Abstract
The molecular mechanism governing the regulated secretion of most exocrine tissues remains elusive, although VAMP8/endobrevin has recently been shown to be the major vesicular SNARE (v-SNARE) of zymogen granules of pancreatic exocrine acinar cells. In this article, we have characterized the role of VAMP8 in the entire exocrine system. Immunohistochemical studies showed that VAMP8 is expressed in all examined exocrine tissues such as salivary glands, lacrimal (tear) glands, sweat glands, sebaceous glands, mammary glands, and the prostate. Severe anomalies were observed in the salivary and lacrimal glands of VAMP8-null mice. Mutant salivary glands accumulated amylase and carbonic anhydrase VI. Electron microscopy revealed an accumulation of secretory granules in the acinar cells of mutant parotid and lacrimal glands. Pilocarpine-stimulated secretion of saliva proteins was compromised in the absence of VAMP8. Protein aggregates were observed in mutant lacrimal glands. VAMP8 may interact with syntaxin 4 and SNAP-23. These results suggest that VAMP8 may act as a v-SNARE for regulated secretion of the entire exocrine system.
INTRODUCTION
Protein and lipid transport in the secretory and endocytic pathways is primarily mediated by shuttling intermediates in the form of small vesicles (50–100 nm in diameter) and/or larger containers (100–2000 nm). Studies over the last three decades have identified molecular machineries and have defined fundamental mechanisms responsible for vesicle-mediated trafficking. Four key events have been described for general vesicle-mediated transport between a donor and a target compartment. Various coat protein complexes function in the process of vesicle formation by causing membrane deformation and selecting cargo proteins into the budding vesicle at a donor compartment. The resulting vesicles/containers are delivered to the target compartment, a process facilitated by the cytoskeleton network. The tethering event acts to position the vesicles/containers in the precise vicinity of the target compartment and is mediated by various tethering proteins. The fusion of vesicles/containers with the target compartment is catalyzed by SNARE (N-ethylmaleimide–sensitive factor [NSF]-attachment protein [SNAP] receptor) complexes. The coat proteins, tethering proteins and SNAREs are converging points for the cell to regulate vesicle trafficking. The pairing of vesicular SNARE (v-SNARE) on the vesicle/container with t-SNARE on the target compartment is the core event in membrane fusion (Barr, 2000; Bonifacino and Glick 2004; Hong, 2005; Jahn and Scheller, 2006; McNiven and Thompson, 2006).
SNAREs mediate secretion of small molecules and polypeptides in many physiological processes. Synaptic vesicles of neurons store neurotransmitters and are triggered by the action potential to fuse with nerve terminals. The core SNARE complex mediating the release of neurotransmitters is well defined and is known to be regulated by many accessory proteins (Söllner et al., 1993; Jahn and Sudhof, 1999; Schoch et al., 2001; Rettig and Neher, 2002; Chandra et al., 2005; Rizo et al., 2006). VAMP2/synaptobrevin2 acts as the v-SNARE of synaptic vesicles, whereas syntaxin 1 and SNAP-25 interact to form the t-SNARE complex on the plasma membrane of nerve terminals. The interaction of VAMP2 with the syntaxin 1-SNAP-25 complex catalyzes the release of neurotransmitters. In contrast to the small synaptic vesicles used by neurons, endocrine and exocrine cells use larger containers called secretory granules to store proteins and bioactive components, and their fusion with the plasma membrane is regulated by various stimuli.
Despite the identification of ∼38 SNAREs and the detailed studies of the SNARE complex mediating the synaptic transmission, the molecular mechanism and the SNARE complexes responsible for regulated exocytosis of the exocrine tissues remain elusive. Using the VAMP8 knockout mice generated recently (Wang et al., 2004), we have examined the hypothesis that VAMP8 may participate in regulated exocytosis of the entire exocrine system. Our study suggests that VAMP8 plays an important role in parotid and lacrimal acinar cells and that it functions in other exocrine tissues as well. This discovery will facilitate further analysis of the molecular mechanism responsible for regulated exocytosis of the exocrine system.
MATERIALS AND METHODS
Antibodies
The VAMP8 polyclonal antibody was raised in rabbits with the cytosolic N-terminal region of human VAMP8 as antigen (Wong et al., 1998). Other antibodies were purchased from commercial suppliers: rabbit anti-amylase from Calbiochem (La Jolla, CA); rabbit anti-KLK9 from Fitzgerald Industries (Concord, MA); rabbit anti-syntaxin4, syntaxin13, and rabbit anti-SNAP23 from Synaptic Systems (Göttingen, Germany); mAb to syntaxin6 from AbCam (Cambridge, United Kingdom).
Mice
The VAMP8 knockout mice have been described previously (Wang et al., 2004). In this study, we crossed the original VAMP8 knockout mouse line with the cre-transgenic strain (Schwenk et al., 1995) to remove the neomycin resistance cassette. The neo-deleted VAMP8 knockout line was then bred onto a 129/SvJ background for five generations. Sex- and age-matched 3–5-mo-old adult mice were used for experiments. Male mice were used unless otherwise indicated.
Histological Analysis and Immunofluorescence Microscopy
Organs were fixed with 4% paraformaldehyde (PFA) in PBS and embedded in paraffin. Sections were stained with hematoxylin and eosin (H&E) or PAS. For immunofluorescence studies, PFA-fixed organs were embedded in OCT compound (Sakura Finetek USA, Torrance, CA). Cryosections were mounted on polylysine-coated slides and stained with primary antibodies followed by appropriate FITC-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Nuclei were stained with DAPI or TO-PRO-3 (Molecular Probes, Eugene, OR).
For morphological electron microscopy (EM) study, fresh tissues were fixed with 4% PFA and 0.5% glutaraldehyde in PBS and embedded in Spurrs' resin. Ultrathin sections were stained with uranyl acetate and lead citrate before being observed with a transmission electronic microscope.
