Scholar]
- North G. Eukaryotic topoisomerases come into the limelight. Nature. 1985 Aug 1;316(6027):394–395. doi: 10.1038/316394a0. [DOI] [PubMed] [Google Scholar]
- Osheroff N., Shelton E. R., Brutlag D. L. DNA topoisomerase II from Drosophila melanogaster. Relaxation of supercoiled DNA. J Biol Chem. 1983 Aug 10;258(15):9536–9543. [PubMed] [Google Scholar]
- Otter R., Cozzarelli N. R. Escherichia coli DNA gyrase. Methods Enzymol. 1983;100:171–180. doi: 10.1016/0076-6879(83)00053-1. [DOI] [PubMed] [Google Scholar]
- Rusquet R., Bonhommet M., David J. C. Quinolone antibiotics inhibit eucaryotic DNA polymerase alpha and beta, terminal deoxynucleotidyl transferase but not DNA ligase. Biochem Biophys Res Commun. 1984 Jun 29;121(3):762–769. doi: 10.1016/0006-291x(84)90744-7. [DOI] [PubMed] [Google Scholar]
- Seibert G., Limbert M., Klesel N. Comparison of the antibacterial in vitro and in vivo activity of ofloxacin (HOE 280 DL 8280) and nalidixic acid analogues. Eur J Clin Microbiol. 1983 Dec;2(6):548–553. doi: 10.1007/BF02016563. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T. Mutational resistance to 4-quinolone antibacterial agents. Eur J Clin Microbiol. 1984 Aug;3(4):347–350. doi: 10.1007/BF01977492. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Sung S. C. Effect of novobiocin on DNA-dependent DNA polymerases from developing rat brain. Biochim Biophys Acta. 1974 Aug 15;361(1):115–117. doi: 10.1016/0005-2787(74)90214-7. [DOI] [PubMed] [Google Scholar]
- Udvardy A., Schedl P., Sander M., Hsieh T. S. Novel partitioning of DNA cleavage sites for Drosophila topoisomerase II. Cell. 1985 Apr;40(4):933–941. doi: 10.1016/0092-8674(85)90353-8. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Weymouth L. A., Loeb L. A. Mutagenesis during in vitro DNA synthesis. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1924–1928. doi: 10.1073/pnas.75.4.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright H. T., Nurse K. C., Goldstein D. J. Nalidixic acid, oxolinic acid, and novobiocin inhibit yeast glycyl- and leucyl-transfer RNA synthetases. Science. 1981 Jul 24;213(4506):455–456. doi: 10.1126/science.7017932. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- Yang L., Rowe T. C., Nelson E. M., Liu L. F. In vivo mapping of DNA topoisomerase II-specific cleavage sites on SV40 chromatin. Cell. 1985 May;41(1):127–132. doi: 10.1016/0092-8674(85)90067-4. [DOI] [PubMed] [Google Scholar]