Abstract
The ADP ribosylation factor (Arf)1 orthologue in the divergent eukaryote Trypanosoma brucei (Tb) shares characteristics with both Arf1 and Arf6 and has a vital role in intracellular protein trafficking. TbARF1 is Golgi localized in trypanosomes but associates with the plasma membrane when expressed in human cells. Depletion of TbARF1 by RNA interference causes a major decrease in endocytosis, which correlates with Rab5 dissociation from early endosomes. Although the Golgi remains intact, parasites display enlarged flagellar pockets and intracellular flagella. An increase in active GTP-bound TbARF1 in bloodstream parasites is rapidly lethal, correlating with a defect in Golgi-to-lysosome transport. We conclude that the essential Golgi-localizing T. brucei ARF1 has a primary role in the maintenance of both post-Golgi transport and endocytosis and that it is significantly divergent from other characterized ARFs.
INTRODUCTION
The infective bloodstream form (BSF) of the protozoan parasite Trypanosoma brucei (Tb) displays exceptionally high rates of protein trafficking that are proposed to aid survival in the host (Engstler et al., 2004). The intracellular machinery driving this process is highly specialized. Due to restrictions imposed by a dense array of subpellicular microtubules, the parasite is highly polarized, with all exocytic and endocytic transport occurring exclusively at the flagellar pocket, an invagination in the plasma membrane at the base of the flagellum. T. brucei BSF cells internalize and recycle large quantities of GPI-anchored proteins (GPI-APs), predominantly the coat protein variant surface glycoprotein (VSG) (Ferguson et al., 1994). Internalization of GPI-APs in mammalian cells is proposed to occur predominantly via a clathrin-independent, noncaveolar pathway (Sabharanjak et al., 2002). However, all endocytosis in T. brucei is clathrin dependent (Allen et al., 2003). Furthermore, there are no homologues of either the AP-2 adaptin complex or caveolin encoded by the T. brucei genome (Berriman et al., 2005), indicating divergent mechanisms of both clathrin-mediated uptake and lipid raft function in the parasite.
Advances in genetic manipulation and completion of the genome sequence have facilitated the use of T. brucei as a model system for the study of trafficking and organelle development in lower eukaryotes. Studying duplication of the single trypanosome Golgi apparatus by videomicroscopy has provided evidence for de novo biogenesis, in which a new Golgi grows next to the old Golgi, guided by a previously undescribed Centrin2-containing bilobed structure, which is distinct from the basal body (He et al., 2004, 2005). Less is known about trypanosome-specific Golgi functions and the role these functions play in maintaining the exceptionally high rate of trafficking within the cell.
The ADP-ribosylation factors (Arfs) are a highly conserved family of eukaryotic small GTP-binding proteins with roles in membrane dynamics and vesicle trafficking. The six mammalian ARFs have been divided into three classes based principally on primary amino acid sequence: class I (Arf1-3), class II (Arf4-5), and class III (Arf6) (Tsuchiya et al., 1991). The most studied member of this protein family, Arf1, has many roles, including the recruitment of COPI, AP-1, and GGA proteins to the Golgi apparatus and activation of the phospholipid-modifying enzymes, phospholipase D, and phosphatidylinositol 5-kinase (Donaldson et al., 2005). Gene knockdown experiments in mammalian cells demonstrate that human class I and II Arfs work in cooperating pairs in specific stages of the secretory pathway but have little or no effect on receptor-mediated endocytosis of transferrin (Volpicelli-Daley et al., 2005). In contrast, the most divergent of the mammalian Arf proteins, Arf6, localizes to the plasma membrane and endosomes, and it has roles in the rearrangement of the actin cytoskeleton at the plasma membrane and mediation of both clathrin-dependent and -independent endocytosis (D'Souza-Schorey et al., 1995; Donaldson, 2003; Naslavsky et al., 2004; Lay et al., 2005).
Gene deletion studies on the yeast isoform of Arf6, Arf3p, which also localizes to the plasma membrane, initially showed no role for this protein in endocytosis, possibly due to the operation of redundant endocytic pathways. Instead, Arf3p was shown to have an actin-independent function in polarizing the growth of emerging buds (Huang et al., 2003). However, further analysis has revealed that Arf3p directly binds to and is required for the correct localization of Lsb5p, an adaptor protein required for endocytosis in yeast (Costa et al., 2005). To date, no class III Arfs have been described in protists.
Here, we describe the functional characterization of a “nonclassical” Arf1 orthologue in T. brucei, which shares high sequence identity with other Arf1 proteins and shows Golgi localization in the cell but, like class III Arfs, performs a vital role in maintenance of the endocytic system.
MATERIALS AND METHODS
Parasite Culture
The T. brucei bloodstream form strains 90-13 and Lister 427 (Wirtz et al., 1999) were maintained in vitro as described previously (Price et al., 2005).
DNA Constructs
The plasmid vectors pM2cC and p2T7Ti were gifts from David Horn and Sam Alsford (London School of Hygiene and Tropical Medicine, London, United Kingdom) and Doug LaCount (PULSe, Purdue University, West Lafayette, IN) respectively. Construct pEGFPC31Rab5a was a gift from Bassam Ali (Imperial College London, London, United Kingdom). The T. brucei Arf1 (TbARF1) open reading frame was amplified from genomic DNA using suitable primers and cloned into the plasmid vector pM2cC (Price et al., 2005). A G2A mutation was also introduced by polymerase chain reaction (PCR), and the amplified fragment was cloned as described above. The resulting constructs, pM2TbARF1 and pM2TbARF1-G2A, encode the target protein with a C-terminal myc epitope tag under the control of a tetracycline-inducible T7 promoter. T31N and Q71L mutations were introduced using the GeneTailor site-directed mutagenesis system (Invitrogen, Paisley, United Kingdom). Amplified ARF1 fragments were also ligated into pcDNA3.1/Myc-His A (Invitrogen). The human ARF1 and ARF6 open reading frames were amplified from human lymph node cDNA (Clontech, Mountain View, CA) and cloned into pcDNA3.1/Myc-His A and pcDNA3.1/CT-GFP-TOPO (Invitrogen). A nonconserved region of the T. brucei ARF1 open reading frame (residues 101-225) was selected for RNA interference (RNAi) by using the program RNAit (Redmond et al., 2003). This region was amplified from T. brucei genomic DNA and cloned into p2T7Ti (Price et al., 2005), producing the construct p2T7ARF1.