Immunoblot Analysis
Fresh tissues were homogenized in a modified RIPA buffer (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 2 mM DTT, 1% deoxycholic acid, 1% Triton X-100, 0.1% SDS, 1 mM PMSF, and Complete proteinase inhibitors from Roche Diagnostics, Mannheim, Germany). Proteins were transferred onto Hybond-C extra membranes (Amersham, Piscataway, NJ) after being separated by SDS-polyacrylamide gels. Protein identities were determined with specific primary antibodies and appropriate peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Peroxidase activity was detected with the SuperSignal substrate and enhancer (Pierce, Rockford, IL) and visualized on x-ray films. Quantitative analysis was carried out with a GS-800 calibrated densitometer from Bio-Rad (Hercules, CA).
Saliva Collection under Resting and Pilocarpine-stimulation Conditions
Mice were anesthetized with Avertin (tribromoethanol from Morre Technology, Union, New Jersey), and saliva was then collected from the oral cavity with microcapillaries. Otherwise, mice were first anesthetized with Avertin and then injected with pilocarpine at 1 mg/kg i.p. (intraperitoneally) or 10 μl of 1 mg/ml pilocarpine at a location close to salivary glands. Saliva secreted into the oral cavity was collected with microcapillaries at 5-min intervals until 30-min after injection. Fifty to 100 μl of saliva was collected from each mouse. The saliva volume of mutant mice was not overtly different from that of normal mice.
GST Pulldown Analysis
The GST pulldown experiment was performed as previously described (Wang et al., 2004). Briefly, salivary glands were homogenized in homogenization buffer (20 mM HEPES, pH 7.4, 10 mM sucrose, 10 mM KCl, 2 mM EDTA, 2 mM EGTA, 6 mM MgCl2, 1 mM DTT, 1 mM PMSF, Complete proteinase inhibitors, and 1 mg/ml GST or GST-VAMP8 fusion protein). Nuclei were removed by centrifugation at 500 × g for 5 min, and total membranes were pelleted from the postnucleus supernatant by a spin at 100,000 × g for 1 h. Membranes were washed in washing buffer (500 mM KCl, 20 mM HEPES, 1 mM DTT, 1 mM EDTA, 1 mM PMSF, Complete proteinase inhibitors, 1 mg/ml GST or GST-VAMP8, pH 7.4) and then resuspended in 2 ml binding buffer (20 mM HEPES, 100 mM KCl, 1 mM DTT, 4 mM EGTA, 4 mM MgCl2, 2 mM ATP, 1 mM PMSF, Complete proteinase inhibitors, 1% BSA, 1 mg/ml GST or GST-VAMP8, pH 7.4) and incubated at 37°C for 5 min. After the incubation, the volume of the mixture was topped up to 12 ml with protein-free binding buffer before a spin at 100,000 × g for 1 h. Membranes were resuspended in extraction buffer (20 mM HEPES, 100 mM KCl, 1 mM DTT, 10 mM EDTA, 0.2 mM ATP, 2% Triton X-100, pH 7.4) and incubated at 4°C for 1 h with rotation. Triton-insoluble materials were removed by centrifugation at 200,000 × g for 30 min. Membrane extracts were incubated overnight with glutathione Sepharose 4B beads (Amersham). Beads were washed three times with extraction buffer containing 0.5% Triton followed by three times with Triton-free buffer. All the procedures were carried out at 4°C except binding. Proteins were eluted by boiling the beads for 5 min in SDS gel loading buffer and then were subjected to Western blotting analysis.
Isolation of Protein Aggregates from Lacrimal Glands
Lacrimal glands were homogenized in 280 mM sucrose supplemented with 10 mM HEPES, pH 7.4, 1 mM PMSF, and the Complete proteinase inhibitor (Roche Diagnostics) with a motor homogenizer (model T8.01; IKA Labortechnik, Staufen, Germany). The tissue suspension was then laid on top of a discontinuous sucrose gradient that consisted of 2, 1.5, and 1.0 M sucrose. Samples were centrifuged at 100,000 × g for 1 h. The black band at the interface between 2 and 1.5 M was retrieved and diluted with 2 volumes of 1% Triton X-100. Protein aggregates were pelleted after a spin at 10,000 × g for 5 min. The whole procedure was carried out at 4°C.
RESULTS
VAMP8 Is Required for Regulated Secretion in Salivary Glands
The requirement of VAMP8 in regulated exocytosis of the pancreatic acinar cells (Wang et al., 2004) prompted us to test the hypothesis that VAMP8 may have a more general role in the exocrine system.
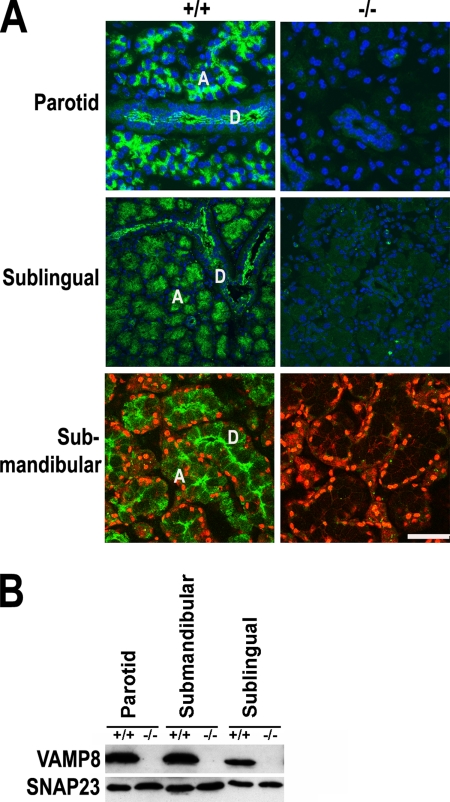
Salivary glands, which consist primarily of parotid, submandibular, and sublingual glands and secrete saliva into the mouth to moisten the mouth, initiate food digestion, and help protect the teeth from decay, were first examined. Immunofluorescence studies with an antibody specifically recognizing VAMP8 showed that VAMP8 was expressed in all acinar cells and lumen-lining duct epithelial cells of the three major salivary glands (Figure 1A). VAMP8 appeared to be enriched in the apical region of acinar cells and duct epithelial cells. The labeling is specific because no signal was detected in VAMP8-null sections. VAMP8 expression in salivary glands was confirmed by immunoblot analysis (Figure 1B).
