Abstract
An array of cell-surface antigens expressed by human cancers have been identified as targets for antibody-based therapies. The great majority of these antibodies do not have specificity for cancer but recognize antigens expressed on a range of normal cell types (differentiation antigens). Over the past two decades, our group has analyzed thousands of mouse monoclonal antibodies for cancer specificity and identified a battery of antibodies with limited representation on normal human cells. The most tumor-specific of these antibodies is 806, an antibody that detects a unique epitope on the epidermal growth factor receptor (EGFR) that is exposed only on overexpressed, mutant, or ligand-activated forms of the receptor in cancer. In vitro immunohistochemical specificity analysis shows little or no detectable 806 reactivity with normal tissues, even those with high levels of wild-type (wt)EGFR expression. Preclinical studies have demonstrated that 806 specifically targets a subset of EGFR expressed on tumor cells, and has significant anti-tumor effects on human tumor xenografts, primarily through abrogation of signaling pathways. The present clinical study was designed to examine the in vivo specificity of a chimeric form of mAb 806 (ch806) in a tumor targeting/biodistribution/pharmacokinetic analysis in patients with diverse tumor types. ch806 showed excellent targeting of tumor sites in all patients, no evidence of normal tissue uptake, and no significant toxicity. These in vitro and in vivo characteristics of ch806 distinguish it from all other antibodies targeting EGFR.
Keywords: tumor, immunotherapy, anti-erbB1, biodistribution
One of the most persistent searches in cancer research has been to find cancer-specific antigens on the surface of human cancer cells to serve as targets for antibody-based therapy. As a consequence of the intense pursuit of this goal, first with heteroimmune sera, then allo- and autoantibodies, and finally monoclonal antibodies, the human cell surface has been mapped in considerable detail. However, virtually all such antigens when analyzed in necessary detail by immunohistochemistry turn out to be normal differentiation antigens, with broad to restricted representation on normal tissues (1, 2). This expression has not limited the clinical application of monoclonal antibodies for cancer therapy, as demonstrated by U.S. Food and Drug Administration approved antibodies against CD20 in lymphoma, epidermal growth factor receptor (EGFR) in solid tumors, and erbB2 in breast cancer, even though the antigens detected by these antibodies are clearly found on a range of normal cell types (2–6). The key issue that cannot be assessed by in vitro analysis of antigen-expression by immunohistochemistry is the in vivo accessibility of antigen in normal tissues and tumors to injected antibody. It may well be that antigens lacking tumor-specific characteristics in vitro could show tumor specificity when analyzed in vivo. For this reason, our first-in-human clinical trials has emphasized the following endpoints: tumor targeting, biodistribution, and pharmacokinetics of trace-radiolabeled antibody. Determining these parameters has been our approach in the clinical evaluation in five antigenic systems: A33 (7, 8), G250 (9), Ley (10), GD3 (11), and FAPα (12, 13). In our opinion, information gained in this way from in vivo specificity analysis is essential for rational development of monoclonal antibodies for therapy, particularly therapies based on monoclonal antibodies as delivery systems for radioisotopes, toxins, or other cytotoxic strategies.
It is known that overexpression of the EGFR has been observed in many epithelial tumors, with increased EGFR expression levels usually correlating with poor clinical outcome (4, 5). Overexpression of the receptor is often caused by amplification of the EGFR gene, an event also linked with EGFR mutation (2, 14–17). The most common EGFR mutation is an extracellular truncation of the EGFR known as the de2-7 EGFR (or EGFRvIII), which is frequently expressed in glioblastoma and possibly some other tumor types including prostate and breast cancer (2, 16). Inhibition of the EGFR by monoclonal antibodies and tyrosine kinase inhibitors is a rational strategy for the development of new cancer therapeutics, because of the high expression on epithelial tumors, and the role of EGFR signaling in maintaining the neoplastic phenotype of cancer cells (2, 4, 5, 18–20). A number of antibodies directed to the extracellular domain of the EGFR have now been tested in the clinic including EMD 72000 (Matuzumab), h-R3 (Nomotuzumab), ABX-EGF (Panitumumab), and C225 (Cetuximab), all of which have displayed anti-tumor activity in patients (4, 5, 18, 21–23). Cetuximab has been approved for use in Europe and the U.S., and Panitumumab has recently been approved for use in the U.S. It has been presumed that the antitumor activity of these antibodies is primarily related to their ability to block ligand binding but other antitumor mechanisms such as immune effector function, receptor down-regulation, induction of inappropriate signaling and interference with receptor dimerization and/or oligomerization could also play a role (4, 5). One limitation of antibodies targeting the wild-type (wt)EGFR is their significant uptake in normal tissues such as the liver and skin, therefore requiring large loading doses to achieve adequate serum concentrations. Targeting of normal tissue (skin) may cause considerable side-effects such as skin rash and gastrointestinal toxicity which may be dose limiting, and side-effects are greater when treatment is combined with chemotherapy and other biologics (1, 8). These side-effects may impact negatively on the ideal therapeutic index of non-tumor-specific EGFR directed therapies. In addition, coupling of drugs or toxins to wtEGFR targeting antibodies is limited by the high uptake of conjugates in normal tissues.