Microscopy
Indirect immunofluorescence assays on parasites were performed as described previously (Price et al., 2005). Primary antibodies were gifts as follows: rabbit anti-TbGRASP from Graham Warren (Department of Cell Biology, Yale University School of Medicine, New Haven, CT), rabbit anti-TbBiP, and mouse anti-p67 from Jay Bangs (Department of Medical Microbiology and Immunology, Madison, WI), rabbit anti-TbRAB5A and anti-TbCLH (clathrin heavy chain) from Mark Field (Department of Pathology, University of Cambridge, Cambridge, United Kingdom), mouse anti-Rod1 from Keith Gull (Sir William Dunn School of Pathology, University of Oxford, Oxford, United Kingdom), and rabbit anti-aldolase from Christine Clayton (ZMBH, Universitat Heidelberg, Heidelberg, Germany). Primary antibodies were detected using Alexa Fluor 488- or 633-conjugated secondary antibodies (Invitrogen). Samples were visualized by confocal microscopy using a Zeiss LSM 510 meta with a Plan-Apochromat 63×/1.4 Oil differential interference contrast I objective lens and images acquired using LSM 510 version 3.2 software (Carl Zeiss, Jena, Germany). A Nikon Eclipse E600 microscope with a Plan-Fluor 100×/1.30 oil objective lens was used for nucleus/kinetoplast counting and concanavalin A (ConA) uptake assays, and images were acquired with a DXM1200 digital camera (Nikon, Tokyo, Japan) using ACT-1 version 2 software (Nikon). BSF electron microscopy was performed as described previously (Price et al., 2003).
Subcellular Localization in T. brucei
Mid-log phase T. brucei BSF strain Lister 427 parasites were electroporated with 10 μg of NotI-digested pM2ARF1 or mutated forms of this construct, as described previously (Price et al., 2005). Expression of myc-tagged proteins was induced in stable cell lines by incubating parasites in 100 ng/ml tetracycline for 0–60 h. Immunoblotting of parasite lysates was performed as described previously (Price et al., 2005), using tetracycline-induced cells grown in the presence or absence of protease inhibitors (Complete mini EDTA-free protease inhibitor cocktail [Roche Diagnostics, Mannheim, Germany], used at one quarter of the recommended concentration). Measurement of endoplasmic reticulum (ER)–Golgi–lysosome transport by pulse-chase labeling and immunoprecipitation of the lysosomal marker p67 was performed as described previously (Price et al., 2005).
Expression of TbARF1 in Human Embryonic Kidney (HEK)293 Cells
HEK293 cells were transfected with pcDNA3.1 and pEGFPC3-1Rab5a constructs described above, using FuGENE 6 Transfection Reagent (Roche Diagnostics) and grown for 36 h. Expressed proteins were detected by indirect immunofluorescence using rabbit polyclonal anti-myc (1:200 dilution; Abcam, Cambridge, United Kingdom) and Alexa Fluor 633 goat anti-rabbit IgG (1:250; Invitrogen,), costained with 4,6-diamidino-2-phenylindole (DAPI), and visualized as described above.
Disruption of TbARF1 Expression by RNAi
Mid-log phase T. brucei BSF strain 90–13 parasites were electroporated with 10 μg of NotI-digested p2T7ARF1 as described previously (Price et al., 2005). Expression of ARF1-specific double-stranded RNA (dsRNA) was induced in stable cell lines by incubating parasites in 100 ng/ml tetracycline. Expression of ARF1 was monitored by quantitative PCR, using SYBR Green Mastermix (Applied Biosystems, Foster City, CA). Total RNA was extracted from parasites using TRIzol Reagent (Invitrogen), treated with DNase I (Ambion, Ausitn, TX), and reverse-transcribed using OmniScript RT (QIAGEN, Valencia, CA). A 66-base pair fragment of TbARF1 was amplified using SYBR Green Mastermix (Applied Biosystems) on a Prism7000 (Applied Biosystems) and compared with levels of the constitutive control, α-tubulin.
Endocytosis and Exocytosis Assays
Receptor-mediated endocytosis of fluorescein isothiocyanate (FITC)-labeled lectin ConA was monitored in mid-log phase BSF cells, tetracycline-induced for 0–36 h, as described previously (Allen et al., 2003; Price et al., 2005). Uptake of the fluorescent lipophilic dye FM4-64FX (Invitrogen) by BSF parasites was monitored as described previously (Field et al., 2004). The movement of 35S-radiolabeled newly synthesized VSG onto the plasma membrane was monitored in mid-log phase BSF cells, either uninduced or tetracycline-induced for 24 h, as described previously (Bangs et al., 1986; Price et al., 2005).
ATP Determination
Parasites were lysed by boiling in 100 mM Tris-HCl and 4 mM EDTA, pH 7.75, for 2 min. ATP concentrations were measured in lysates from 2 × 106 viable cells using an ATP Determination kit (Invitrogen).
RESULTS
The TbARF1 Protein Sequence Has Features of Both ARF1 and ARF6
There are two Arf genes in the T. brucei genome, designated TbARF1 and TbARF2 (Price et al., 2005). TbARF1 shares the greatest level of identity with Arf1 sequences from other species (75% amino acid identity with human ARF1). In correlation, phylogenetic analysis (Supplemental Figure 1A) predicts with high confidence that there is a close relationship between TbARF1 and other Arf1 orthologues, with TbARF2 more distantly related to these and other eukaryotic Arfs. Together with other conserved features, the TbARF1 protein has a predicted N-terminal N-myristoylation motif, in addition to an MXXE motif (Supplemental Figure 1B), which is found in all known Golgi-associating Arfs (but absent from Arf6) and is implicated in binding to the ER-Golgi soluble N-ethylmaleimide-sensitive factor attachment receptor protein (SNARE), membrin (Honda et al., 2005). Unlike other Arf1 proteins, TbARF1 has a basic pI value (9.1), a distinctive feature of class III Arfs (of which Arf6 is representative). All class I and II Arfs have lower pI values of 6–7. The positive charge on class III Arfs is proposed to act in combination with N-myristoylation to allow persistent association with membranes, even in the inactive GDP-bound form (Donaldson, 2003; Macia et al., 2004). Most Arf6 isoforms also contain a conserved QS motif (residues 37–38 in hARF6) adjacent to the Switch I effector binding domain, which confers specific guanine nucleotide binding properties and is essential for mediating actin rearrangement (Al-Awar et al., 2000). However, T. brucei does not demonstrate the genetic capacity for actin-based motility (Berriman et al., 2005), while the QS motif is absent from both TbARF1 and the Saccharomyces cerevisiae actin-independent class III Arf, Arf3p (Supplemental Figure 1B). In summary, the TbARF1 protein sequence contains features characteristic of both mammalian ARF1 and ARF6 isoforms.
