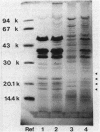
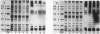Abstract
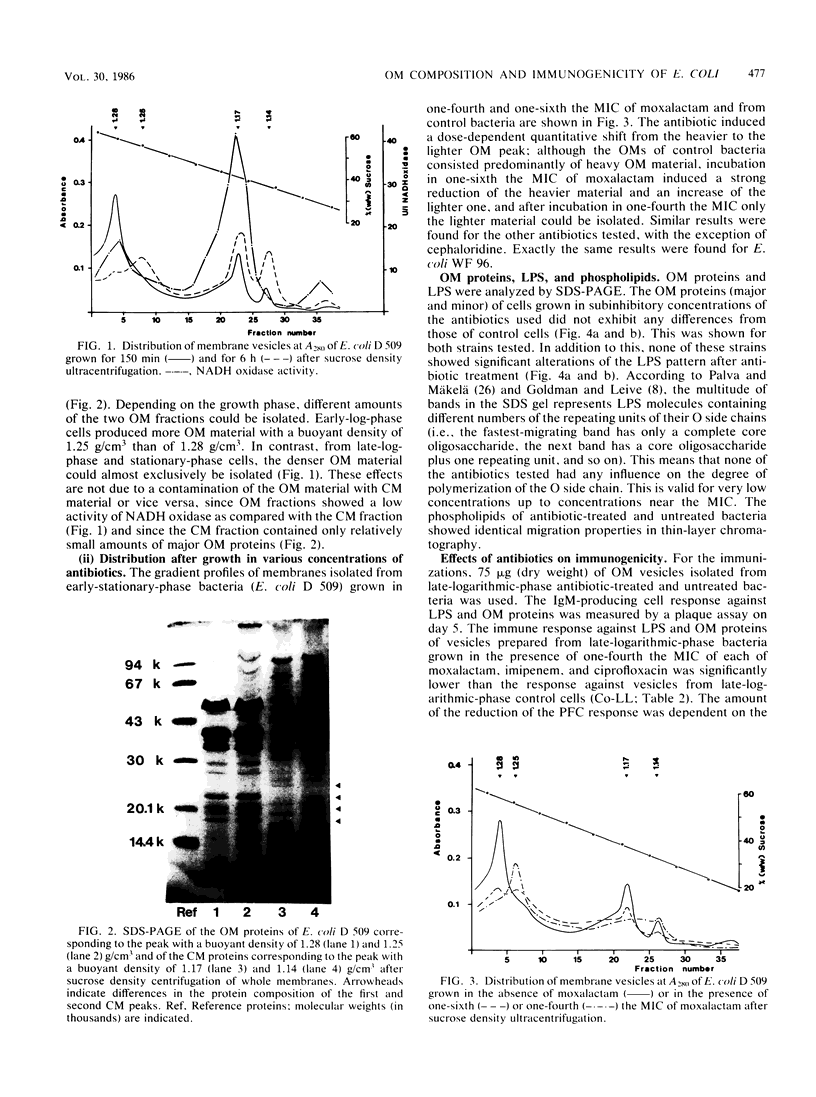
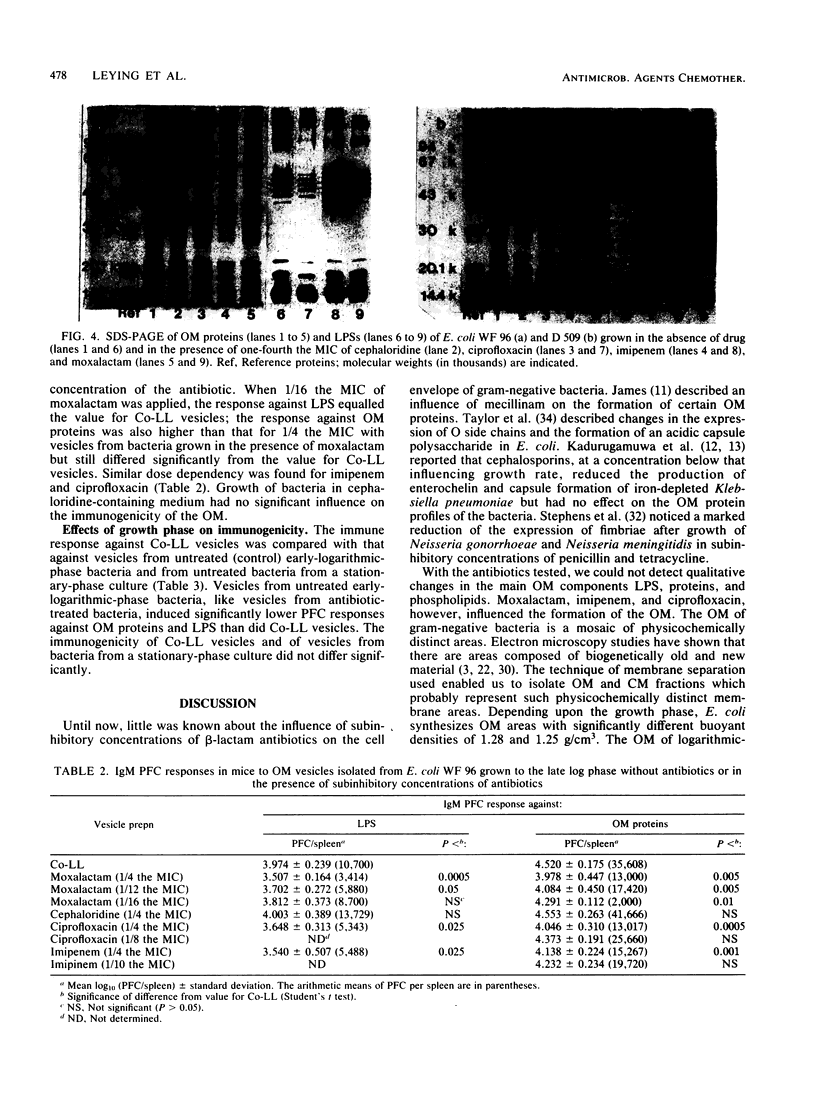
The effects of subinhibitory concentrations of different beta-lactam antibiotics and one quinolone on the sedimentation of outer membranes (OMs) of Escherichia coli and on the qualitative properties and immunogenicity of OM components were studied. Membranes were prepared by osmotic lysis of plasmolyzed bacteria. OM and cytoplasmic membrane vesicles were separated by sucrose density ultracentrifugation. Two peaks of OM vesicles with different buoyant densities could be isolated; the quantitative contribution of these to the total OM varied, depending upon the growth phase. In early log phase, the OM consisted mainly of lighter material; in late log and stationary phases, the OM consisted mainly of heavier material. Moxalactam, imipenem, and ciprofloxacin inhibited the formation of heavier material in all growth phases. The immunogenicity of OM vesicles was tested in mice by the hemolytic plaque test. The lighter OM material was markedly less immunogenic than the heavier OM material. The vesicles from antibiotic-treated bacteria and those from early-log-phase cells were less immunogenic than vesicles from untreated late-log-phase and stationary-phase bacteria. These changes were found for the immune response against lipopolysaccharides, as well as against OM proteins. Thus, the immunogenicity of OM components seems to be dependent upon the quantitative composition of lighter and heavier compounds, which is strongly influenced by growth phase and treatment with certain antibiotics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. G., Gregoriadis G. Liposomes as immunological adjuvants. Nature. 1974 Nov 15;252(5480):252–252. doi: 10.1038/252252a0. [DOI] [PubMed] [Google Scholar]
- Begg K. J., Doanachie W. D. Growth of the Escherichia coli cell surface. J Bacteriol. 1977 Mar;129(3):1524–1536. doi: 10.1128/jb.129.3.1524-1536.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Friedman H., Warren G. H. Antibody-mediated bacteriolysis: enhanced killing of cyclacillin-treated bacteria. Proc Soc Exp Biol Med. 1976 Nov;153(2):301–304. doi: 10.3181/00379727-153-39533. [DOI] [PubMed] [Google Scholar]