The monoclonal antibody (mAb) 806 was raised after immunization of BALB/c mice with mouse fibroblast cells expressing the de2-7 EGFR (24, 25). Unlike other de2-7 EGFR specific antibodies, which all bind the unique de2-7 EGFR junctional peptide, fine epitope mapping of the EGFR-specific mAb 806 revealed that it preferentially recognizes an epitope only exposed on overexpressed, mutant or ligand activated forms of the EGFR (26). Whereas mAb 806 does recognize the de2-7 EGFR, it can also bind a small proportion (5–10%) of the wtEGFR overexpressed on A431 cells when compared with the wtEGFR specific mAb 528 (24). mAb 806 binds specifically and at high levels to xenografts overexpressing the EGFR (24–28). mAb 806 is also rapidly internalized into tumor cells expressing both amplified wtEGFR and de2-7 EGFR both in vitro and in vivo (24). When used as a single agent, mAb 806 has shown significant anti-tumor activity against human xenografts expressing either the de2-7 or amplified wtEGFR (28–30). Intracranial xenografts expressing the de2-7 EGFR have also been shown to be inhibited by mAb 806, and this observation represents the only reported mAb that has such effects on gliomas in vivo (30). In conjunction with other EGFR inhibitors such as the mAb 528, or the tyrosine kinase inhibitor AG1478, mAb 806 shows synergistic effects on xenograft growth, with >60% of established tumours showing complete regressions (29, 31).
Immunohistochemical analyses of frozen sections of normal tissue have shown that mAb 806 does not show significant binding to normal tissue expressing the wtEGFR (25). Tumors demonstrating mAb 806 reactivity include gliomblastoma (≈70%), and between 30% and 70% of squamous cell carcinomas of the head and neck, lung, cervix, esophagus, and bladder (25). A direct correlation has also been demonstrated for mAb 806 reactivity and EGFR gene amplification (25).
To develop a humanized form of mAb 806 suitable for clinical development, a chimeric form of mAb 806 (ch806) has been produced under cGMP conditions (32). Extensive preclinical studies have shown that ch806 has identical specificity to mAb 806 and high affinity for the 806 epitope on EGFR (32). Preclinical in vivo studies have also demonstrated similar tumor growth inhibition of xenografts by ch806 compared with mAb 806 (32). These findings collectively indicate that ch806 has one of the most selective patterns of tumor antigen binding yet reported, and with preclinical data showing potent tumor activity ch806 represents an important potential therapeutic in cancer patients. We report herein the results of a first-in-human trial of ch806 administered in a single dose to patients with advanced tumors expressing the 806 antigen.
Results
Patients.
Eight patients [one female and seven male; mean age of 61 years of age (range 44–75 years of age)] completed the trial (Table 1). Primary tumor sites, prior therapy history, and sites of disease at study entry are also shown in Table 1. All eight patients had 806 antigen positivity in archived tumors (Table 1).
Table 1.
Patient characteristics
| Patient no. | Dose level, mg/m2 | Age, yr | Sex | KPS, % | Site of primary tumor | IHC of positive cells, % | Prior therapies | Disease sites at study entry | Tumor response to ch806 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5 | 71 | M | 10 | NSCLC | 50–75 | RT | Lung, adrenal | PD |
| 8 | 5 | 44 | M | 90 | Anaplastic astrocytoma | >75* | Surgery, RT, CT | Brain | SD |
| 2 | 10 | 49 | F | 80 | SCC anus | <10 | Chemo, RT | LN, lung, bone | SD |
| 3 | 10 | 75 | M | 90 | NSCLC | 50–75 | Surgery, RT | Lung | SD |
| 4 | 20 | 52 | M | 100 | Colon | <10† | Surgery, CT | Lung, LN | PD |
| 5 | 20 | 65 | M | 80 | Mesothelioma | >75 | RT, CT | Lung | SD |
| 6 | 40 | 59 | M | 80 | SCC vocal cord | >75 | Surgery, RT, CT | Soft tissue | SD |
| 7 | 40 | 71 | M | 90 | SCC skin | 50–75 | Surgery, CT | Lung, LN | PD |
F, female; M, male; NSCLC, non-small cell lung carcinoma; SCC, squamous cell carcinoma; RT, radiotherapy; CT, chemotherapy; LN, lymph nodes; PD, progressive disease; SD, stable disease; KPS, Karnofsky performance scale; IHC, immunohistochemistry.
*Positive for de2–7 EGFR expression.
†Positive for EGFR gene amplification.
All patients fulfilled inclusion criteria and, except for patient 8 (who had a primary brain tumor), all had metastatic disease at study entry. Sites of disease classified as target lesions included: lung (five patients), brain (one patient), lymph nodes (one patient), supraglottis (one patient). Other sites of metastatic disease (nontarget lesions) included a suprarenal mass, bone and lymph nodes (Table 1). The median Karnofsky performance status was 90 (range 80–100).
Adverse Events and Human Antichimeric Antibodies (HACA).
Adverse events related to ch806 are listed in Tables 2 and 3. No infusion related adverse events were observed. There was no dose-limiting toxicity (DLT), and therefore maximum tolerated dose (MTD) was not reached. The principal toxicities that in the investigator's opinion were possibly attributable to ch806 were as follows: transient pruritis, mild nausea, fatigue/lethargy, and possible effects on serum alkaline phosphatase (ALP) and γ-glutamyl-transferase (GGT) levels. A common toxicity criteria grade 2 elevation in GGT level in patient 5 was observed; however this rise was on a background of a baseline grade 1 elevation, and was transient in nature. Three serious adverse events were reported but none were attributed to ch806. Overall, ch806 was safe and well tolerated at all dose levels with generally predictable and manageable minor toxicities being observed. Further dose escalation was not performed because of the limited amount of cGMP ch806 available for the trial.
Table 2.