Figure 1.
VAMP8 is expressed in salivary glands. (A) Immunofluorescence staining for VAMP8 in major salivary glands. VAMP8 was stained green with an FITC-conjugated secondary antibody. Nuclei were either stained blue with DAPI or red with TO-PRO-3. D, duct; A, acinus. Scale bar, 50 μm. (B) Western blotting analysis of VAMP8 and SNAP-23 expression in parotid, submandibular, and sublingual glands. +/+, wild-type mice; −/−, VAMP8-null mice.
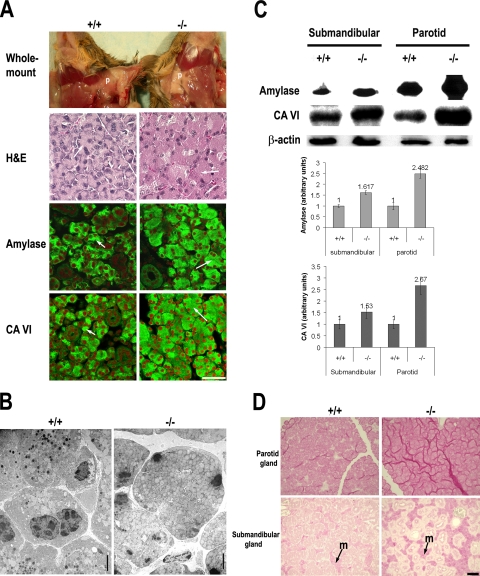
Visual examination of salivary glands revealed that the parotid glands of VAMP8-null mice exhibited an abnormally milky appearance in contrast to the slightly tan appearance of control mice (Figure 2A), a phenotype similar to that previously observed in the pancreas (Wang et al., 2004). H&E staining of parotid gland sections showed that the cytoplasm of normal acinar cells was stained purple, whereas that of VAMP8-null cells appeared pink (Figure 2A), indicating possible accumulation of secretory granules by mutant cells. To verify this possibility, we examined the distribution of secretory granules in parotid glands by immunolabeling their constituents such as amylase and carbonic anhydrase VI. As shown in Figure 2A, both amylase and carbonic anhydrase VI were restricted to the apical region in normal cells. In contrast, they were evenly distributed throughout the entire cytoplasm in VAMP8-null cells. These results are similar to those observed earlier in the pancreas and can be explained by the accumulation of secretory granules in the acinar cells due to defective exocytosis. The overall accumulation of secretory granules by VAMP8-null acinar cells was confirmed by EM analysis (Figure 2B). However, we also noticed that the dense granules were reduced in mutant cells. The cause for this reduction is unknown. We speculate that it might be due to premature activation of enzymes, which we have previously found in the pancreas (Wang et al., 2004). Consistent with a possible exocytosis defect, amylase and carbonic anhydrase VI were substantially increased in mutant parotid glands (Figure 2C). These enzymes were also increased in VAMP8-null submandibular glands, but the difference was not as obvious as in parotid glands (Figure 2C). Because VAMP8 was detected in all the three primary salivary glands, we speculated that VAMP8 might be required not only for secretion of enzymes by serous acinar cells but also for secretion of mucin by mucous cells. PAS staining for carbohydrates showed that VAMP8-null mucous cells in sublingual glands and submandibular glands contained more mucin than their wild-type counterpart (Figure 2D). Taken together, these results suggest that the absence of VAMP8 caused a defect in exocytosis, leading to the accumulation of secretory granules in acinar cells.
Figure 2.
VAMP8-null salivary glands retain secretory granules and accumulate mucin and enzymes. (A) Morphological defects in VAMP8-null parotid glands revealed by conventional histological study (H&E) and immunofluorescent staining for amylase and carbonic anhydrase VI (CA VI). Amylase and CA VI were stained green with a FITC-conjugated secondary antibody, and the nuclei were stained red. Arrows mark apical poles of acinar cells. P, parotid gland. Scale bar, 50 μm. (B) Electron microscopy showing overall accumulation of secretory granules in VAMP8-null acinar cells of parotid glands. The dense granules were, however, substantially reduced in mutant cells, probably due to premature activation of enzymes as described in the pancreas. Scale bar, 5 μm. (C) Western blotting showing that both parotid and submandibular glands of VAMP8-null mice accumulate amylase and CA VI. Quantitative studies on five pairs of mice are shown at the bottom. The average enzyme levels in wild-type mice are arbitrarily defined as one unit. Error bars, SD. p < 0.01 for all the four groups. (D) PAS staining showing that sublingual and submandibular glands of VAMP8-null mice accumulate mucin (stained red). m, mucin acinar cells. Scale bar, 50 μm. +/+, wild-type mice; −/−, VAMP8-null mice.
