Abstract
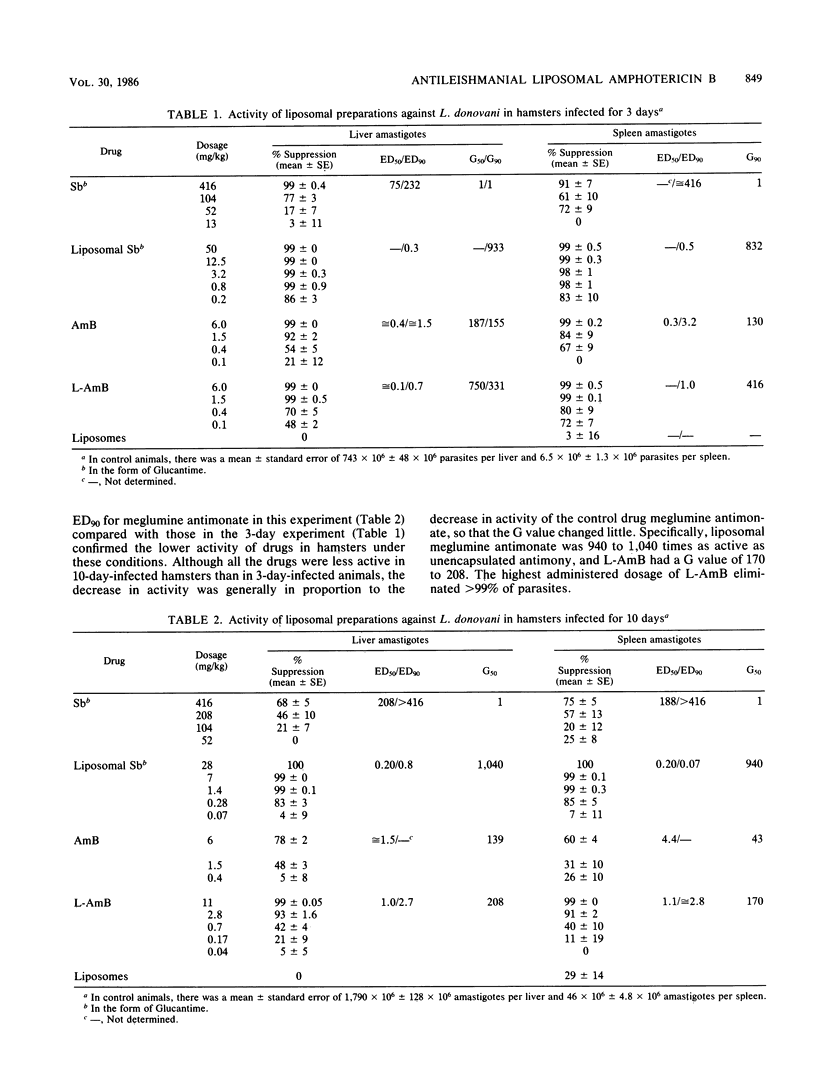
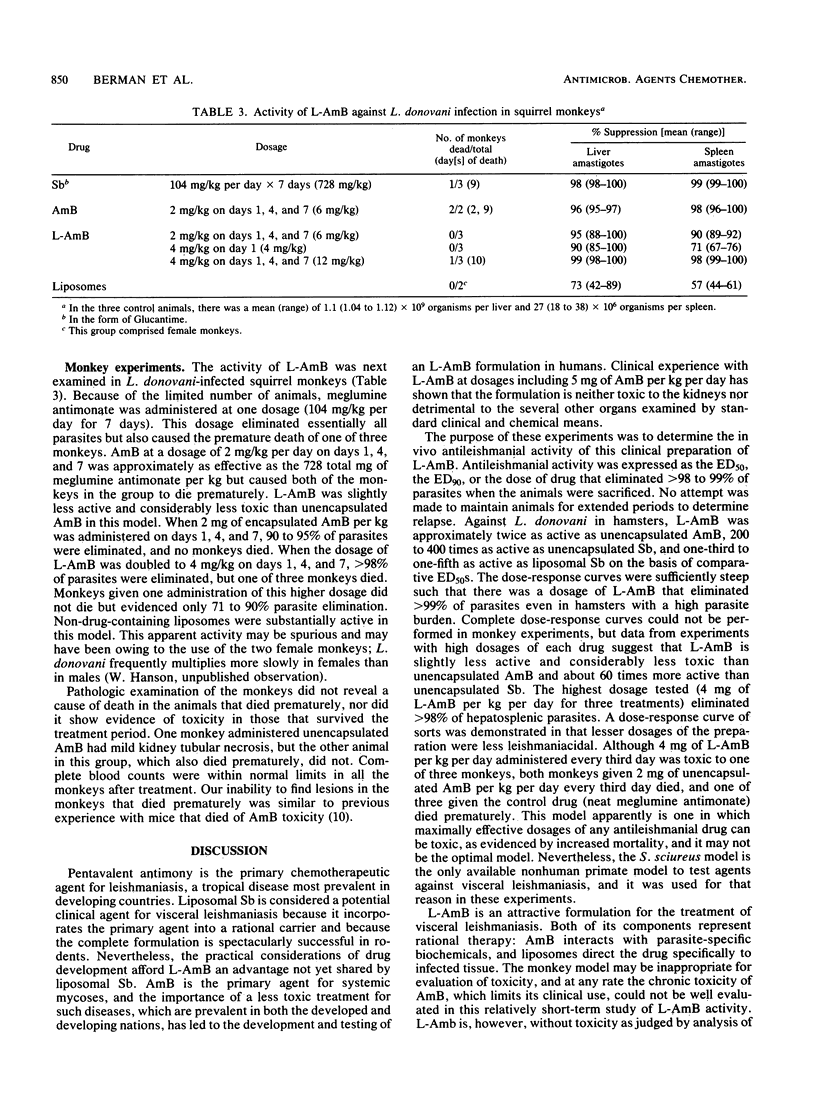
Visceral leishmaniasis (kala-azar) results from parasitization of the macrophages of the liver, spleen, and the rest of the visceral reticuloendothelial system with Leishmania donovani. Pentavalent antimony is the drug of choice for leishmaniasis chemotherapy; amphotericin B (AmB) is active but is rarely used, because of drug toxicity. AmB encapsulated within macrophage-directed carriers (liposomes) has been used to treat humans with systemic mycoses complicating neoplastic diseases; dosages of up to 5 mg of encapsulated AmB per kg per day for greater than 14 days are without apparent kidney or liver toxicity. In the present work, greater than 99% of L. donovani parasites were eliminated from the liver and spleen of infected hamsters by one administration of 1.5 to 11 mg of liposome-encapsulated AmB (L-Amb) per kg. A total of 98 to 99% of hepatosplenic parasites were eliminated from squirrel monkeys by three administrations of 4 mg of L-AmB per kg. L-AmB was 170 to 750 times as active as antimony in hamsters, and approximately 60 times as active as antimony in monkeys. The demonstration that apparently nontoxic human dosages of L-AmB eliminate essentially all hepatosplenic parasites in hamster and primate models suggests that this preparation should be considered for clinical trial against kala-azar.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alving C. R., Steck E. A., Chapman W. L., Jr, Waits V. B., Hendricks L. D., Swartz G. M., Jr, Hanson W. L. Therapy of leishmaniasis: superior efficacies of liposome-encapsulated drugs. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2959–2963. doi: 10.1073/pnas.75.6.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black C. D., Watson G. J., Ward R. J. The use of Pentostam liposomes in the chemotherapy of experimental leishmaniasis. Trans R Soc Trop Med Hyg. 1977;71(6):550–552. doi: 10.1016/0035-9203(77)90155-9. [DOI] [PubMed] [Google Scholar]
- Goad L. J., Holz G. G., Jr, Beach D. H. Sterols of Leishmania species. Implications for biosynthesis. Mol Biochem Parasitol. 1984 Feb;10(2):161–170. doi: 10.1016/0166-6851(84)90004-5. [DOI] [PubMed] [Google Scholar]