Occurrence of adverse events related to ch806
| Adverse event | Dose level, mg/m2* |
Total no. of episodes of each event | |||
|---|---|---|---|---|---|
| 5 | 10 | 20 | 40 | ||
| Dizziness | 0 | 0 | 0 | 1 | 1 |
| Fatigue | 0 | 0 | 1 | 0 | 1 |
| Lethargy | 0 | 0 | 0 | 1 | 1 |
| Appetite suppressed | 0 | 0 | 0 | 1 | 1 |
| Nausea | 0 | 1 | 0 | 1 | 2 |
| Pruritis | 1 | 0 | 0 | 0 | 1 |
| ALP-elevated | 0 | 0 | 1 | 0 | 1 |
| GGT-elevated | 0 | 0 | 1 | 0 | 1 |
| Total | 1 | 1 | 3 | 4 | 9 |
*Numbers represent no. of episodes of any event at each dose level.
Table 3.
No. of patients with indicated maximum CTC grade toxicity (distribution of study agent-related adverse events)
| Dose level, mg/m2 | CTC grade toxicity |
|||
|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| 5 | 1 | 0 | 0 | 0 |
| 10 | 1 | 0 | 0 | 0 |
| 20 | 2 | 1 | 0 | 0 |
| 40 | 4 | 0 | 0 | 0 |
| Overall | 8 | 1 | 0 | 0 |
Grade 1, mild; grade 2, moderate; grade 3, severe; grade 4, life-threatening; CTC, common toxicity criteria.
A positive immune response to ch806 (with concordance of both ELISA and BIAcore methodologies) was observed in only one of the eight patients (patient 1).
Radiolabeling of ch806.
There were a total of eight infusions of 111In-ch806 [Indium-111, 111In (200–280 MBq; 5–7 mCi; 1 Ci = 37 GBq)] administered during the trial. The mean (±SD) radiochemical purity and immunoreactivity of 111In-ch806 was measured to be 99.3 ± 0.1% and 77.4 ± 7.0%, respectively.
Biodistribution of ch806.
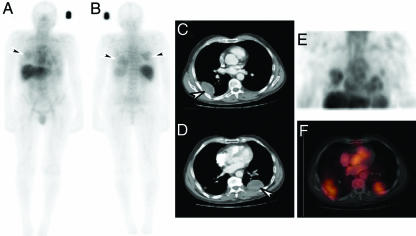
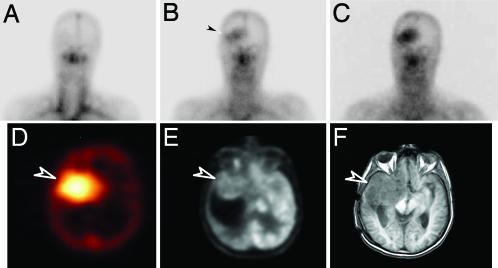
The initial pattern of 111In-ch806 biodistribution in patients at all dose levels was consistent with blood pool activity, which cleared gradually with time. Over the 1-week period after injection, the uptake of 111In-ch806 in liver and spleen was consistent with the normal clearance of 111In-chelate metabolites through the reticuloendothelial system. Specific localization of 111In-ch806 was observed in target lesions (≥2 cm) of all patients at all dose levels (Fig. 1), including target lesions located in the lungs (patients 1, 3, 4, 5, and 7), the abdomen (patients 1 and 2), and the supraglottic region in the right side of the neck (patient 6). High uptake of 111In-ch806 in a brain tumor (patient 8) was also demonstrated (Fig. 2). Importantly, uptake of 111In-ch806 in tumor was not dependent on a the level of 806 antigen expression. For example, patient 4 demonstrated high uptake by both lung target lesions, despite <10% positivity by immunohistochemistry for 806 reactivity in archived tumor [supporting information (SI) Fig. 4]. This degree of uptake of 111In-ch806 in target lesions in patient 4 was comparable with that seen in patient 3, where 50–75% of tumor cells were positive for 806 antigen staining on archived sample immunohistochemistry (SI Fig. 4).
Fig. 1.
Biodistribution and tumor localization of 111In-ch806. (A and B) Whole body gamma camera images of patient 7, anterior (A) and posterior (B), on day 5 after infusion of 111In-ch806. High uptake of 111In-ch806 in metastatic lesions in the lungs (arrows) is evident. (C and D) Metastatic lesions (arrows) on scan. (E) 3D SPECT images of the chest. (F) Coregistered transaxial images of SPECT and CT showing specific uptake of 111In-ch806 in metastatic lesions.
Fig. 2.
ch806 targeting of glioma. (A–C) Planar images of the head and neck of patient 8 obtained on day 0 (A), day 3 (B), and day 7 (C) after infusion of 111In-ch806. Initial blood pool activity is seen on day 0, and uptake of 111In-ch806 in an anaplastic astrocytoma in the right frontal lobe is evident by day 3 (arrow) and increases by day 7. (D–F) Specific uptake of 111In-ch806 is confirmed in SPECT image of the brain (D) (arrow), at the site of tumor (arrow) evident in 18F-FDG (FDG, Fluorodeoxyglucose) positron emission tomography (E), and MRI (F).
Pharmacokinetics.
Individual patient pharmacokinetic parameters t1/2α and t1/2β (half lives of the initial and terminal phases of disposition); volume of central compartment (V1); maximum serum concentration (Cmax); area under the serum concentration curve extrapolated to infinite time (AUC); and total serum clearance (CL) for the single infusion of 111In-ch806 are shown in Table 4. The Kruskal–Wallis rank sum test was applied to the α and β half lives, V1, and clearance. No significant difference between dose levels was observed (P > 0.05).