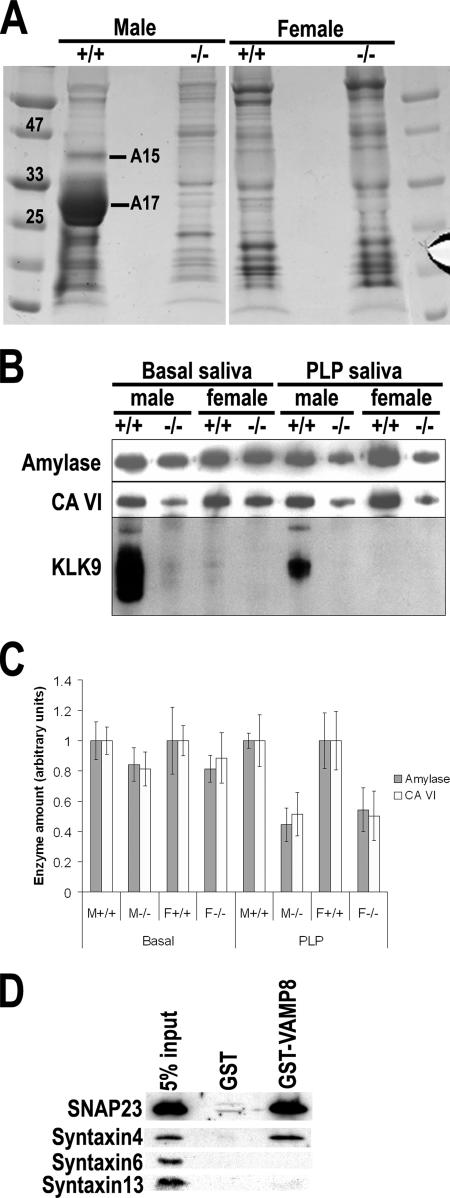
If exocytosis was affected, we would expect some alterations in the protein profile of saliva. Therefore, we compared the saliva proteins of control and VAMP-null mice. After resolving saliva proteins on a SDS-polyacrylamide gel, we observed a dramatic difference between normal and VAMP8-null male mice (Figure 3A). The difference for female mice was, however, subtle. There was also a prominent sexual difference: the protein profiles of normal male and female saliva were distinct. It was clear that several proteins were underrepresented in the saliva of VAMP8-null male mice. Mass spectrometry analysis of these protein bands identified A15 as carbonic anhydrase VI (also called carbonate dehydratase VI) and A17 as kallikrein 9 (KLK9 or KLK-L3). Carbonic anhydrase VI is a zinc metalloenzyme that catalyzes the reversible hydration of CO2 to form HCO3− and H+ and therefore plays a role in regulating the pH of saliva. The accumulation of carbonic anhydrase VI in the acinar cells of VAMP8-null parotid glands (Figure 2) suggested that the reduced level of this protein in the saliva was due, at least in part, to its defective exocytosis. KLK9 is a recently identified member of the kallikrein family of serine proteases. This protein was abundant in normal male saliva but could hardly be detected in VAMP8-null male mice (Figure 3A). Saliva collected over a period of 4 h under resting condition from control and VAMP8-null male and female mice was also analyzed by immunoblot (Figure 3B). Consistent with the results of mass spectroscopic analysis, KLK9 was abundant in normal male saliva but hardly detected in the saliva of VAMP8-null male mice. It was absent from female saliva regardless of genotypes (Figure 3B). These results suggest that KLK9 is likely a male-specific salivary protein whose secretion depends on VAMP8. Unlike the strict VAMP8 dependence of KLK9 secretion, the levels of amylase and carbonic anhydrase IV in saliva were only partially reduced in mutant mice (Figure 3B), and the differences in these two enzymes between female mice were not statistically significant, suggesting that VAMP8 is not the primary v-SNARE mediating the constitutive exocytosis of these two proteins.
Figure 3.
VAMP8 plays a role in protein secretion by salivary glands. (A) Protein profiles of saliva resolved by SDS-PAGE. Lanes 1 and 6, protein markers 25, 33, and 47 are 25, 33, and 47 kDa, respectively. +/+, wild-type mice; −/−, VAMP8-null mice. Bands A15 and A17 were identified as CA VI and KLK9, respectively. (B) Western blotting analysis showing amylase, carbonic anhydrase VI (CAVI), and kallikrein 9 (KLK9) in basal and PLP-stimulated saliva. +/+, wild-type mice; −/−, VAMP8-null mice. An equal volume of saliva was loaded on each lane. (C) Quantitative analysis of salivary levels of amylase and carbonic anhydrase IV (CAVI). The average enzyme levels for wild-type samples are arbitrarily defined as one unit. Error bars, SD. n = 5. The values for basal saliva are as follows: M+/+ (amylase) = 1.00 ± 0.123, M−/− (amylase) = 0.844 ± 0.111, p = 0.069<0.10; M+/+ (CA VI) = 1.00 ± 0.088, M−/− (CA VI) = 0.813 ± 0.111, p = 0.040 < 0.05; F+/+ (amylase) = 1.00 ± 0.222, F−/− (amylase) = 0.814 ± 0.088, p = 0.140 > 0.10; F+/+ (CA VI) = 1.00 ± 0.100, F−/− (CA VI) = 0.886 ± 0.166, p = 0.226>0.10. The values for PLP-stimulated saliva are: M+/+ (amylase) = 1.00 ± 0.049, M−/− (amylase) = 0.445 ± 0.108, p<0.01; M+/+ (CA VI) = 1.00 ± 0. 172, M−/− (CA VI) = 0.514 ± 0.143, p < 0.01; F+/+ (amylase) = 1.00 ± 0.182, F−/− (amylase) = 0.545 ± 0.145, p < 0.01; F+/+ (CA VI) = 1.00 ± 0.192, F−/− (CA VI) = 0.504 ± 0.165, p < 0.01. +/+, wild-type; −/−, VAMP8-null. M, male; F, female. PLP, PLP-stimulated saliva. (D) GST pulldown experiment showing the interaction SANP23 and syntaxin4 with VAMP8.
Pilocarpine (PLP) is an alkaloid obtained from plants of the genus Pilocarpus (family Rutaceae). It stimulates secretion by the salivary and lacrimal glands by mimicking the effects of acetylcholine. It is a cholinergic drug used to treat xerostomia (dry mouth) and dry eyes caused by Sjögren's syndrome and radiation therapy for cancers of the head and neck. To provide more direct evidence for a role of VAMP8 in regulated exocytosis of salivary glands, we examined the saliva elicited by pilocarpine. Mice were administrated with 1 mg/kg pilocarpine. Saliva was collected for a period of 30 min so that regulated secretion of secretory proteins triggered by pilocarpine could be analyzed. As observed for saliva collected over a period of 4 h under resting conditions, acute secretion of KLK9 stimulated by pilocarpine was detected only in normal male mice. Under this condition, salivary levels of both amylase and carbonic anhydrase IV were significantly reduced in VAMP8-null mice compared with the sex-matched normal mice. The results are quantified and shown in Figure 3C. The results clearly demonstrate that pilocarpine-stimulated exocytosis of amylase and carbonic anhydrase VI was severely compromised in the absence of VAMP8 in both male and female mice, suggesting that VAMP8 plays a key role in regulated exocytosis of secretory proteins of salivary glands.