- Hanson W. L., Chapman W. L., Jr, Kinnamon K. E. Testing of drugs for antileishmanial activity in golden hamsters infected with Leishmania donovani. Int J Parasitol. 1977 Dec;7(6):443–447. doi: 10.1016/0020-7519(77)90004-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi G. S., Medoff G. Antifungal agents: recent developments. Annu Rev Microbiol. 1977;31:291–308. doi: 10.1146/annurev.mi.31.100177.001451. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Fainstein V., Hopfer R., Mehta K., Sullivan M. P., Keating M., Rosenblum M. G., Mehta R., Luna M., Hersh E. M. Liposomal amphotericin B for the treatment of systemic fungal infections in patients with cancer: a preliminary study. J Infect Dis. 1985 Apr;151(4):704–710. doi: 10.1093/infdis/151.4.704. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Hopfer R. L., Mehta R., Mehta K., Hersh E. M., Juliano R. L. Liposome-encapsulated amphotericin B for treatment of disseminated candidiasis in neutropenic mice. J Infect Dis. 1984 Aug;150(2):278–283. doi: 10.1093/infdis/150.2.278. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Kasi L., Rosenblum M. G., Haynie T., Jahns M., Glenn H., Mehta R., Mavligit G. M., Hersh E. M. Clinical pharmacology of 99mTc-labeled liposomes in patients with cancer. Cancer Res. 1984 Jan;44(1):375–378. [PubMed] [Google Scholar]
- Lopez-Berestein G., Mehta R., Hopfer R. L., Mills K., Kasi L., Mehta K., Fainstein V., Luna M., Hersh E. M., Juliano R. Treatment and prophylaxis of disseminated infection due to Candida albicans in mice with liposome-encapsulated amphotericin B. J Infect Dis. 1983 May;147(5):939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- Madindou T. J., Hanson W. L., Chapman W. L., Jr Chemotherapy of visceral leishmaniasis (Leishmania donovani) in the squirrel monkey (Saimiri sciureus). Ann Trop Med Parasitol. 1985 Feb;79(1):13–19. doi: 10.1080/00034983.1985.11811884. [DOI] [PubMed] [Google Scholar]
- New R. R., Chance M. L., Heath S. Antileishmanial activity of amphotericin and other antifungal agents entrapped in liposomes. J Antimicrob Chemother. 1981 Nov;8(5):371–381. doi: 10.1093/jac/8.5.371. [DOI] [PubMed] [Google Scholar]
- New R. R., Chance M. L., Thomas S. C., Peters W. Antileishmanial activity of antimonials entrapped in liposomes. Nature. 1978 Mar 2;272(5648):55–56. doi: 10.1038/272055a0. [DOI] [PubMed] [Google Scholar]
- Panosian C. B., Barza M., Szoka F., Wyler D. J. Treatment of experimental cutaneous leishmaniasis with liposome-intercalated amphotericin B. Antimicrob Agents Chemother. 1984 May;25(5):655–656. doi: 10.1128/aac.25.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay C., Barza M., Fiore C., Szoka F. Efficacy of liposome-intercalated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob Agents Chemother. 1984 Aug;26(2):170–173. doi: 10.1128/aac.26.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]