Table 4.
Mean ± SD pharmacokinetic parameter estimates for 111In-ch806 in each dose level and across all dose levels
| Dose level, mg/m2 |
t½α, hr |
t½β, hr |
V1, ml |
CL, ml/hr |
AUC, hr × mg/ml |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| 5 | 10.91 | 3.4 | 183.87 | 110.22 | 2,963.06 | 493.23 | 21.97 | 16.59 | 541.17 | 371.75 |
| 10 | 11.75 | 4.4 | 124.54 | 9.25 | 3,060.29 | 721.70 | 28.58 | 8.60 | 566.79 | 26.39 |
| 20 | 9.34 | 8.3 | 125.26 | 73.66 | 2,902.06 | 1,064.77 | 30.98 | 21.65 | 1,438.12 | 957.18 |
| 40 | 8.95 | 3.2 | 133.94 | 10.79 | 4,742.42 | 169.10 | 37.99 | 6.47 | 2,269.04 | 381.68 |
| All | 10.24 | 1.32 | 141.90 | 28.30 | 3,416.96 | 886.04 | 29.88 | 6.61 | ||
The pharmacokinetic curve fit to the pooled population ELISA data are shown in SI Fig. 5. The mean ± SD pharmacokinetic parameters were t1/2α 29.16 ± 21.12 h, t1/2β 172.40 ± 90.85 h, V1 2984.59 ± 91.91 ml, and CL 19.44 ± 4.05 ml/hr. Measured peak and trough ch806 serum concentrations (Cmax and Cmin) data are presented in Table 5 for each patient. As expected, linear relationships were observed for Cmax and Cmin with each dose level. The mean ± SD values determined for the ch806 ELISA pharmacokinetic data were in good agreement with the values obtained for the 111In-ch806 pharmacokinetic data (Table 4).
Table 5.
Cmax and Cmin serum ch806 levels determined by ELISA
| Patient no. | Dose level, mg/m2 | Cmax,* mg/ml | Cmin,* mg/ml |
|---|---|---|---|
| 1 | 5 | 1.38 ± 0.02 | 0.10 ± 0.05† |
| 8 | 5 | 1.52 ± 0.17 | 0.96 ± 0.08 |
| 2 | 10 | 5.92 ± 0.11 | 1.50 ± 0.01 |
| 3 | 10 | 6.27 ± 0.45 | 1.83 ± 0.20 |
| 4 | 20 | 12.25 ± 0.66 | 4.05 ± 0.05 |
| 5 | 20 | 11.22 ± 0.77 | 1.58 ± 0.04 |
| 6 | 40 | 27.76 ± 2.10 | 6.90 ± 0.38 |
| 7 | 40 | 32.32 ± 0.84 | 6.80 ± 0.13 |
*Cmax, 60 min after injection; Cmin, day 7.
†Day 8 serum level.
Dosimetry of 111In-ch806.
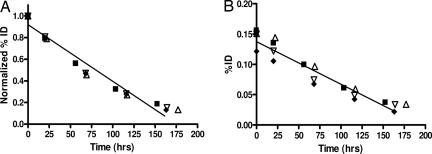
Whole body clearance was similar in all patients across all dose levels, with a t1/2 biologic (mean ± SD) of 948.6 ± 378.6 hr. Because of the relatively short physical half-life, calculation of biological halftime was extremely sensitive to small changes in effective halftime. There was no statistical significant difference in whole body clearance between dose levels (Kruskal–Wallis rank sum test: P = 0.54) (Fig. 3).
Fig. 3.
Individual patient results for normalized whole body clearance (A) and hepatic clearance (B) of 111In-ch806 at the 5 mg/m2 (■), 10 mg/m2 (▵), 20 mg/m2 (▿), and 40 mg/m2 (⧫) dose levels. Linear regression for data sets is indicated in each image [r2 = 0.9595 (A); r2 = 0.9415 (B)].
The clearance of 111In-ch806 from normal organs (liver, lungs, kidney and spleen) showed no difference between dose levels, and the mean t1/2 effective was calculated to be 78.3, 48.6, 69.7, and 66.2 h, respectively. There was no statistically significant difference in clearance between these normal organs. In particular, liver clearance showed no difference between dose levels (Fig. 3), indicating no saturable antigen compartment in the liver for ch806.
Tumor dosimetry analysis was completed for six patients. Patients 1 and 2 had target lesions close to the cardiac blood pool, or motion during some image acquisitions, which prevented accurate analysis. The measured peak uptake of 111In-ch806 occurred 5–7 days after infusion, and ranged from 5.2 to 13.7 × 10−3 % injected dose per gram of tumor tissue.
Assessment of Clinical Activity.
At the completion of this one month study period five patients were found to have stable disease, and three patients progressive disease (Table 1). Interestingly, one patient (patient 7, 40 mg/m2 dose level) had clinical evidence of transient shrinkage of a palpable auricular lymph node (proven to be metastatic squamous-cell carcinoma on fine needle aspiration) during the study period, which suggests possible biologic activity of ch806. However, this patient had confirmed progressive disease by RECIST at study completion.