Previous studies of pancreatic acinar cells indicates that VAMP8 may act as a vesicular SNARE (v-SNARE) of zymogen granules and mediate exocytosis by interacting with the target SNAREs (t-SNARE) syntaxin 4 and SNAP-23 on the plasma membrane. To assess whether a similar mechanism exists in salivary glands, we used a GST-VAMP8 fusion protein in affinity coisolation analysis to test if VAMP8 could interact with syntaxin 4 and SNAP-23. As shown in Figure 3D, syntaxin 4 and SNAP-23 were efficiently corecovered with GST-VAMP8, whereas syntaxin6 and syntaxin13 were not corecovered.
Ablation of VAMP8 Leads to Accumulation of Secretory Granules and Protein Aggregates in Lacrimal Glands
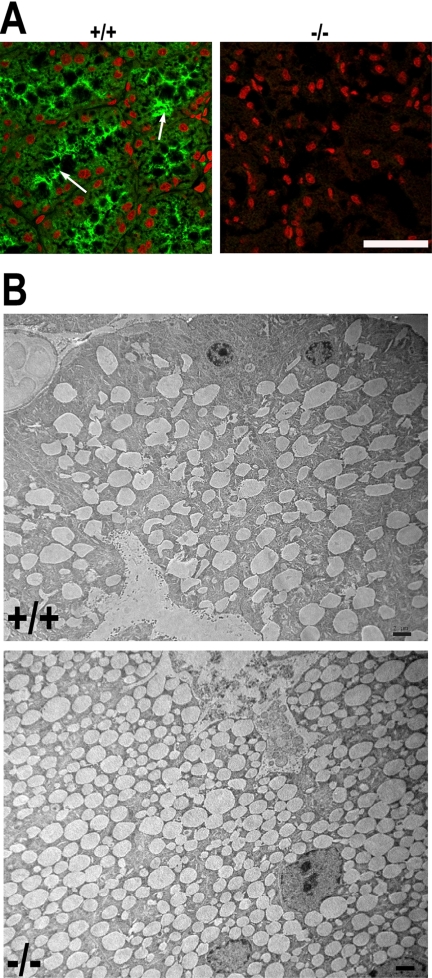
Lacrimal (tear) glands are responsible for secretion of lubrication material for the eyes. VAMP8 expression was also detected in the acinar cells of this gland (Figure 4A). VAMP8 was primarily distributed in the apical pole of acinar cells. EM revealed accumulation of secretory granules in VAMP8-null acinar cells (Figure 4B). Unlike normal acinar cells whose secretory granules were restricted to the apical region, mutant cells had secretory granules distributed all over the cytoplasm. The overall number of secretary granules was obviously increased in VAMP8-null cells. This phenotype suggests that VAMP8 might be required for exocytosis in lacrimal glands.
Figure 4.
Ablation of VAMP8 led to accumulation of secretory granules in lacrimal gland acinar cells. (A) Immunofluorescence staining showing VAMP8 expression in the apical region of lacrimal gland acinar cells. VAMP8 was stained green, whereas the nuclei are red. Arrows indicate apical poles of acinar cells. Scale bar, 50 μm. (B) Electron microscopy showing accumulation of secretory granules in VAMP8-null acinar cells of lacrimal glands. Scale bar, 2 μm. +/+, wild-type; −/−, VAMP8-null.
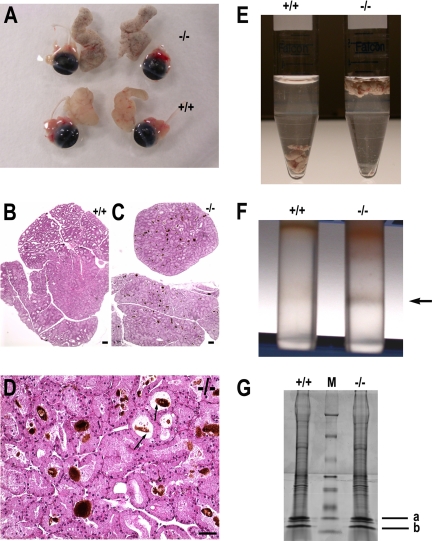
The gross appearance of VAMP-null lacrimal glands was distinguishable from that of normal lacrimal glands: whereas normal glands were translucent and tan to slightly gray, mutant glands were gray and opaque, with dark spots of varying sizes present throughout the entire glands (Figure 5A). For an unknown reason, the relative density of mutant glands was reduced. As shown in Figure 5E, mutant glands floated on the surface in PBS but normal glands sank to the bottom. H&E staining of lacrimal gland sections revealed accumulation of dark-brown deposits in the lumen of acini and ducts (Figure 5, C and D) of VAMP8-null glands, whereas there were no such deposits in normal lacrimal glands (Figure 5B). We then attempted to determine the identity of the deposits. After fractionation of gland homogenate by a sucrose density gradient, a dark-gray to black band was observed in mutant samples at the interface between 2 and 1.5 M sucrose (Figure 5F). The corresponding band in normal samples was only 10–20% in size relative to the mutant band and could hardly be seen (Figure 5F). The precipitates were resistant to 1% Triton X-100 but were completely dissolved in 1% SDS, suggesting that the major constituents of the precipitates might be protein. SDS-PAGE showed that the precipitates contained multiple proteins with two major small peptides (Figure 5G). Mass spectrometry analysis identified peptide “a” as histone 1 and H2b and peptide “b” as hemoglobin beta-1 subunit. The yellow to brown pigment that ran just ahead of bromphenol blue was probably heme. It seems that hemoglobin combined with heme is the major constituent that produced the dark-brown to black color. Although much less than in mutant glands, this kind of protein aggregates was also present in normal glands, and these aggregates were similar to those found in mutant glands, regarding their constituents (Figure 5G). Histone is a well-known component of tear, but hemoglobin has not been reported in tear although some plasma proteins, such as albumin and immunoglobulin, are abundant in the tear (Zhou et al., 2006). Hemoglobin probably has escaped from detection because of its low concentration and/or propensity to form aggregates with histone. The source of tear plasma proteins is unclear. We suspect that plasma proteins and hemoglobin are probably derived from the blood. These proteins might somehow be selectively picked up by acinar cells and/or duct cells and then be transported into the lumen of acini and ducts. Whatever the source of hemoglobin is, VAMP8 did not seem to be involved in the trafficking of this protein because the protein deposits were found outside cells. Under normal condition, most hemoglobin might be flushed out of the lacrimal gland, whereas a small portion forms fine deposits and provides the gland with a light gray color. These tiny aggregates can hardly be observed on H&E-stained sections. When secretion from acinar cells is reduced, hemoglobin may accumulate and form large aggregates in the lumen of acini and ducts. The defects described above suggest that exocytosis of lacrimal acinar cells is compromised in the absence of VAMP8, indicating a role for VAMP8 in tear secretion.