- Gemmell C. G., Peterson P. K., Schmeling D., Mathews J., Quie P. G. Antibiotic-induced modification of Bacteroides fragilis and its susceptibility to phagocytosis by human polymorphonuclear leukocytes. Eur J Clin Microbiol. 1983 Aug;2(4):327–334. doi: 10.1007/BF02019462. [DOI] [PubMed] [Google Scholar]
- Goding J. W. The chromic chloride method of coupling antigens to erythrocytes: definition of some important parameters. J Immunol Methods. 1976;10(1):61–66. doi: 10.1016/0022-1759(76)90007-7. [DOI] [PubMed] [Google Scholar]
- Goldman R. C., Leive L. Heterogeneity of antigenic-side-chain length in lipopolysaccharide from Escherichia coli 0111 and Salmonella typhimurium LT2. Eur J Biochem. 1980;107(1):145–153. doi: 10.1111/j.1432-1033.1980.tb04635.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb C. F. Application of transformations to normalize the distribution of plaque-forming cells. J Immunol. 1974 Jul;113(1):51–57. [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R. Identification of an outer membrane protein of Escherichia coli, with a role in the coordination of deoxyribonucleic acid replication and cell elongation. J Bacteriol. 1975 Nov;124(2):918–929. doi: 10.1128/jb.124.2.918-929.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Anwar H., Brown M. R., Zak O. Effect of subinhibitory concentrations of cephalosporins on surface properties and siderophore production in iron-depleted Klebsiella pneumoniae. Antimicrob Agents Chemother. 1985 Feb;27(2):220–223. doi: 10.1128/aac.27.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Anwar H., Brown M. R., Zak O. Protein antigens of encapsulated Klebsiella pneumoniae surface exposed after growth in the presence of subinhibitory concentrations of cephalosporins. Antimicrob Agents Chemother. 1985 Aug;28(2):195–199. doi: 10.1128/aac.28.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Gmeiner J., Nixdorff K. Alteration of the immunoglobulin G subclass responses in mice to lipopolysaccharide: effects of nonbacterial proteins and bacterial membrane phospholipids or outer membrane proteins of Proteus mirabilis. Infect Immun. 1983 Apr;40(1):157–165. doi: 10.1128/iai.40.1.157-165.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch H., Nixdorff K. Antibody-producing cell responses to an isolated outer membrane protein and to complexes of this antigen with lipopolysaccharide or with vesicles of phospholipids from Proteus mirabilis. Infect Immun. 