Discussion
This study represents the first reported demonstration of the biodistribution and tumor targeting of a chimeric antibody against an epitope only exposed on overexpressed, mutant or ligand activated forms of the EGFR. At doses up to 40 mg/m2, ch806 was well tolerated, no DLT was observed, and MTD was not reached. The principle toxicities that were possibly attributable to ch806 were transient pruritis, mild nausea, fatigue/lethargy, and possible effects on serum ALP and GGT levels. The advanced nature of these patient's malignancies meant their disease could also have been contributing factors to these adverse events. Of the adverse events that were possibly related to study drug, all were mild, many were self-limiting, and none required any active treatment. Importantly, no skin rash or gastrointestinal tract disturbances were observed in any patient, even at the highest dose level. The excellent tolerability of ch806 in this single-dose study justifies the next step of testing in repetitive dose trials.
The biodistribution of ch806 in all patients showed gradual clearance of blood pool activity, and no definite normal tissue uptake of 111In-ch806. Excellent tumor uptake of ch806 was also evident in all patients, including lung, lymph node, and adrenal metastases, and in mesothelioma and glioma. This tumor uptake was observed at all dose levels including 5 mg/m2 (the lowest dose studied), which is 1/10th to 1/20th of the dose required to visualize uptake in tumor by other antibodies to wtEGFR (33). This difference in uptake of ch806 compared with antibodies to wtEGFR can be attributed to their substantial normal tissue (liver and skin) uptake because of wtEGFR acting as an antigen sink (33). In addition, the localization of 111In-ch806 was high even in patients with low expression of 806 assessed by immunohistochemistry of archived tumor samples (SI Fig. 4). The uptake of 111In-ch806 in glioma was particularly impressive (Fig. 2), and comparable with any published data on antibody targeting of brain tumor after systemic or even locoregional infusion. These data support the unique selectivity of ch806 to EGFR expressed by a broad range of tumors, and confirms the lack of normal tissue uptake of this antibody in human.
Pharmacokinetic analyses showed that ch806 has a terminal half-life of more than a week, and no dose dependence of 111In-ch806 serum clearance. Linear relationships also were observed for AUC, Cmax, and Cmin, with dose levels >10 mg/m2 achieving trough serum concentrations >1 μg/ml. The V1, CL, t1/2α, and t1/2β values were consistent between dose levels and in keeping with typical IgG1 human antibodies (8, 9, 11). The clearance of ch806 was also determined to be slower when ELISA ch806 calculations were compared with 111In-ch806 measurements. Whereas this difference may be explained by the small number of patients studied, the longer sampling time points for the ch806 ELISA would support this value as being more representative of true ch806 clearance. The pharmacokinetic values for ch806 are comparable with other chimeric antibodies reported to date (9, 11), and supports a weekly dosing schedule of ch806.
The quantitative dosimetry and pharmacokinetic results indicate that there is no saturable normal tissue compartment for ch806 for the dose levels assessed in this trial. Importantly, the lack of dose dependence on pharmacokinetic and whole body and liver organ clearance is in marked contrast to all reported studies of antibodies to wtEGFR (4, 33–37), supporting the tumor specificity and lack of normal tissue binding of ch806 in humans. These observations provide compelling evidence of the potential for ch806 (or humanized forms) to selectively target EGFR in tumor, avoid the normal toxicity of other EGFR antibodies and kinase inhibitors (particularly skin) (38, 39), and potentially achieve greater therapeutic effect. Moreover, the possibility of payload delivery (because of the rapid internalization of mAb 806 in tumor cells), and combination treatment with other biologics such as EGFR antibodies and tyrosine kinase inhibitors where combined toxicity is likely be minimized, is strongly supported by the data from this trial. This study provides clear evidence of the ability to target an epitope on EGFR that is specific for tumor, and further clinical development of this unique approach to cancer therapy is ongoing.
Materials and Methods
Trial Design.
This first-in-human trial was an open label, dose escalation phase I study. The primary objective was to evaluate the safety of a single infusion of ch806 in patients with advanced tumors expressing the 806 antigen. The secondary study objectives were to determine the biodistribution, pharmacokinetics, and tumor uptake of 111In-ch806; determine the patient's immune response to ch806; and to assess early evidence of clinical activity of ch806. A single dose was chosen for this study to optimally assess the in vivo specificity of ch806 for EGFR expressed on tumor. The protocol was approved by the Human Research and Ethics Committee of the Austin Hospital before study commencement. The trial was performed under the Australian Therapeutic Goods Administration Clinical Trials Exemption scheme. All patients gave written informed consent.
Eligibility criteria included the following: advanced or metastatic tumors positive for 806 antigen expression based on chromogenic in situ hybridization or immunohistochemistry of archived tumor samples (tumors were defined as 806 positive if immunohistochemical assessment of archived tumor samples showed any cells positive for 806 expression; see SI Materials and Methods); histological or cytologically proven malignancy; measurable disease on CT scan with at least one lesion ≥2 cm; expected survival of at least 3 months; Karnofsky performance scale ≥70; adequate hematologic, hepatic, and renal function; age >18 yr; and able to give informed consent. Exclusion criteria included the following: active central nervous system metastases (unless adequately treated and stable); chemotherapy, immunotherapy, biologic therapy, or radiation therapy within 4 weeks before study entry; prior antibody exposure (unless no evidence of HACA); failure to fully recover from effects of prior cancer therapy; concurrent use of systemic corticosteroids or immunosuppressive agents; uncontrolled infection or other serious disease; pregnancy or lactation; women of childbearing potential not using medically acceptable means of contraception.