TbARF1 Localizes to the Golgi Apparatus in T. brucei but Is Found at the Plasma Membrane in Human Cells
To establish the subcellular localization of TbARF1, the protein was expressed with a C-terminal myc-tag using a tetracycline-inducible system in T. brucei BSF parasites. The protein was detected in a small perinuclear region proximal to the location of the Golgi matrix protein, TbGRASP (Figure 1A, top). Analysis of 20 cells showed ∼32% colocalization of the myc-tagged protein with GRASP. TbARF1 is therefore found either at the Golgi apparatus, or in an adjacent location, possibly in the ERGIC or Golgi-proximal endosomes. However, there is no colocalization between TbARF1 and the ER marker BiP (data not shown). A G2A mutant form of the protein, which is not a substrate for N-myristoylation (Price et al., 2005), was detected in a punctate pattern throughout the cell (Figure 1A, bottom). This mutant form of the protein was found exclusively in the soluble fraction of cell lysates (Supplemental Figure 3).
Figure 1.
Localization of TbARF1 in T. brucei and HEK293 cells. (A) Immunofluorescence of T. brucei BSF-transfected lines 427/pM2/ARF1/WT and 427/pM2/ARF1/G2A (16 h posttetracycline induction) using anti-myc (green) and anti-GRASP (red) and costained with DAPI (blue). Bar, 2.5 μm. (B) Immunofluorescence of HEK293 cells expressing myc-tagged TbARF1 or human ARF6 (hARF6). Cells in columns 1 and 4 were cotransfected with a GFP-Rab5 construct. Cells in columns 2 and 3 were treated immediately before fixing with either 2 μg/ml BFA for 10 min or 1 μM CD for 30 min, respectively. All cells were costained with DAPI (blue). Bar, 10 μm. (C) Total cell lysates (1 × 106 cells/lane) were immunoblotted and probed with rabbit anti-myc (top; 1:2000 dilution) (Abcam) or with mouse anti-cytokeratin (bottom; 1:50,000) (Sigma Chemical, Poole, Dorset, United Kingdom) to monitor equal sample loading. 1, untransfected HEK293; 2–4, HEK293 expressing myc-tagged hARF6, TbARF1, and TbARF1-G2A, respectively.
To further investigate overlapping Arf1/Arf6 function, TbARF1 was expressed in human HEK293 cells (Figure 1B), in which it localized not to the Golgi apparatus as in trypanosomes but to the plasma membrane and cytoplasm. Thus, the presence of a MXXE motif, which is sufficient to target a hybrid hARF6 molecule to the Golgi membrane (Honda et al., 2005), does not seem to influence the localization of TbARF1 in the same manner. As in the parasite, the localization of TbARF1 in HEK293 cells was dependent on N-myristoylation, because G2A mutant protein was detected as punctate staining in the cytoplasm (Figure 1B). Both T. brucei proteins were expressed at a lower level than the smaller myc-tagged human ARF6, as determined by immunoblotting (Figure 1C).
Treatment of mammalian cells with the fungal toxin brefeldin A (BFA) causes inhibition of Arf1-dependent trafficking processes by sequestering the Arf molecules in a complex with GDP and the Sec7 domain-containing guanine nucleotide exchange factors (GEFs) (Peyroche et al., 1999). In contrast, Arf6 uses BFA-resistant GEFs (Jackson and Casanova, 2000), but it is sensitive to cytochalasin D (CD), which inhibits actin filament function by depolymerization of actin stress fibers. In this study, TbARF1 localization in HEK293 cells resembled that of human ARF6, being resistant to treatment with BFA but sensitive to the presence of CD (Figure 1B). In the presence of BFA, both hARF6 and TbARF1 showed uniform distribution on the plasma membrane (PM) in 96 and 90% of transfected cells, respectively. After CD treatment, both hARF6 and TbARF1 proteins translocated to endosomes and protrusions on the PM, in 100 and 96% of transfected cells, respectively. Therefore, TbARF1 may preferentially interact with Arf6-specific effectors at the PM of the mammalian cell. Neither BFA nor CD have any significant effect on T. brucei cell growth or on the localization of TbARF1 protein in the parasite (data not shown).
These observations suggest that TbARF1 shares characteristics of both Arf1 and Arf6 proteins. Because T. brucei is an ancient organism, TbARF1 may represent an ancestral Arf that diverged before the emergence of the three Arf classes in higher eukaryotes.
ARF1 Is Essential for Viability in T. brucei BSF
The function of TbARF1 was investigated by disrupting expression using tetracycline-inducible RNAi. This caused a rapidly lethal phenotype in BSF parasites, with a significant decrease in cell division by 24 h after induction (Supplemental Figure 2A). Quantitative PCR analysis showed degradation of TbARF1-specific RNA by 4 h after induction (Supplemental Figure 2B).
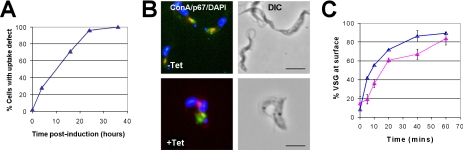
RNAi resulted in a steady accumulation of cells with multiple nuclei (Supplemental Figure 2C) and highly abnormal morphology, most notably the appearance of rounded cells (Figure 2A and Supplemental Figure 2D) in which the cytoplasm is dominated by a large vacuole-like structure. This phenotype was first described as “BigEye” when observed in T. brucei BSF subjected to RNAi of the coat protein, clathrin (Allen et al., 2003). In these cells, the large vacuole was identified as a grossly enlarged flagellar pocket, forced to invaginate and occupy areas of the cytoplasm due to loss of vesicular transport, increase in internal pressure, and accumulation of excess membrane. This phenotype has also been observed in T. brucei BSF after RNAi to disrupt expression of actin, Rab5 isoforms, and Rab11 (Garcia-Salcedo et al., 2004; Hall et al., 2004a, 2005). In the present study, this round vacuolar phenotype was displayed by 86% of the cell population lacking TbARF1 by 24 h after induction (Supplemental Figure 4D).
Figure 2.
Electron micrographs of BSF-transfected line 9013/p2T7/ARF1 grown in the presence of tetracycline for 24 h. B and D are enlarged (3.75 and 5 times, respectively) views of the boxed areas in A and C, respectively. F, flagellum; FP, flagellar pocket; N, nucleus; SM, subpellicular microtubules; G, Golgi. Bar, 1 μm (A and C), 0.5 μm (E–G), or 0.2 μm (B and D).
Transmission electron microscopy showed a number of abnormal features in these cells (Figure 2), including the enlarged flagellar pocket, and, unexpectedly, the appearance of intracellular flagella in ∼20% of cells, features that are also detectable by immunofluorescence (Figure 3). Furthermore, some of these flagella, unusually, are closely associated with the subpellicular microtubules that enclose the parasite (Figure 2, B and D).