Figure 5.
Protein deposits (aggregates) in VAMP8-null lacrimal glands. (A) Gross appearance of VAMP8-null (top) and wild-type (bottom) lacrimal glands together with eyeballs. (B) H&E staining of normal lacrimal gland section. (C) H&E staining of VAMP8-null lacrimal gland section showing accumulation of dark-brown deposits. (D) High magnification of an H&E-stained section of VAMP8-null lacrimal glands showing protein deposits in the lumen of ducts and acini. Arrows indicate lumen of the ducts. Scale bar, 50 μm. (E) Photography of lacrimal glands in PBS showing gravity density difference between normal (+/+) and mutant (−/−) lacrimal glands. (F) Sucrose density fractionation of lacrimal gland homogenate showing a dark-gray band at the interface between 2 and 1.5 M sucrose (indicated by an arrow). (G) SDS-PAGE showing protein components in the deposits. From bottom to top, the molecular weights of the protein markers (M) were 6.5, 16.5, 25, 32.5, 47.5, 63, 83, and 175 kDa, respectively. Bands were identified by mass spectrometry as histone 1 and H2b (a) and hemoglobin beta-1 subunit (b).
VAMP8 Expression in Other Exocrine Tissues
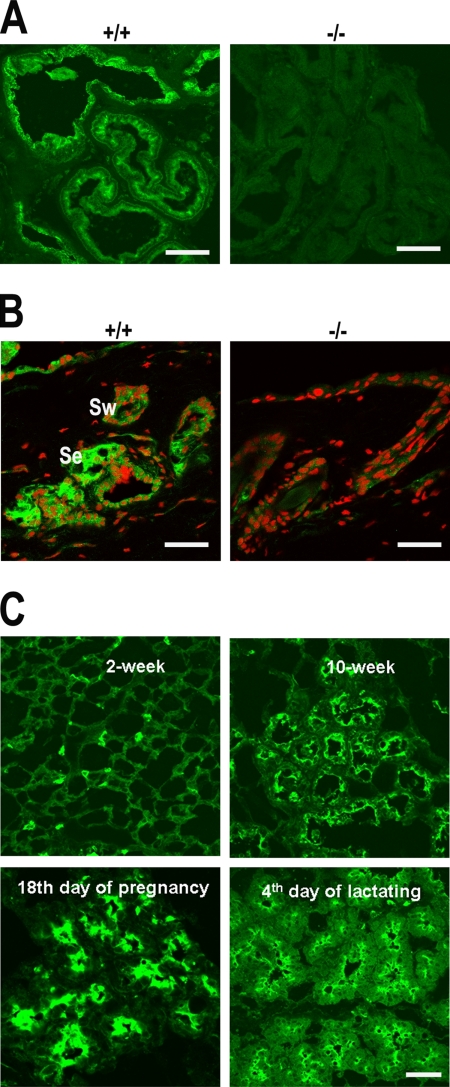
VAMP8 was also detected in the prostate (Figure 6A) and the exocrine glands of the skin (Figure 6B). In the prostate, VAMP8 is primarily present in the apical region of the lumen-lining epithelial gland cells (Figure 6A).The sebaceous glands, which secrete an oily substance called sebum into the follicular canal and eventually onto the surface of the skin, expressed VAMP8 (Figure 6B, indicated as Se). The eccrine sweat glands, which secrete sweat to cool the body, also expressed VAMP8 (Figure 6B, indicated as Sw). The detection of VAMP8 in the sebaceous and sweat glands was specific because no labeling was detected in VAMP8-null mice.
Figure 6.
VAMP8 expression in other exocrine tissues. Immunofluorescent labeling showing VAMP8 expression in (A) prostate, (B) sebaceous glands (Se) and sweat glands (Sw), and (C) mammary glands at different development stages and under different physiological conditions. VAMP8 was stained green. Nuclei were stained red or unstained. +/+, wild-type; −/−, VAMP8-null. Scale bar, 50 μm.
In the mammary gland, VAMP8 expression was regulated during development and pregnancy (Figure 6C). Low levels of expression were detected in 2-wk-old mice, and the level of expression increased substantially in 10-wk-old mice. The highest level of expression of VAMP8 was detected in mice at the 18th day of pregnancy, and VAMP8 was seen to be concentrated in the apical pole of the alveoli cells (Figure 6C). High and more diffuse expression of VAMP8 was detected in lactating alveoli. Again, the detection is specific as no labeling was observed in VAMP8-null mice (data not shown).