1981 Mar;31(3):862–867. doi: 10.1128/iai.31.3.862-867.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kroll H. P., Bhakdi S., Taylor P. W. Membrane changes induced by exposure of Escherichia coli to human serum. Infect Immun. 1983 Dec;42(3):1055–1066. doi: 10.1128/iai.42.3.1055-1066.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mug-Opstelten D., Witholt B. Preferential release of new outer membrane fragments by exponentially growing Escherichia coli. Biochim Biophys Acta. 1978 Apr 4;508(2):287–295. doi: 10.1016/0005-2736(78)90331-0. [DOI] [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Eisenstein B. I., Alkan M. L., Sharon N. Suppression of bacterial adherence by subminimal inhibitory concentrations of beta-lactam and aminoglycoside antibiotics. Rev Infect Dis. 1979 Sep-Oct;1(5):832–837. doi: 10.1093/clinids/1.5.832. [DOI] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Palva E. T., Mäkelä P. H. Lipopolysaccharide heterogeneity in Salmonella typhimurium analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Eur J Biochem. 1980;107(1):137–143. doi: 10.1111/j.1432-1033.1980.tb04634.x. [DOI] [PubMed] [Google Scholar]
- Ruttkowski E., Nixdorff K. Qualitative and quantitative changes in the antibody producing cell response to lipopolysaccharide induced after incorporation of the antigen into bacterial membrane phospholipid vesicles. J Immunol. 1980 Jun;124(6):2548–2551. [PubMed] [Google Scholar]
- Ryter A., Shuman H., Schwartz M. Intergration of the receptor for bacteriophage lambda in the outer membrane of Escherichia coli: coupling with cell division. J Bacteriol. 1975 Apr;122(1):295–301. doi: 10.1128/jb.122.1.295-301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens D. S., Krebs J. W., McGee Z. A. Loss of pili and decreased attachment to human cells by Neisseria meningitidis and Neisseria gonorrhoeae exposed to subinhibitory concentrations of antibiotics. Infect Immun. 1984 Nov;46(2):507–513. doi: 10.1128/iai.46.2.507-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Houte A. J., Snippe H., Schmitz M. G., Willers J. M. Characterization of immunogenic properties of haptenated liposomal model membranes in mice. V. Effect of membrane composition on humoral and cellular immunogenicity. Immunology. 1981 Nov;44(3):561–568. [PMC free article] [PubMed] [Google Scholar]