Figure 3.
Immunofluorescence analysis of BSF transfected line 9013/p2T7/ARF1 grown in the absence (−Tet) or presence (+Tet) of tetracycline for 16 and 24 h. Cells were stained with antibodies against the ER marker BiP (red) + paraflagellar rod protein Rod1 (green); Golgi marker GRASP; early endosomal marker Rab5; clathrin heavy chain, CLH; and the glycosomal marker aldolase. Cells in the last four panels were costained with DAPI (blue). Bar, 2.5 μm.
Loss of TbARF1 Does Not Result in Golgi Disassembly
Both electron microscopy (Figure 2, E–G) and immunofluorescence assays with the Golgi matrix marker GRASP (Figure 3) show that the Golgi structure in the ARF1–RNAi cells remains intact. In mammalian cells, the attenuation of Arf1 function by treatment with BFA, expression of Arf1, Arf3, or Arf4 dominant-negative mutants or knockdown of both Arf1 and Arf4 causes the dispersal of COPI proteins into the cytoplasm and collapse of the Golgi (Fujiwara et al., 1988; Lippincott-Schwartz et al., 1989; Volpicelli-Daley et al., 2005). We have previously shown that knockdown of the related protein TbARL1 in T. brucei BSF parasites does cause Golgi disintegration (Price et al., 2005). Therefore, it is feasible that recruitment of coat proteins and effector molecules and maintenance of Golgi integrity are mediated in BSF cells by TbARL1 or the currently unstudied TbARF2 rather than by TbARF1.
TbARF1 Negatively Regulates Glycosome Proliferation
Trypanosomes contain peroxisome-like microbodies, glycosomes, which contain the first seven enzymes of the glycolytic pathway (Blattner et al., 1998). A consequence of TbARF1 depletion by RNAi is an increase in glycosome number in BSF parasites, detectable using antibody to the glycosomal marker aldolase (Figure 3). The mean number of glycosomes per cell (counted in 70 cells per experimental group) increases significantly from 4.7 in uninduced cells to 7.8 in parasites induced for 24 h (p < 0.01). These observations suggest that TbARF1 has a negative effect on glycosome proliferation, similar to the observed effect of knockdown of the yeast class III Arf ScARF3 on peroxisomes (Lay et al., 2005).
TbARF1 Is Essential for Endocytosis in BSF
Crucial to our understanding of TbARF1 function, immunofluorescence data show that Rab5-positive endosomes are rapidly disassembled after the induction of TbARF1 RNAi in BSF cells (Figure 3). The distribution of clathrin is also altered, from accumulation around the expanding flagellar pocket at 16 h after induction to relocalization in small punctate peripheral structures by 24 h (Figure 3). To establish the impact of these changes on endocytosis, we monitored the uptake and trafficking of FITC-labeled ConA in T. brucei BSF. After induction of TbARF1 double-stranded RNA production, there was a steady accumulation of cells with a severe defect in receptor-mediated uptake of ConA from the cell surface, affecting >98% of cells by 24 h (Figure 4A). This phenotype correlates with the observed increase in cells displaying an enlargement of the flagellar pocket (Supplemental Figure 2D). Uptake of ConA was severely impaired in affected cells, with retention of the ligand in the flagellar pocket (Figure 4B). The uptake of the lipid probe FM4-64 by bulk lipid endocytosis was also inhibited by 24 h after induction in these cells (data not shown). These observations are in consensus with previous data from RNAi of clathrin and Rab5 in T. brucei BSF (Allen et al., 2003; Hall et al., 2004a) and indicate that TbARF1 is essential for maintaining the integrity of Rab5-positive early endosomes and in regulating the distribution of clathrin to enable endocytosis in the bloodstream stage of the organism.
Figure 4.
Effects of ARF1 depletion on endocytosis and exocytosis (A) Receptor-mediated endocytosis was analyzed by monitoring the uptake of FITC-labeled ConA in BSF line 9013/p2T7/ARF1 grown in the presence of tetracycline for 0–48 h. Cells labeled with FITC-ConA but with no colocalization with p67 were classified as having an endocytic defect. More than 100 cells were counted per experimental group. (B) Typical cells, either uninduced (−Tet) or induced with tetracycline for 16 h (+Tet), the latter showing a defect in ConA uptake. Cells are labeled with FITC-ConA (green), p67 (red), and DAPI (blue). Bar, 5 μm. (C) The movement of newly synthesized VSG from the ER to the cell surface was monitored in BSF-transfected line 9013/p2T7/ARF1 grown in the absence (blue, Δ) and presence (pink, ▲) of tetracycline for 24 h. VSG was isolated from lysates of cells labeled by pulse-chase with 35S-labeled methionine and cysteine. Radiolabeled products were separated by SDS-PAGE, detected by autoradiography, and quantified by densitometry. Data are presented as the percentage of total radiolabeled VSG exocytosed onto the cell surface. Mean values from two independent experiments are shown.
Loss of TbARF1 Causes a Minor Delay in Exocytosis of VSG
We monitored the movement of newly synthesized radiolabeled VSG (the major surface protein of BSF cells) on to the parasite surface by pulse-chase analysis as described previously (Price et al., 2005). There is a minor retardation in VSG trafficking from the site of synthesis to the plasma membrane in ARF1 RNAi cells by 24 h after induction (Figure 4C). However, this is not as pronounced as in other studies, such as the knockdown of the phosphatidylinositol 3-kinase Vsp34 (Hall et al., 2006), so may be a secondary effect caused by disruption of other mechanisms in cellular traffic.
Overexpression of TbARF1 Is Lethal in BSF Cells
As an alternative approach to gene knockdown, we studied the effects of tetracycline-inducible overexpression of wild-type and mutant forms of TbARF1 in BSF parasites. Overexpression of wild-type TbARF1 caused a rapid cessation in cell division and an increase in cell death, compared with uninduced cells, whereas overexpression of a G2A mutant, which cannot be N-myristoylated, had no effect on cell growth (Supplemental Figure 3A). Thus, increasing the levels of the low abundance protein TbARF1 (Supplemental Figure 3) is detrimental to the cell but only if the additional protein is capable of being N-myristoylated, enabling correct localization and/or protein interactions. The overexpression level of wild-type myc-tagged TbARF1 was still relatively low and could only be detected by immunoblotting after incubation of induced cells in the presence of protease inhibitors for 8 h (Supplemental Figure 3C). The nontoxic 25-kDa G2A mutant protein was more abundant but was also further stabilized in cells treated with protease inhibitors (Supplemental Figure 3C), suggesting that the recombinant ARF1 proteins are highly susceptible to proteolysis.