Our findings that VAMP8 is crucially involved in the regulated exocytosis of the parotid and tear glands and it is widely expressed in all the exocrine tissues examined together with its established function in the pancreatic exocrine cells (Wang et al., 2004), suggest that VAMP8 may play a role in regulated exocytosis of the entire exocrine system.
DISCUSSION
The SNARE proteins were initially discovered as an essential component of the secretory pathway. They were later discovered to mediate release of neurotransmitters. It has been well established that SNARE proteins function as engines for membrane fusion (Jahn and Scheller, 2006). The focus of the SNARE field has recently shifted to the regulation of SNARE functions (Giraudo et al., 2006). Another challenge in the field is to define the physiological role of individual SNAREs. This kind of studies will advance our understanding of human diseases. Actually, mutations in trafficking proteins have already been shown to be the bases of many human diseases. However, of the some 38 SNAREs identified, only a few have been studied in whole animals. It has been shown by gene-targeting that synaptobrevin/VAMP2 is important for neurotransmitter release (Schoch et al., 2001), whereas syntaxin4 is required for surface deployment of GLUT4 (Yang et al., 2001a). Epimorphin/syntaxin2 negatively regulates epithelium growth (Wang et al., 2006). Despite its wide expression, VAMP3 seems to be nonessential (Yang et al., 2001b), and Vti1b plays only a minor role in the endocytic pathway (Atlashkin et al., 2003). VAMP8 has recently been shown to play an important role in regulated secretion of pancreas and platelets (Wang et al., 2004; Ren et al., 2006). In this study, we extend our investigation into the role of VAMP8 in the whole exocrine system.
Saliva has lubricative, protective, and digestive functions and is produced by various salivary glands associated with the oral cavity. The parotid, submandibular, and sublingual glands represent the major salivary glands with independent excretory ducts, whereas minor salivary glands are located throughout the oral mucosa and the tongue. Based on secreted materials, salivary glands are described as serous or mucous glands. Serous cells secret a thin watery fluid rich in enzymes, whereas mucous cells produce a thick viscid secretion containing a large amount of glycoproteins. The parotid gland is predominantly composed of serous acini, whereas the sublingual gland is mainly mucous and the submandibular gland is a mixture of serous and mucous cells. VAMP8 was expressed in all three major salivary glands. Two lines of evidence support a role for VAMP8 in exocytosis of salivary glands. First, mucin and enzymes such as amylase and carbonate anhydrase VI accumulated in VAMP8-null glands due to accumulation of secretory granules. Second, mutant saliva protein levels were substantially reduced, especially in pilocarpine (PLP)-stimulated saliva. In our study, PLP-induced secretion was measured within a period of 30 min compared with a period of 4 h for basal secretion. As such, we were measuring acute regulated exocytosis by the major salivary glands. Under this condition, basal secretion is less significant than PLP-stimulated secretion. The result is the key evidence suggesting that VAMP8 is more important for regulated secretion than for constitutive secretion. This interpretation is supported by other morphological (including EM) analysis. In line with earlier analysis of pancreatic acinar cells (Wang et al., 2004), VAMP8 is likely to act as the v-SNARE of the secretory granule in salivary glands as well. Because the difference between normal and VAMP8-null saliva amylase and CA VI are more significant in PLP-stimulated secretion than in constitutive secretion, we conclude that VAMP8 is more important for regulated exocytosis than for basal secretion.
KLK9 was molecularly identified in 2000 (Diamandis et al., 2000; Yousef and Diamandis, 2000), but its function remains to be determined. Our results here suggest that KLK9 is likely a male-specific salivary protein whose secretion is strictly dependent on VAMP8. In contrast to the partially reduced secretion of well-characterized salivary proteins such as amylase and carbonic anhydrase VI, KLK9 in saliva was essentially abolished in the absence of VAMP8. This disparity between KLK9 and amylase (or carbonic anhydrase VI) suggests that KLK9 and amylase (or carbonic anhydrase VI) might be present in different secretary granules that express distinct sets of v-SNAREs. In fact, it is well known that there are different types of secretory granules that response to different stimuli (Castle et al., 2002). An interesting question to be answered in future studies is whether the secretion of other KLKs in salivary glands and other tissues is also dependent on VAMP8.
The cornea of the eye is covered by a tear film which keeps the eye moist and washes away dirty and small particles. The tear film consists of three layers, with the middle aqueous layer of tear fluid being composed of water, salt, proteins and other substances that help to lubricate the eye (Zhou et al., 2006). Tear fluid is primarily formed in the lacrimal tear glands; it is secreted by the tubuloalveolar acinar cells and transported via a number of ducts to the eye's surface. In the absence of VAMP8, tear secretion might be compromised, although no overt injury has been observed. Consistent with this is the cellular observation that acinar cells accumulated secretory granules, indicating that exocytosis was defective. Furthermore, the lacrimal glands of VAMP8 knockout mice accumulate dark brown protein aggregates in the lumen of the acinus and duct, which may partially affect the flow of tear. These defects ultimately result from the lack of VAMP8 that is important for fusion of secretory granules with the plasma membrane. VAMP2, VAMP7, and VAMP8 have been proposed to be candidate v-SNAREs in lacrimal glands (Wu et al., 2006). Our study shows that VAMP8 is a major if not the only v-SNARE of lacrimal glands.
In human, there is a disease called Sjögren's syndrome. Patients of this syndrome suffer from dry mouth and dry eyes. Because the PLP-stimulated saliva volume was not significantly different between normal mice and VAMP8 knockout mice, despite the difference in protein composition, we do not believe the VAMP8 knockout mouse line is a perfect model for human Sjögren's syndrome. But human with defective VAMP8 might suffer from a mild symptom of dry mouth and dry eyes.
