Abstract
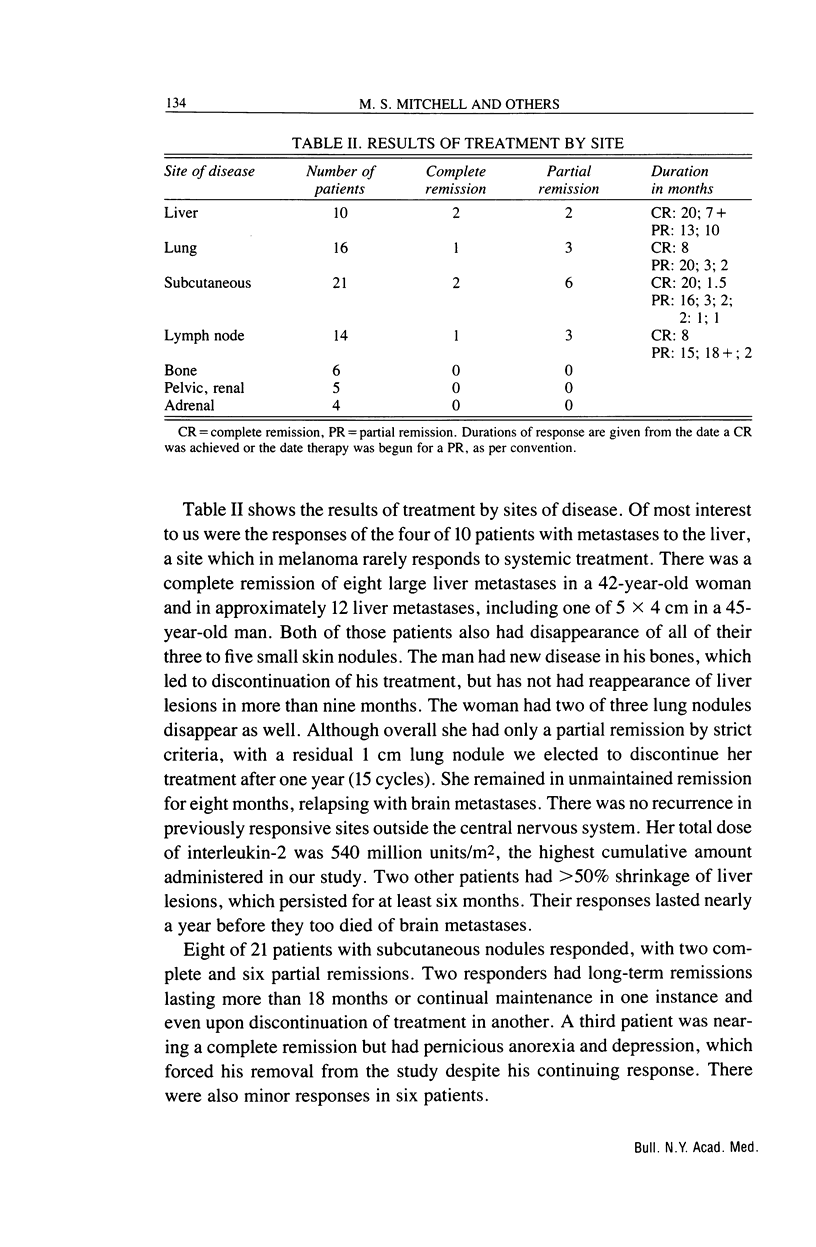
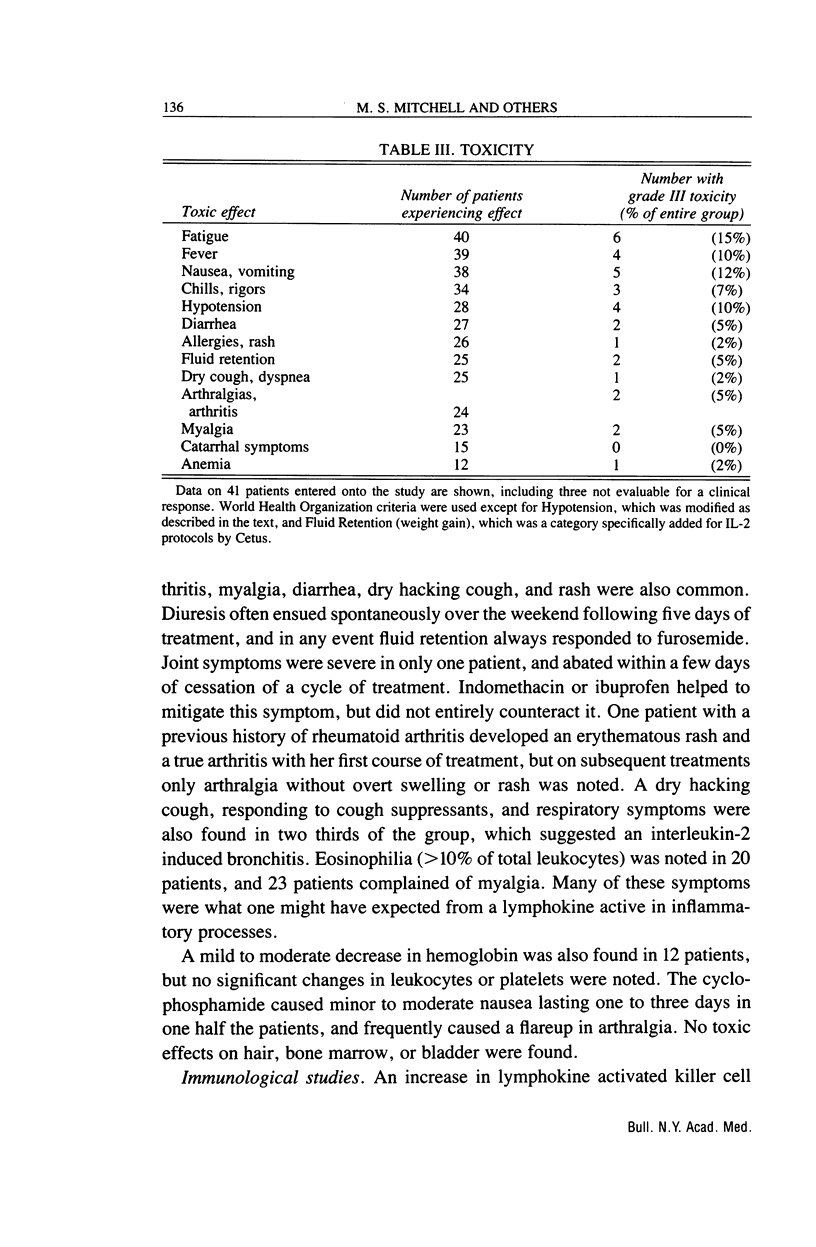
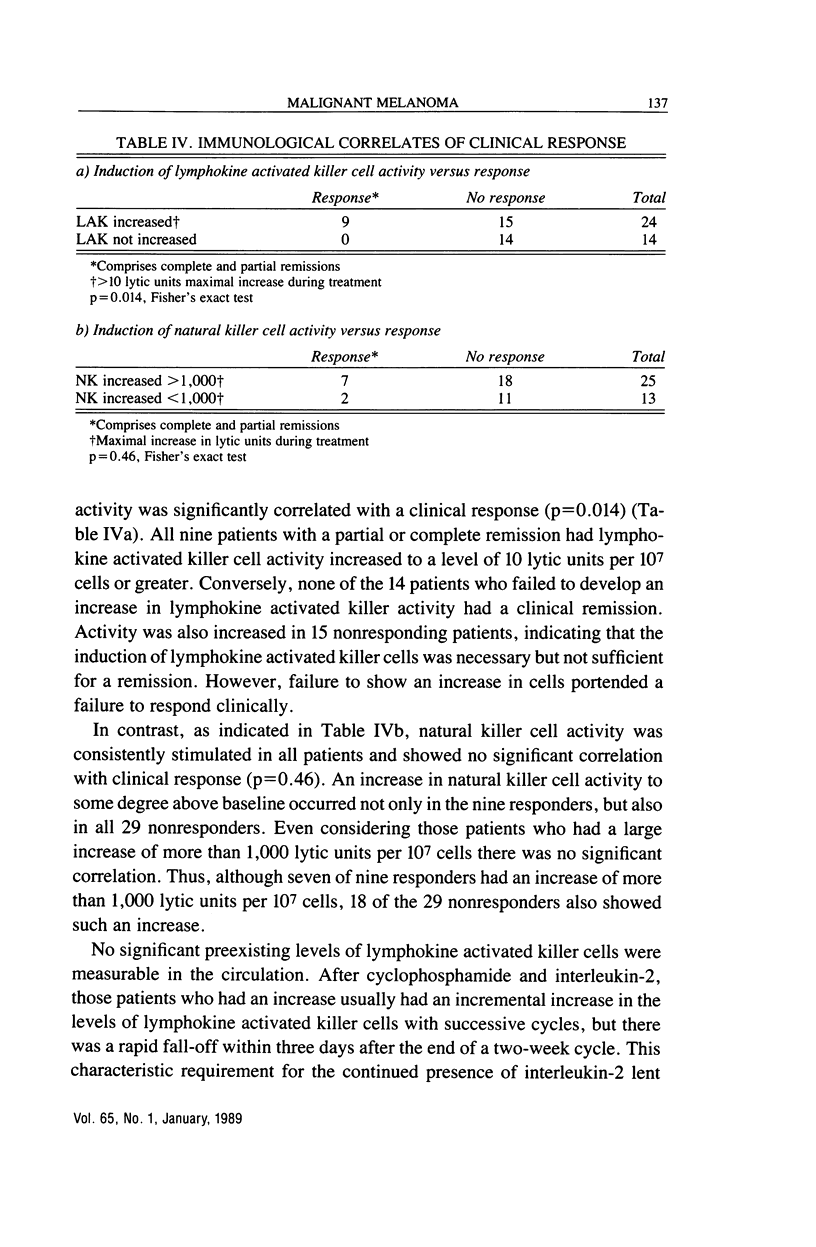
We have studied the effects of low-dose recombinant interleukin-2 preceded by low-dose cyclophosphamide on malignant melanoma. Thirty eight outpatients aged from 25 to 75 years were treated with interleukin-2, 3.6 million Cetus units/m2 i.v. daily for five days on two successive weeks beginning three days after 350 mg/m2 of intravenous cyclophosphamide. This schedule was repeated at least twice more with a one-week interval between cycles, usually at the same dosage level. Ten of the 38 patients (26.3%) had clinically significant remissions: two complete (5.3%), seven partial (18.4%), and one ongoing, long-term (greater than 18 mo) "minor" response (2.6%). Four others (10.5%) had shorter minor responses and four (10.5%) a mixed response. One patient with disease restricted to the skin had a complete remission, while the other patient with a complete remission had had three lung nodules and an enlarged hilar lymph node. It was gratifying that one of the major sites of disease responding to treatment was the liver. Two complete and two partial remissions (i.e., greater than 50% regressions for greater than four weeks at this site) were obtained in 10 patients with liver involvement. Lung metastases also responded in four of 16 patients (one complete and three partial remissions). Subcutaneous nodules responded in seven of 21 patients (two complete, five partial remissions), while lymph node metastases diminished significantly in four of 14 patients (one complete, three partial remissions). The median duration of response was nine months (range, 1.5-20 months), with four patients treated for more than