Overexpression of TbARF1 Inhibits Golgi-to-Lysosome Transport
Electron microscopy revealed that cells expressing myc-tagged ARF1 have altered Golgi morphology (Figure 5, A–C), with cisternae occurring either circular or folding back on themselves, compared with more regular structures in uninduced cells (Figure 5D). These circular Golgi stacks also have few if any vesicles budding from them, indicative of a severe disruption in transport processes. A similar phenotype has been described in rat hepatocytes treated with cyclohexamide to clear proteins in transit from the Golgi (Taylor et al., 1997).
Figure 5.
Effects of expression of myc-tagged TbARF1 in T. brucei. (A–D) Electron micrographs of BSF-transfected line 427/pM2/ARF1/WT grown in the presence (A–C) or absence (D) of tetracycline for 16 h. G, Golgi; F, flagellum. Bar, 0.5 μm. (E) Immunoprecipitation of p67, following radioactive labeling of BSF lines 427/pM2/ARF1/WT and 427/pM2/ARF1/G2A (16 h after induction), by using 35S-labeled methionine and cysteine, with a pulse of 7 min and chase time of up to 3 h. Proteins were separated by SDS-PAGE, and radiolabeled products were detected by autoradiography. p67 is detected as a 100-kDa protein (ER), 150-kDa glycoform (Golgi) and four smaller fragments (lysosomal) of which 32- and 28-kDa products are clearly visible. (F) p67 immunoprecipitation results were quantified by densitometry to determine the percentages of total radiolabeled p67 present as the 100-kDa (ER), 150-kDa (Golgi), and proteolytically cleaved (lysosomal) glycoforms in the parasite lines 427/pM2/ARF1/WT (pink, ▲), 427/pM2/ARF1/G2A (blue, ♦), and parental cell line 427s (gray, ■). Data are presented as the percentage of total radiolabeled p67 represented by the 150-kDa Golgi-associated glycoform (left) and the percentage present as the proteolytically cleaved lysosomal glycoforms (right), during the 3-h pulse-chase time course. Results shown are the mean values of two independent experiments. (G) Immunofluorescence assays of BSF lines 427/pM2/ARF1/WT and 427/pM2/ARF1/G2A stained with an antibody against the lysosomal marker p67 (red). Cells were costained with DAPI (blue). Bar, 5 μm.
The effects of TbARF1 overexpression were investigated further by monitoring the trafficking of the lysosomal marker p67 by pulse-chase labeling (Figure 5, E and F). This protein is synthesized as a gp100 glycoform in the ER, which is delivered to the Golgi for N-glycan processing, generating a gp150 glycoform. After transport to the lysosome, the protein is proteolytically cleaved into four fragments (Alexander et al., 2002).
Cells expressing wild-type myc-tagged TbARF1 produced the Golgi-associated gp150 glycoform of p67 at a slightly reduced rate compared with the parental line and cells expressing the G2A mutant. In contrast, cells expressing the wild-type myc-tagged TbARF1 processed the gp150 glycoform at a significantly reduced rate into the proteolytic products seen in the lysosome, compared with the other cell lines. By 3-h chase time, 70% of the total radiolabeled p67 in the G2A cells was represented by proteolytic fragments, similar to the levels seen in the parental line, compared with only 34% in cells expressing wild-type TbARF1 (Figure 5, E and F). These data correlate with immunofluorescence showing that most p67 is concentrated in a small dense region at the posterior region of G2A cells, indicative of lysosomal localization, whereas the p67 signal is fainter and more dispersed in cells expressing wild-type myc-tagged TbARF1 (Figure 2G). Thus, increasing the levels of N-myristoylated Golgi-associated TbARF1 in BSF parasites severely inhibits Golgi to lysosome transport, which may in turn lead to cell death. In comparison, TbARF1 overexpression has no significant effect on the receptor-mediated endocytosis of concanavalin A (data not shown).
Expression of Nucleotide-locked Forms of TbARF1
To further examine the effects of TbARF1 on trafficking, we overexpressed nucleotide-locked mutant forms of the protein in BSF cells. The effects of expressing these mutant proteins are summarized in Table 1, with additional data in Supplemental Figures 3 and 4. Expression of GDP-locked (T31N), GTP-locked (Q71L), and nonmyristoylated GTP-locked (G2A Q71L) mutant forms of TbARF1 caused cell death within 24 h of tetracycline induction. However, overexpression of a nonmyristoylated GDP-locked (G2A T31N) mutant protein was tolerated by parasites, with no effects on cell growth, confirming the importance of the active protein in the phenotype observed (Supplemental Figure 3A).
Table 1.
Summary of the effects of expression of mutant proteins and RNA interference of ARF1 in T. brucei bloodstream form parasites
| Mutant | Predicted effect on protein | Location | Phenotype |
||
|---|---|---|---|---|---|
| Cell viability | Golgi–lysosome trafficking of p67 | Receptor-mediated endocytosis | |||
| ARF1 wild type | Golgi | Death 24 h | Defect | Normal | |
| G2A | Nonmyristoylated | Cytosol | Normal | Normal | Normal |
| T31N | GDP locked | Membranes + cytosol | Death 24 h | Normal | Normal |
| Q71L | GTP locked | Golgi | Death 2 h | Defect | Variable |
| G2A T31N | Nonmyristoylated GDP locked | Membranes + cytoso | Normal | Normal | Normal |
| G2A Q71L | Nonmyristoylated GTP locked | Membranes + cytosol | Death 24 h | Defect | Normal |
| RNAi | Inhibition of expression | Death 24 h | Normal | Severe inhibition | |
A GTP-locked Mutant Form of TbARF1 Is Rapidly Lethal
Expression of the GTP-locked, constitutively active mutant (Q71L) of TbARF1 was exceptionally toxic to BSF parasites, reducing the number of motile cells to <50% by 2 h after induction (Figure 6A). The speed of this effect after induction of gene expression is unprecedented to date, suggesting a crucial role for active cycling TbARF1 in the BSF trypanosome. Induced cells were also analyzed using a fluorescent two-color cell viability assay (Figure 6B). By 2 h after induction, 80% of cells remained viable using this method, a figure that dropped sharply to <20% by 4 h after induction. Comparison of motility and viability data for these cells shows a loss of motility phenotype before death. This may indicate an insufficient supply of ATP to activate the dynein motors that drive flagellum beating (Summers and Gibbons, 1971). Our results show ATP depletion in induced cells, although this is not statistically significant until 3 h after induction (data not shown).
Figure 6.
In vivo expression of GTP-locked TbARF1 in T. brucei. (A) Growth of the BSF transfected line 427/pM2/ARF1/Q71L in the absence (▲) and presence (■) of tetracycline, monitored over 8 h. (B) Live/dead two-color cell viability assay of the cell line described above, grown in the presence of tetracycline for 0–5 h. Live cells (♦), dead cells (■), and unlabeled cells (▲).