Patients received a single infusion of ch806 trace labeled with 111In by i.v. infusion in normal saline/5% human serum albumin over 60 min. The planned dose escalation meant patients were enrolled into one of four dose levels: 5, 10, 20, and 40 mg/m2. These doses were chosen to allow assessment of the specificity of ch806 to EGFR expressed on tumor and to determine whether any normal tissue compartment binds ch806 (and affects pharmaco kinetics or biodistribution) in vivo. Biodistribution, pharmaco kinetics, and immune response were evaluated in all patients.
Whole body gamma camera imaging for assessment of biodistribution and tumor uptake was performed on day 0, day 1, day 2 or 3, day 4 or 5, and day 6 or 7 after 111In-ch806 infusion. Blood samples for pharmacokinetics were obtained at these time points, and additionally on day 14 (±2 days) and day 21 (±2 days). Blood samples for assessment of HACA levels were obtained at baseline and weekly until day 30. Toxicity assessment was performed at each study visit. Physical examination and routine hematology and biochemistry were performed weekly until end of study (day 30). Restaging was performed on day 30.
Dose Escalation Criteria.
The first patient at each dose level was observed for 4 weeks before enrollment of any additional patients. If no DLT was observed in any of the first two patients within 4 weeks of the infusion of ch806, four patients were then to be entered on the next highest dosage tier. If one patient in any cohort of two patients experienced a DLT within 4 weeks from the first dose, an additional four patients (maximum of six) were entered at that dosage level. If no more than one patient of six in any dose level experienced grade 3 toxicity or higher, subsequent patients were entered at the next dose level.
DLT was defined as grade 3 nonhematological toxicity or grade 4 hematological toxicity as defined by the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE v3.0). MTD was defined as the ch806 dose below that where two or more patients of six experienced DLT.
Radiolabeling of ch806.
Clinical grade ch806 was produced in the Biological Production Facility of the Ludwig Institute for Cancer Research. The antibody ch806 was labeled with 111In (MDS Nordion, Ottawa, ON, Canada) via the bifunctional metal ion chelate CHX-A″-DTPA according to methods described previously (10, 11).
Gamma Camera Imaging.
Whole body images of 111In-ch806 biodistribution were obtained in all patients on day 0 after infusion of 111In-ch806, and on at least three further occasions up to day 7 after infusion. Single-photon emission computed tomography (SPECT) images of a region of the body with known tumor were also obtained on at least one occasion during this period. All gamma camera images were acquired on a dual-headed gamma camera (Picker International, Cleveland, OH).
Pharmacokinetics.
Blood for pharmacokinetic analysis was collected on day 0 before 111In-ch806 infusion; then at 5 min, 60 min, 2 h and 4 h after 111In-ch806 infusion, day 1, day 2 or 3, day 4 or 5, and day 6 or 7. Further blood for pharmacokinetics of ch806 protein was also obtained on day 14 (±2 days), day 21 (±2 days), and day 30 (±2 days).
Serum samples were aliquotted in duplicate and counted in a gamma scintillation counter (Packard Instruments, Melbourne, Australia), along with appropriate 111In standards. The results of the serum were expressed as % injected dose per liter. Measurement of patient serum ch806 protein levels after each infusion was performed by using a validated protocol for the immunochemical measurement of ch806 protein in human serum (40). The limit of quantitation for ch806 in serum samples was 70 ng/ml. All samples were assayed in triplicate and diluted by a factor of at least 1:2. Measured serum levels of ch806 were expressed as μg/ml.
Pharmacokinetic calculations were performed on serum 111In-ch806 measurements after the infusion, and ELISA determined patient sera ch806 protein levels, by using a curve fitting program (WinNonlin Pro Node 5.0.1; Pharsight Co., Mountain View, CA). Estimates were determined for the following parameters: t1/2α and t1/2β (half lives of the initial and terminal phases of disposition); V1, Cmax, AUC, and CL.
Whole Body Clearance and Tumor and Organ Dosimetry of 111In-ch806.
Whole body and normal organ (liver, lungs, kidney, and spleen) dosimetry calculations were performed based on regions of interest in each individual patient 111In-ch806 infusion image data set, allowing calculation of cumulated activity and analysis for final dosimetry results (41). Regions of interest were also defined for suitable tumors at each time point on 111In-ch806 image data sets, corrected for background and attenuation, and dosimetry calculation was performed to derive the concentration of 111In-ch806 in tumor/gram (8). This value was converted to micrograms of ch806 per gram of tumor tissue based on the injected milligram of ch806 protein dose.
HACA Analysis.
Blood samples for HACA assessment were taken before ch806 infusion, then weekly until 30 days after ch806 infusion. Samples were analyzed by ELISA and by surface plasmon resonance technology by using a BIAcore2000 instrument, as described (8, 40, 42).
Supplementary Material
Acknowledgments
We acknowledge the role of Elizabeth Stockert, Ph.D., in the discovery and characterization of mAb 806. We thank George Demetri, M.D., for his insightful comments on the manuscript. This work was supported in part by National Health and Medical Research Grants 280912 and 234709; a project grant from The Garnett Passe and Rodney Williams Foundation; and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Abbreviations
- EGFR
EGF receptor
- HACA
human antichimeric antibodies
- DLT
dose-limiting toxicity
- ALP
alkaline phosphatase
- GGT
γ-glutamyl-transferase
- MTD
maximum tolerated dose
- SPECT
single-photon emission computed tomography
- V1
volume of central compartment
- Cmax
maximum serum concentration
- Cmin
minimum serum concentration
- AUC
area under the serum concentration curve extrapolated to infinite time
- CL
total serum clearance
- 111In
Indium-111
- wt
wild type.