one year. Toxicity was moderate and controllable, and only two patients required hospitalization, both overnight. Lymphokine activated killer cell activation was induced in 24 of 38 patients, including all nine of the major responders. Conversely, none of 14 patients without lymphokine activated killer cell activation had a significant clinical remission. This regimen appeared to be as effective in melanoma as those involving ex vivo activation of lymphokine activated killer cells, and was more tolerable than therapy with high doses of interleukin-2.
Full text
PDF













Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berd D., Maguire H. C., Jr, Mastrangelo M. J. Impairment of concanavalin A-inducible suppressor activity following administration of cyclophosphamide to patients with advanced cancer. Cancer Res. 1984 Mar;44(3):1275–1280. [PubMed] [Google Scholar]
- Berd D., Maguire H. C., Jr, Mastrangelo M. J. Potentiation of human cell-mediated and humoral immunity by low-dose cyclophosphamide. Cancer Res. 1984 Nov;44(11):5439–5443. [PubMed] [Google Scholar]
- Grimm E. A., Mazumder A., Zhang H. Z., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982 Jun 1;155(6):1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotze M. T., Chang A. E., Seipp C. A., Simpson C., Vetto J. T., Rosenberg S. A. High-dose recombinant interleukin 2 in the treatment of patients with disseminated cancer. Responses, treatment-related morbidity, and histologic findings. JAMA. 1986 Dec 12;256(22):3117–3124. [PubMed] [Google Scholar]
- Mitchell M. S., Kan-Mitchell J., Kempf R. A., Harel W., Shau H. Y., Lind S. Active specific immunotherapy for melanoma: phase I trial of allogeneic lysates and a novel adjuvant. Cancer Res. 1988 Oct 15;48(20):5883–5893. [PubMed] [Google Scholar]