Immunofluorescence showed that p67 was dispersed in parasites expressing the Q71L mutant (Supplemental Figure 4B), confirming that the post-Golgi trafficking defect described above is due to an excess in functionally active Arf1.
The endocytosis of several florescence-labeled markers was studied in the Q71L mutant cell line (data not shown), but results proved inconclusive and difficult to analyze due to the rapid death phenotype. Concanavalin A was highly toxic to induced cells, causing rapid parasite lysis. The uptake of transferrin by receptor-mediated endocytosis was highly compromised, whereas induced cells incubated with tomato lectin produced two approximately equal subpopulations, one showing a significant increase in fluorescence. There was no change in the uptake of dextran by fluid-phase endocytosis. Therefore, the effects of expressing the TbARF1 Q71L mutant on endocytic pathways are complex, suggesting an underlying diversity of mechanism.
DISCUSSION
In the present study, we have characterized T. brucei ARF1, which has molecular properties of both class I and III Arfs. We show evidence that the protein localizes at or close to the Golgi apparatus. The primary effect of TbARF1 knockdown is a severe inhibition of endocytosis, correlating with Rab5 dissociation from the early endosomes. Further studies are required to establish whether TbARF1 resides in Golgi-proximal endosomes and has a direct effect on endocytosis, or the protein is Golgi-localized and indirectly influences the endocytic pathway through the regulation of post-Golgi transport. Nonetheless, we have demonstrated that TbARF1 has a central nonredundant role in maintaining the uptake of essential components and altering levels of this protein has severe consequences for the parasite. This is in contrast to studies in yeast and mammalian cells, in which Arf1 has a highly conserved role in regulation of the secretory system (Donaldson et al., 2005).
The T. brucei genome encodes two putative Arf genes, of which TbARF1 shares the highest level of identity with other Arf1 isoforms, particularly at the Switch I and II effector binding domains. At the molecular level, TbARF1 is likely to act in a similar way to other Arfs, but the environment in which it operates is highly unusual, with a requirement to support extremely rapid rates of endocytosis and recycling within the bloodstream trypanosome. The functions of TbARF1 will be additionally influenced by interacting effector molecules, although identifiable orthologues of many known Arf effector proteins, including phospholipase D and arfophilins, are absent from the T. brucei proteome. Because all endocytosis in T. brucei is proposed to be clathrin dependent (Allen et al., 2003), the interactions between ARF1 and the clathrin-associated adaptor complexes may be more critical than in other species. As a consequence, the downstream effects of perturbing TbARF1 expression are wide ranging.
Another highly conserved eukaryotic protein, actin, has a specialized role in the BSF cell. Trypanosomes have a largely tubulin-based cytoskeleton, whereas actin localizes predominantly to the endosomal system in BSF parasites, with a conserved role in endocytosis (Garcia-Salcedo et al., 2004). Arf1 and actin are functionally linked in mammalian cells, in which the formation of discrete pools of actin at the Golgi membrane is Arf dependent, through the binding of coatomer and the Rho GTPase Cdc42, the latter acting via the Arp2/Arp3 pathway (Fucini et al., 2000, 2002). Perhaps actin and TbARF1 also interact directly or indirectly in the parasite to maintain the endocytic pathway. Disrupting actin expression by RNAi results in gross enlargement of the flagellar pocket (Garcia-Salcedo et al., 2004) as for TbARF1, but the onset of cell death is more rapid in the TbARF1-depleted parasites studied here.
It is intriguing to note that knockdown of TbARF1 results in the formation of intracellular flagella. Uncoated internal flagella have also been observed in a subset of BSF parasites subjected to RNAi of the VSG (Sheader et al., 2005). These VSG RNAi cells exhibit arrest in cell division at a precise K2N2 precytokinesis stage. Cells committed to cytokinesis at the time of induction continue to divide but are proposed to have a deficit in the VSG required to coat the newly forming flagellum, causing limited internal flagellar extension (Sheader et al., 2005). The ARF1 RNAi cells also have uncoated intracellular flagella, which may have been forced to extend internally due to defects in VSG trafficking mechanisms. However, this phenotype has not been described in cells deficient in other proteins associated with the endocytic pathway: clathrin, actin, Rab5, or Rab11 (Allen et al., 2003; Garcia-Salcedo et al., 2004; Hall et al., 2004a,2005), and so is unlikely to be a secondary effect of disrupting endocytosis alone. In addition, the appearance of intracellular flagella in response to TbARF1 knockdown does not correlate with growth arrest at K2N2 as in the VSG RNAi cells (Supplemental Figure 4C). The novel finding of some internal flagella that seem to be integrated with the subpellicular microtubules (Figure 2, B and D) suggests that loss of TbARF1 may also influence specific microtubule positioning or basal body segregation, perhaps as an indirect effect of multiple defects in transport mechanisms.
Another important observation from our data are the inhibition of lysosomal trafficking by an increase in active TbARF1 in the parasite. Trypanosomes do not contain GGA proteins, Arf1 effector proteins that function in sorting of mannose 6-phosphate receptors and other cargo at the trans-Golgi network in higher eukaryotes (Ghosh et al., 2003). However, the parasite does contain components of the AP-1 complex, which is implicated in tyrosine-based sorting of cargo for the lysosomal compartment. This mechanism is conserved throughout eukaryotes, even in the early branching protist Giardia (Touz et al., 2004). In mammalian cells, both Arf1 and Rab4 are involved in AP-1 recruitment. However, in T. brucei BSF cells, disrupting the expression of Rab4 by RNAi has the same phenotype as overexpressing active TbARF1: there is reduced transport of p67 from the Golgi to the lysosome (Hall et al., 2004b). If TbARF1 binding to AP-1 blocks Rab4 interaction, then the presence of excess active ARF1 may have negative downstream effects on Rab4 function.
In addition to multiple roles in the Golgi, Arfs are essential for the recruitment of coatomer to peroxisomal membranes in mammalian cells (Anton et al., 2000; Lay et al., 2005; Passreiter et al., 1998). Peroxisome proliferation in yeast is also regulated by Arfs, although the mechanisms involved are still unclear. ScARF1 is proposed to activate a pathway that up-regulates peroxisome proliferation, whereas ScARF3 either negatively regulates this pathway or triggers a mechanism of peroxisome degradation (Lay et al., 2005). In T. brucei, the peroxisome-like microbodies called glycosomes are essential for the viability of BSF cells, which are dependent on glycolysis for ATP production. Our data indicate that knockdown of TbARF1 correlates with a significant increase in the number of glycosomes in the BSF parasite, as quantified by the number of microbodies positive for the matrix enzyme aldolase. This suggests that TbARF1 could act in a similar way to the class III Arf ScARF3. However, trypanosomes are well adapted to modify their metabolism to cope with environmental changes (Michels et al., 2006), so we cannot discount that the observed up-regulation in glycosome proliferation may instead be a stress response in these dying cells.