Footnotes
Conflict of interest statement: A.M.S., A.A.J., A.W.B., T.G.J., and L.J.O. are coinventors of a patent for monoclonal antibody 806. A.M.S. and F.E.S. are consultants to Life Sciences Pharmaceuticals, which has the license for ch806. L.J.O. is a noncompensated board member of Life Sciences Pharmaceuticals.
This article contains supporting information online at www.pnas.org/cgi/content/full/0611693104/DC1.
References
- 1.Rettig WJ, Old LJ. Annu Rev Immunol. 1989;7:481–511. doi: 10.1146/annurev.iy.07.040189.002405. [DOI] [PubMed] [Google Scholar]
- 2.Van den Eynde BJ, Scott AM. In: Encylopedia of Immunology. Roitt DPJ, Roitt IM, editors. London: Academic; 1998. pp. 2424–2431. [Google Scholar]
- 3.Maloney DG, Grillo-Lopez AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, Janakiraman N, Foon KA, Liles TM, Dallaire BK, et al. Blood. 1997;90:2188–2195. [PubMed] [Google Scholar]
- 4.Baselga J, Artega CL. J Clin Oncol. 2005;23:2445–2449. doi: 10.1200/JCO.2005.11.890. [DOI] [PubMed] [Google Scholar]
- 5.Voldborg BR, Damstrup L, Spang-Thomsen M, Poulsen HS. Ann Oncol. 1997;8:1197–1206. doi: 10.1023/a:1008209720526. [DOI] [PubMed] [Google Scholar]
- 6.Baselga J, Tripathy D, Mendelsohn J, Baughman S, Benz CC, Dantis L, Sklarin NT, Seidman AD, Hudis CA, Moore J, et al. J Clin Oncol. 1996;14:737–744. doi: 10.1200/JCO.1996.14.3.737. [DOI] [PubMed] [Google Scholar]
- 7.Welt S, Divgi CR, Real FX, Yeh SD, Garin-Chesa P, Finstad CL, Sakamoto J, Cohen A, Sigurdson ER, Kemeny N, et al. J Clin Oncol. 1990;8:1894–1906. doi: 10.1200/JCO.1990.8.11.1894. [DOI] [PubMed] [Google Scholar]
- 8.Scott AM, Lee F-T, Jones R, Hopkins W, MacGregor D, Cebon JS, Hannah A, Chong G, U P, Papenfuss A, et al. Clin Cancer Res. 2005;11:4810–4817. doi: 10.1158/1078-0432.CCR-04-2329. [DOI] [PubMed] [Google Scholar]
- 9.Steffens MG, Boerman OC, Oosterwijk-Wakka JC, Oosterhof GO, Witjes JA, Koenders EB, Oyen WJ, Buijs WC, Debruyne FM, Corstens FH, et al. J Clin Oncol. 1997;15:1529–1537. doi: 10.1200/JCO.1997.15.4.1529. [DOI] [PubMed] [Google Scholar]
- 10.Scott AM, Geleick D, Rubira M, Clarke K, Nice EC, Smyth FE, Stockert E, Richards EC, Carr FJ, Harris WJ, et al. Cancer Res. 2000;60:3254–3261. [PubMed] [Google Scholar]
- 11.Scott AM, Lee F-T, Hopkins W, Cebon JS, Wheatley JM, Liu Z, Smyth FE, Murone C, Sturrock S, MacGregor D, et al. J Clin Oncol. 2001;19:3976–3987. doi: 10.1200/JCO.2001.19.19.3976. [DOI] [PubMed] [Google Scholar]
- 12.Welt S, Divgi CR, Scott AM, Garin-Chesa P, Finn RD, Graham M, Carswell EA, Cohen A, Larson SM, Old LJ. J Clin Oncol. 1994;12:1193–1203. doi: 10.1200/JCO.1994.12.6.1193. [DOI] [PubMed] [Google Scholar]
- 13.Scott AM, Wiseman G, Welt S, Adjei A, Lee FT, Hopkins W, Divgi CR, Hanson LH, Mitchell P, Gansen DN, et al. Clin Cancer Res. 2003;9:1639–1647. [PubMed] [Google Scholar]
- 14.Sugawa N, Ekstrand AJ, James CD, Collins VP. Proc Natl Acad Sci USA. 1990;87:8602–8606. doi: 10.1073/pnas.87.21.8602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong AJ, Bigner SH, Bigner DD, Kinzler KW, Hamilton SR, Vogelstein B. Proc Natl Acad Sci USA. 1987;84:6899–6903. doi: 10.1073/pnas.84.19.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishikawa R, Ji XD, Harmon RC, Lazar CS, Gill GN, Cavenee WK, Huang HJ. Proc Natl Acad Sci USA. 1994;91:7727–7731. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hills D, Rowlinson-Busza D, Gullick WJ. Int J Cancer. 1995;63:537–543. doi: 10.1002/ijc.2910630414. [DOI] [PubMed] [Google Scholar]
- 18.Sridhar SS, Seymour L, Shepherd FA. Lancet Oncol. 2003;4:397–406. doi: 10.1016/s1470-2045(03)01137-9. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 20.Humphrey PA, Wong AJ, Vogelstein B, Zalutsky MR, Fuller GN, Archer GE, Friedman HS, Kwatra MM, Bigner SH, Bigner DD. Proc Natl Acad Sci USA. 1990;87:4207–4211. doi: 10.1073/pnas.87.11.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, D'Andrea G, Seidman A, Norton L, Gunnett K, et al. J Clin Oncol. 2000;18:904–914. doi: 10.1200/JCO.2000.18.4.904. [DOI] [PubMed] [Google Scholar]