Our current study suggests that VAMP8 is important for regulated exocytosis of salivary glands and lacrimal glands, in addition to its role in regulated exocytosis of pancreatic acinar cells (Wang et al., 2004). In the context of its wide expression in all other exocrine tissues examined, we believe that VAMP8 may also participate in regulated secretion of additional exocrine tissues. It is reasonable to speculate that VAMP8 is likely a common v-SNARE responsible for regulated exocytosis in the entire exocrine system.
ACKNOWLEDGMENTS
We thank staff of the Biological Resource Center (BRC) for maintaining the VAMP8 knockout mice. We thank Singh Paramjeet for reading the manuscript. This work is funded by Agency for Science, Technology, and Research (A*Star).
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-10-0974) on January 10, 2007.
REFERENCES
- Atlashkin V., Kreykenbohm V., Eskelinen E. L., Wenzel D., Fayyazi A., Fischer von Mollard G. Deletion of the SNARE vti1b in mice results in the loss of a single SNARE partner, syntaxin 8. Mol. Cell. Biol. 2003;23:5198–5207. doi: 10.1128/MCB.23.15.5198-5207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr F. Vesicular transport. Essays Biochem. 2000;36:37–46. doi: 10.1042/bse0360037. [DOI] [PubMed] [Google Scholar]
- Bonifacino J. S., Glick B. S. The mechanisms of vesicle budding and fusion. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- Castle A. M., Huang A. Y., Castle J. D. The minor regulated pathway, a rapid component of salivary secretion, may provide docking/fusion sites for granule exocytosis at the apical surface of acinar cells. J. Cell Sci. 2002;115:2963–2973. [Google Scholar]
- Chandra S., Gallardo G., Fernandez-Chacon R., Schluter O. M., Sudhof T. C. Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration. Cell. 2005;123:383–396. doi: 10.1016/j.cell.2005.09.028. [DOI] [PubMed] [Google Scholar]
- Diamandis E. P., et al. New nomenclature for the human tissue kallikrein gene family. Clin. Chem. 2000;46:1855–1858. [PubMed] [Google Scholar]
- Giraudo C. G., Eng W. S., Melia T. J., Rothman J. E. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- Hong W. SNAREs and traffic. Biochim. Biophys. Acta. 2005;1744:493–517. [PubMed] [Google Scholar]
- Jahn R., Scheller R. H. SNAREs—engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- Jahn R., Sudhof T. C. Membrane fusion and exocytosis. Annu. Rev. Biochem. 1999;68:863–911. doi: 10.1146/annurev.biochem.68.1.863. [DOI] [PubMed] [Google Scholar]
- McNiven M. A., Thompson H. M. Vesicle formation at the plasma membrane and trans-Golgi network: the same but different. Science. 2006;313:1591–1594. doi: 10.1126/science.1118133. [DOI] [PubMed] [Google Scholar]
- Ren Q., Barber H. K., Crawford G. L., Karim Z. A., Zhao C., Choi W., Wang C.-C., Hong W., Whiteheart S. W. Endobrevin/VAMP-8 Is the Primary v-SNARE for the Platelet Release Reaction. Mol. Biol. Cell. 2007;18:24–33. doi: 10.1091/mbc.E06-09-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J., Neher E. Emerging roles of presynaptic proteins in Ca++-triggered exocytosis. Science. 2002;298:781–785. doi: 10.1126/science.1075375. [DOI] [PubMed] [Google Scholar]
- Rizo J., Chen X., Arac C. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Schwenk F., Baron U., Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S., Deak F., Konigstorfer A., Mozhayeva M., Sera Y., Sudhof T. C., Kavalali E. T. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart S. W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J. E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Wang C.-C., Ng C. P., Lu L., Atlashkin V., Zhang W., Seet L.-F., Hong W. A role of VAMP8/endobrevin in regulated exocytosis of pancreatic acinar cells. Dev. Cell. 2004;7:359–371. doi: 10.1016/j.devcel.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang L., Iordanov H., Swietlicki E. A., Zheng Q., Jiang S., Tang Y., Levin M. S., Rubin D. C. Epimorphin(−/−) mice have increased intestinal growth, decreased susceptibility to dextran sodium sulfate colitis, and impaired spermatogenesis. J. Clin. Invest. 2006;116:1535–1546. doi: 10.1172/JCI25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. H., Zhang T., Xu Y., Subramaniam V. N., Griffiths G., Hong W. Endobrevin, a novel synaptobrevin/VAMP-like protein preferentially associated with the early endosome. Mol. Biol. Cell. 1998;9:1549–1563. doi: 10.1091/mbc.9.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Jerdeva G. V., da Costa S. R., Sou E., Schechter J. E., Hamm-Alvarez S. F. Molecular mechanisms of lacrimal acinar secretory vesicle exocytosis. Exp. Eye Res. 2006;83:84–96. doi: 10.1016/j.exer.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Yang C., Coker K. J., Kim J. K., Mora S., Thurmond D. C., Davis A. C., Yang B., Williamson R. A., Shulman G. I., Pessin J. E. Syntaxin 4 heterozygous knockout mice develop muscle insulin resistance. J. Clin Invest. 2001a;107:1311–1318. doi: 10.1172/JCI12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Mora S., Ryder J. W., Coker K. J., Hansen P., Allen L. A., Pessin J. E. VAMP3 null mice display normal constitutive, insulin- and exercise-regulated vesicle trafficking. Mol. Cell. Biol. 2001b;21:1573–1580. doi: 10.1128/MCB.21.5.1573-1580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef G. M., Diamandis E. P. The expanded human kallikrein gene family: locus characterization and molecular cloning of a new member, KLK-L3 (KLK9) Genomics. 2000;65:184–194. doi: 10.1006/geno.2000.6159. [DOI] [PubMed] [Google Scholar]
- Zhou L., Beueman R. W., Foo Y., Liu S., Ang L. P., Tan D. T. Characterisation of human tear proteins using high-resolution mass spectrometry. Ann. Acad. Med. Singapore. 2006;35:400–407. [PubMed] [Google Scholar]