In summary, our data demonstrate that, unlike other Arf1 orthologues, TbARF1 is not important in regulating ER-to-Golgi transport and in maintaining Golgi integrity. Instead, we propose that TbARF1 is a novel example of a Golgi-proximal Arf protein that is essential for the maintenance of the endocytic pathway and may be key to unraveling the evolution of this critical family of small GTPases.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge contributions from the following colleagues: Sam Alsford and David Horn for the pM2cC vector; George Cross and Doug LaCount for parasite strains and the p2T7Ti vector; Bassam Ali for the pEGFPC3-1Rab5a construct; Loraine Karran for lymph node cDNA; and Graham Warren, Jay Bangs, Mark Field, Keith Gull, and Christine Clayton for primary antibodies. We also thank David Goulding, Karen Chance, Karen Hogg, Graeme Park, Cristina Guerra, Marc Pypaert, Belinda Hall, Clare Allen, Mark Field, Markus Engstler, Nathalie Signoret, Lorna McLean, and Paul Robinson for technical advice and helpful discussions. This work was funded by the Wellcome Trust Grants 061343 and 077503.
Abbreviations used:
- Arf
ADP ribosylation factor
- BFA
brefeldin A
- BSF
bloodstream form
- ConA
concanavalin A
- CD
cytochalasin D
- GEF
guanine nucleotide exchange factor
- RNAi
RNA interferencep
- VSG
variant surface glycoprotein.
Footnotes
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E06-08-0736) on December 20, 2006.
 The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
REFERENCES
- Al-Awar O., Radhakrishna H., Powell N. N., Donaldson J. G. Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant. Mol. Cell. Biol. 2000;20:5998–6007. doi: 10.1128/mcb.20.16.5998-6007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander D. L., Schwartz K. J., Balber A. E., Bangs J. D. Developmentally regulated trafficking of the lysosomal membrane protein p67 in Trypanosoma brucei. J. Cell Sci. 2002;115:3253–3263. doi: 10.1242/jcs.115.16.3253. [DOI] [PubMed] [Google Scholar]
- Allen C. L., Goulding D., Field M. C. Clathrin-mediated endocytosis is essential in Trypanosoma brucei. EMBO J. 2003;22:4991–5002. doi: 10.1093/emboj/cdg481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton M., Passreiter M., Lay D., Thai T. P., Gorgas K., Just W. W. ARF- and coatomer-mediated peroxisomal vesiculation. Cell Biochem. Biophys. 2000 Spring;32:27–36. doi: 10.1385/cbb:32:1-3:27. [DOI] [PubMed] [Google Scholar]
- Bangs J. D., Doering T. L., Englund P. T., Hart G. W. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. J. Biol. Chem. 1986;261:13813–13819. [PubMed] [Google Scholar]
- Berriman M., et al. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- Blattner J., Helfert S., Michels P., Clayton C. Compartmentation of phosphoglycerate kinase in Trypanosoma brucei plays a critical role in parasite energy metabolism. Proc. Natl. Acad. Sci. USA. 1998;95:11596–11600. doi: 10.1073/pnas.95.20.11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R., Warren D. T., Ayscough K. R. Lsb5p interacts with actin regulators Sla1p and Las17p, ubiquitin and Arf3p to couple actin dynamics to membrane trafficking processes. Biochem. J. 2005;387:649–658. doi: 10.1042/BJ20041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G. Multiple roles for Arf6, sorting, structuring, and signaling at the plasma membrane. J. Biol. Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Honda A., Weigert R. Multiple activities for Arf1 at the Golgi complex. Biochim. Biophys. Acta. 2005;1744:364–373. doi: 10.1016/j.bbamcr.2005.03.001. [DOI] [PubMed] [Google Scholar]
- D'Souza-Schorey C., Li G., Colombo M. I., Stahl P. D. A regulatory role for ARF6 in receptor-mediated endocytosis. Science. 1995;267:1175–1178. doi: 10.1126/science.7855600. [DOI] [PubMed] [Google Scholar]
- Engstler M., Thilo L., Weise F., Grunfelder C. G., Schwarz H., Boshart M., Overath P. Kinetics of endocytosis and recycling of the GPI-anchored variant surface glycoprotein in Trypanosoma brucei. J. Cell Sci. 2004;117:1105–1115. doi: 10.1242/jcs.00938. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., et al. Glycosyl-phosphatidylinositol molecules of the parasite and the host. Parasitology. 1994;108(suppl):S45–S54. doi: 10.1017/s0031182000075715. [DOI] [PubMed] [Google Scholar]
- Field M. C., Allen C. L., Dhir V., Goulding D., Hall B. S., Morgan G. W., Veazey P., Engstler M. New approaches to the microscopic imaging of Trypanosoma brucei. Microsc. Microanal. 2004;10:621–636. doi: 10.1017/S1431927604040942. [DOI] [PubMed] [Google Scholar]
- Fucini R. V., Chen J. L., Sharma C., Kessels M. M., Stamnes M. Golgi vesicle proteins are linked to the assembly of an actin complex defined by mAbp1. Mol. Biol. Cell. 2002;13:621–631. doi: 10.1091/mbc.01-11-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucini R. V., Navarrete A., Vadakkan C., Lacomis L., Erdjument-Bromage H., Tempst P., Stamnes M. Activated ADP-ribosylation factor assembles distinct pools of actin on Golgi membranes. J. Biol. Chem. 2000;275:18824–18829. doi: 10.1074/jbc.M000024200. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Oda K., Yokota S., Takatsuki A., Ikehara Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 1988;263:18545–18552. [PubMed] [Google Scholar]
- Garcia-Salcedo J. A., Perez-Morga D., Gijon P., Dilbeck V., Pays E., Nolan D. P. A differential role for actin during the life cycle of Trypanosoma brucei. EMBO J. 2004;23:780–789. doi: 10.1038/sj.emboj.7600094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P., Griffith J., Geuze H. J., Kornfeld S. Mammalian GGAs act together to sort mannose 6-phosphate receptors. J. Cell Biol. 2003;163:755–766. doi: 10.1083/jcb.200308038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B., Allen C. L., Goulding D., Field M. C. Both of the Rab5 subfamily small GTPases of Trypanosoma brucei are essential and required for endocytosis. Mol. Biochem. Parasitol. 2004a;138:67–77. doi: 10.1016/j.molbiopara.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Hall B. S., Gabernet-Castello C., Voak A., Goulding D., Natesan S. K., Field M. C. TbVps34, the trypanosome orthologue of Vps34, is required for Golgi complex segregation. J. Biol. Chem. 2006;281:27600–27612. doi: 10.1074/jbc.M602183200. [DOI] [PubMed] [Google Scholar]