- Pross H. F., Baines M. G., Rubin P., Shragge P., Patterson M. S. Spontaneous human lymphocyte-mediated cytotoxicity against tumor target cells. IX. The quantitation of natural killer cell activity. J Clin Immunol. 1981 Jan;1(1):51–63. doi: 10.1007/BF00915477. [DOI] [PubMed] [Google Scholar]
- Ribi E., Cantrell J. L., Takayama K., Qureshi N., Peterson J., Ribi H. O. Lipid A and immunotherapy. Rev Infect Dis. 1984 Jul-Aug;6(4):567–572. doi: 10.1093/clinids/6.4.567. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Chang A. E., Avis F. P., Leitman S., Linehan W. M., Robertson C. N., Lee R. E., Rubin J. T. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987 Apr 9;316(15):889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Lotze M. T., Muul L. M., Leitman S., Chang A. E., Ettinghausen S. E., Matory Y. L., Skibber J. M., Shiloni E., Vetto J. T. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N Engl J Med. 1985 Dec 5;313(23):1485–1492. doi: 10.1056/NEJM198512053132327. [DOI] [PubMed] [Google Scholar]
- Shou L., Schwartz S. A., Good R. A. Suppressor cell activity after concanavalin A treatment of lymphocytes from normal donors. J Exp Med. 1976 May 1;143(5):1100–1110. doi: 10.1084/jem.143.5.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg E., Lubera J. A. Cancer statistics, 1988. CA Cancer J Clin. 1988 Jan-Feb;38(1):5–22. doi: 10.3322/canjclin.38.1.5. [DOI] [PubMed] [Google Scholar]
- West W. H., Tauer K. W., Yannelli J. R., Marshall G. D., Orr D. W., Thurman G. B., Oldham R. K. Constant-infusion recombinant interleukin-2 in adoptive immunotherapy of advanced cancer. N Engl J Med. 1987 Apr 9;316(15):898–905. doi: 10.1056/NEJM198704093161502. [DOI] [PubMed] [Google Scholar]