- 22.Graeven U, Kremer B, Sudhoff T, Kiling B, Rojo F, Weber D, Tillner J, Unal C, Schmiegel W. Br J Cancer. 2006;94:1293–1299. doi: 10.1038/sj.bjc.6603083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramos TC, Figueredo J, Catala M, Gonzales S, Selva JC, Cruz TM, Toldeo C, Silva S, Pestano Y, Ramos M, et al. Cancer Biol Ther. 2006;5:375–379. doi: 10.4161/cbt.5.4.2522. [DOI] [PubMed] [Google Scholar]
- 24.Johns TG, Stockert E, Ritter G, Jungbluth AA, Huang HJ, Cavenee WK, Smyth FE, Hall CM, Watson N, Nice EC, et al. Int J Cancer. 2002;98:398–408. doi: 10.1002/ijc.10189. [DOI] [PubMed] [Google Scholar]
- 25.Jungbluth AA, Stockert E, Huang HJ, Collins VP, Coplan K, Iversen K, Kolb D, Johns TG, Scott AM, Gullick WJ, et al. Proc Natl Acad Sci USA. 2003;100:639–644. doi: 10.1073/pnas.232686499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johns TG, Adams TE, Wittrup KD, Hall NE, Hoyne PA, Cochrane JR, Olsen MJ, Kim YS, Rothacker J, Nice EC, et al. J Biol Chem. 2004;279:30375–30384. doi: 10.1074/jbc.M401218200. [DOI] [PubMed] [Google Scholar]
- 27.Johns TG, Mellman I, Cartwright GA, Ritter G, Old LJ, Burgess AW, Scott AM. FASEB J. 2005;19:780–782. doi: 10.1096/fj.04-1766fje. [DOI] [PubMed] [Google Scholar]
- 28.Luwor RB, Johns TG, Murone C, Huang HJ, Cavenee WK, Ritter G, Old LJ, Burgess AW, Scott AM. Cancer Res. 2001;61:5355–5361. [PubMed] [Google Scholar]
- 29.Johns TG, Luwor RB, Murone C, Walker F, Weinstock J, Vitali AA, Perera RM, Jungbluth AA, Stockert E, Old LJ, et al. Proc Natl Acad Sci USA. 2003;100:15871–15876. doi: 10.1073/pnas.2036503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mishima K, Johns TG, Luwor RB, Scott AM, Stockert E, Jungbluth AA, Ji XD, Suvarna P, Voland JR, Old LJ, et al. Cancer Res. 2001;61:5349–5354. [PubMed] [Google Scholar]
- 31.Perera RM, Narita Y, Furnari FB, Tavernasi ML, Luwor RB, Burgess AW, Stockert E, Jungbluth AA, Old LJ, Cavenee WK, et al. Clin Cancer Res. 2005;11:6390–6399. doi: 10.1158/1078-0432.CCR-04-2653. [DOI] [PubMed] [Google Scholar]
- 32.Panousis C, Rayzman VM, Johns TG, Renner C, Liu Z, Cartwright G, Lee F-T, Wang D, Kypridis A, Smyth FE, et al. Br J Cancer. 2005;92:1069–1077. doi: 10.1038/sj.bjc.6602470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Divgi CR, Welt S, Kris M, Real FX, Yeh SD, Gralla R, Merchant B, Schweighart S, Unger M, Larson SM, et al. J Natl Cancer Inst. 1991;83:97–104. doi: 10.1093/jnci/83.2.97. [DOI] [PubMed] [Google Scholar]
- 34.Baselga J. Eur J Cancer. 2001;37(Suppl 4):S16–S22. doi: 10.1016/s0959-8049(01)00233-7. [DOI] [PubMed] [Google Scholar]
- 35.Gibson TB, Ranganathan A, Grothey A. Clin Colorectal Cancer. 2006;6:29–31. doi: 10.3816/CCC.2006.n.01. [DOI] [PubMed] [Google Scholar]
- 36.Rowinsky EK, Schwartz GH, Gollob JA, Thompson JA, Vogelzang NJ, Figlin R, Bukowski R, Haas N, Lockbaum P, Li YP, et al. J Clin Oncol. 2004;22:3003–3015. doi: 10.1200/JCO.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 37.Tan AR, Moore DF, Hidalgo M, Doroshow JH, Polpin EA, Goodin S, Mauro D, Rubin EH. Clin Cancer Res. 2006;12:6517–6522. doi: 10.1158/1078-0432.CCR-06-0705. [DOI] [PubMed] [Google Scholar]
- 38.Lacouture AE. Nat Rev Cancer. 2006;6:803–812. doi: 10.1038/nrc1970. [DOI] [PubMed] [Google Scholar]
- 39.Adams GP, Weiner LM. Nat Biotechnol. 2005;23:1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 40.Liu Z, Panousis C, Smyth FE, Murphy R, Wirth V, Cartwright G, Johns TG, Scott AM. Hybrid Hybridomics. 2003;22:219–228. doi: 10.1089/153685903322328947. [DOI] [PubMed] [Google Scholar]
- 41.Stabin MG, Sparks RB, Crowe E. J Nucl Med. 2005;46:1023–1027. [PubMed] [Google Scholar]
- 42.Ritter G, Cohen LS, Williams C, Jr, Richards EC, Old LJ, Welt S. Cancer Res. 2001;61:685–6859. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.