- Hall B. S., Pal A., Goulding D., Field M. C. Rab4 is an essential regulator of lysosomal trafficking in trypanosomes. J. Biol. Chem. 2004b;279:45047–45056. doi: 10.1074/jbc.M407271200. [DOI] [PubMed] [Google Scholar]
- Hall B. S., Smith E., Langer W., Jacobs L. A., Goulding D., Field M. C. Developmental variation in Rab11-dependent trafficking in Trypanosoma brucei. Eukaryot Cell. 2005;4:971–980. doi: 10.1128/EC.4.5.971-980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C. Y., Ho H. H., Malsam J., Chalouni C., West C. M., Ullu E., Toomre D., Warren G. Golgi duplication in Trypanosoma brucei. J. Cell Biol. 2004;165:313–321. doi: 10.1083/jcb.200311076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C. Y., Pypaert M., Warren G. Golgi duplication in Trypanosoma brucei requires Centrin2. Science. 2005;310:1196–1198. doi: 10.1126/science.1119969. [DOI] [PubMed] [Google Scholar]
- Honda A., Al-Awar O. S., Hay J. C., Donaldson J. G. Targeting of Arf-1 to the early Golgi by membrin, an ER-Golgi SNARE. J. Cell Biol. 2005;168:1039–1051. doi: 10.1083/jcb.200409138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. F., Liu Y. W., Tung L., Lin C. H., Lee F. J. Role for Arf3p in development of polarity, but not endocytosis, in Saccharomyces cerevisiae. Mol. Biol. Cell. 2003;14:3834–3847. doi: 10.1091/mbc.E03-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C. L., Casanova J. E. Turning on ARF: the Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 2000;10:60–67. doi: 10.1016/s0962-8924(99)01699-2. [DOI] [PubMed] [Google Scholar]
- Lay D., Grosshans B. L., Heid H., Gorgas K., Just W. W. Binding and functions of ADP-ribosylation factor on mammalian and yeast peroxisomes. J. Biol. Chem. 2005;280:34489–34499. doi: 10.1074/jbc.M503497200. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Yuan L. C., Bonifacino J. S., Klausner R. D. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E., Luton F., Partisani M., Cherfils J., Chardin P., Franco M. The GDP-bound form of Arf6 is located at the plasma membrane. J. Cell Sci. 2004;117:2389–2398. doi: 10.1242/jcs.01090. [DOI] [PubMed] [Google Scholar]
- Michels P. A., Bringaud F., Herman M., Hannaert V. Metabolic functions of glycosomes in trypanosomatids. Biochim. Biophys. Acta. 2006;1763:1463–1477. doi: 10.1016/j.bbamcr.2006.08.019. [DOI] [PubMed] [Google Scholar]
- Naslavsky N., Weigert R., Donaldson J. G. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol. Biol. Cell. 2004;15:3542–3552. doi: 10.1091/mbc.E04-02-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passreiter M., Anton M., Lay D., Frank R., Harter C., Wieland F. T., Gorgas K., Just W. W. Peroxisome biogenesis: involvement of ARF and coatomer. J. Cell Biol. 1998;141:373–383. doi: 10.1083/jcb.141.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyroche A., Antonny B., Robineau S., Acker J., Cherfils J., Jackson C. L. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol. Cell. 1999;3:275–285. doi: 10.1016/s1097-2765(00)80455-4. [DOI] [PubMed] [Google Scholar]
- Price H. P., Menon M. R., Panethymitaki C., Goulding D., McKean P. G., Smith D. F. Myristoyl-CoA:protein N-myristoyltransferase, an essential enzyme and potential drug target in kinetoplastid parasites. J. Biol. Chem. 2003;278:7206–7214. doi: 10.1074/jbc.M211391200. [DOI] [PubMed] [Google Scholar]
- Price H. P., Panethymitaki C., Goulding D., Smith D. F. Functional analysis of TbARL1, an N-myristoylated Golgi protein essential for viability in bloodstream trypanosomes. J. Cell Sci. 2005;118:831–841. doi: 10.1242/jcs.01624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond S., Vadivelu J., Field M. C. RNAit: an automated web-based tool for the selection of RNAi targets in Trypanosoma brucei. Mol. Biochem. Parasitol. 2003;128:115–118. doi: 10.1016/s0166-6851(03)00045-8. [DOI] [PubMed] [Google Scholar]
- Sabharanjak S., Sharma P., Parton R. G., Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell. 2002;2:411–423. doi: 10.1016/s1534-5807(02)00145-4. [DOI] [PubMed] [Google Scholar]
- Sheader K., Vaughan S., Minchin J., Hughes K., Gull K., Rudenko G. Variant surface glycoprotein RNA interference triggers a precytokinesis cell cycle arrest in African trypanosomes. Proc. Natl. Acad. Sci. USA. 2005;102:8716–8721. doi: 10.1073/pnas.0501886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers K. E., Gibbons I. R. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc. Natl. Acad. Sci. USA. 1971;68:3092–3096. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. S., Jones S. M., Dahl R. H., Nordeen M. H., Howell K. E. Characterization of the Golgi complex cleared of proteins in transit and examination of calcium uptake activities. Mol. Biol. Cell. 1997;8:1911–1931. doi: 10.1091/mbc.8.10.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touz M. C., Kulakova L., Nash T. E. Adaptor protein complex 1 mediates the transport of lysosomal proteins from a Golgi-like organelle to peripheral vacuoles in the primitive eukaryote Giardia lamblia. Mol. Biol. Cell. 2004;15:3053–3060. doi: 10.1091/mbc.E03-10-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya M., Price S. R., Tsai S. C., Moss J., Vaughan M. Molecular identification of ADP-ribosylation factor mRNAs and their expression in mammalian cells. J. Biol. Chem. 1991;266:2772–2777. [PubMed] [Google Scholar]
- Volpicelli-Daley L. A., Li Y., Zhang C. J., Kahn R. A. Isoform-selective effects of the depletion of Arfs1-5 on membrane traffic. Mol. Biol. Cell. 2005;16:4495–4508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz E., Leal S., Ochatt C., Cross G. A. Trypanosoma brucei variant surface glycoprotein regulation involves coupled activation/inactivation and chromatin remodeling of expression sites. Mol. Biochem. Parasitol. 1999;18:2265–2272. doi: 10.1093/emboj/18.8.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.