Abstract
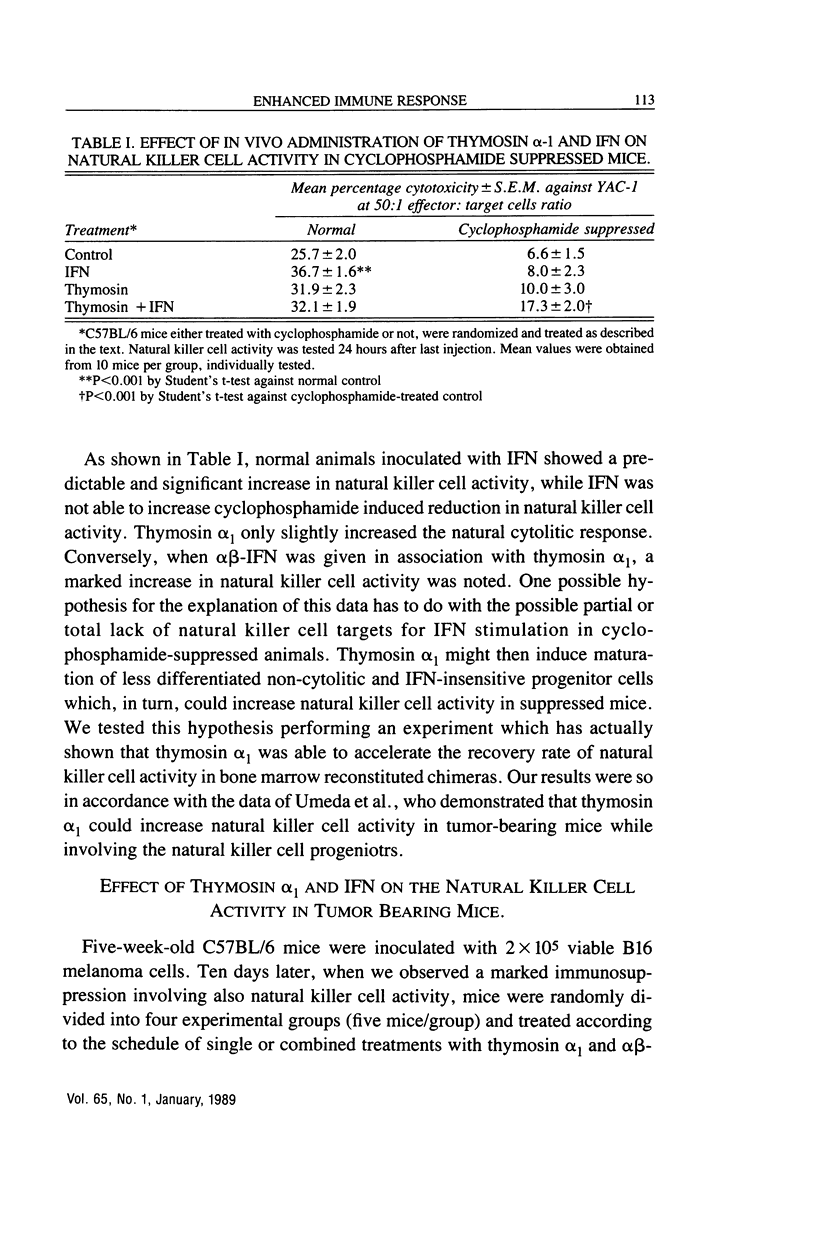
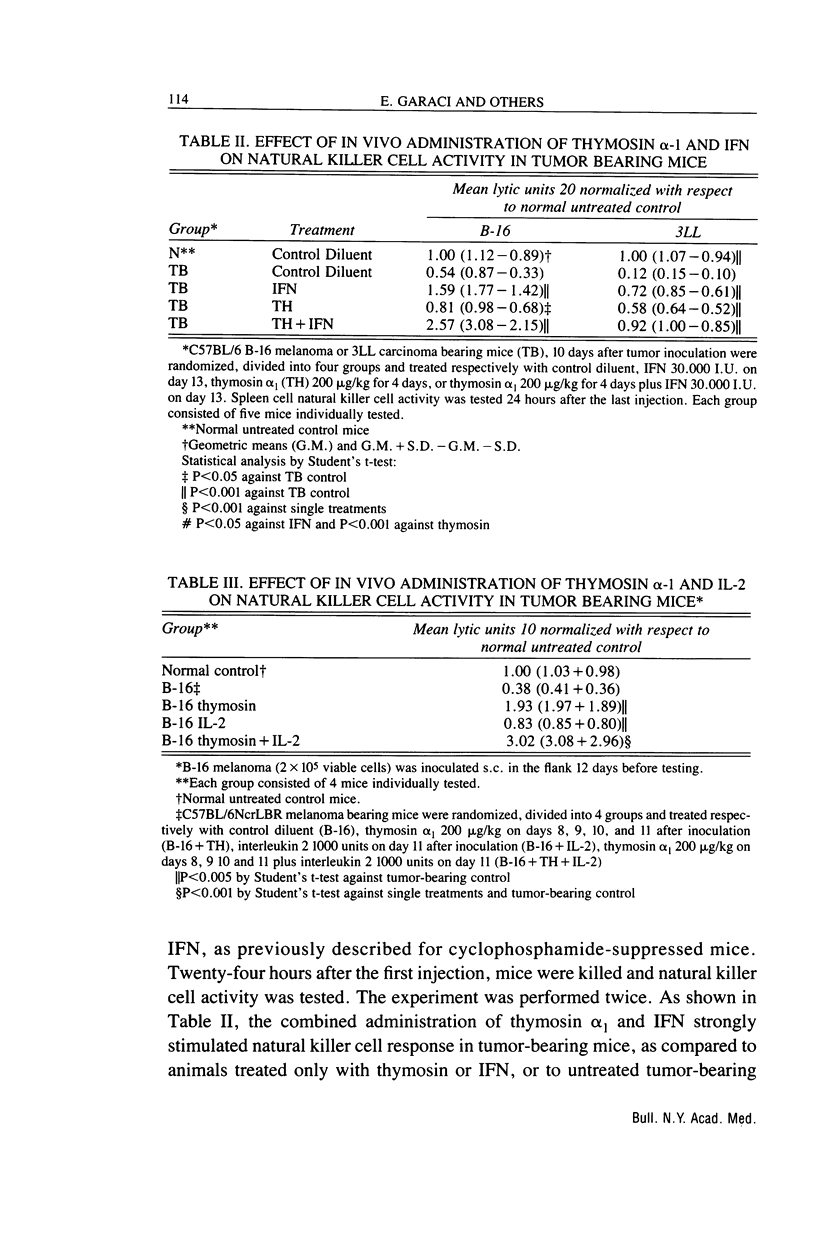
We have studied the effects of combination therapy with thymosin alpha 1 and IFN or IL-2 on natural killer cell activity in both normal and immunosuppressed animals after cyclophosphamide treatment and during B-16 melanoma and 3LL tumor growth. Our results suggest that while the combined treatment does not substantially modify the depressed natural killer cell response, thymosin alpha 1 pre-treatment significantly restores the boosting capacity of the two cytokines, IL-2 and IFN. Since thymosin alpha 1 proved capable of accelerating natural killer cell activity recovery in animals irradiated and reconstituted with symgenic marrow cells, we hypothesize that the synergistic effect between thymosin alpha 1 and IFN could result from the differentiation of natural killer cell lines by thymosin alpha 1 which can then become sensitive to IFN. Furthermore, we have demonstrated a good correlation between restoration of natural killer cell activity and regulation of tumor growth. Thus, these results may have important implications in tumor immunotherapy and patients with infectious diseases such as AIDS which is associated with low natural killer cell activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. W. Manipulation of host resistance in cancer therapy. Springer Semin Immunopathol. 1982;5(2):113–125. doi: 10.1007/BF00199791. [DOI] [PubMed] [Google Scholar]
- Borden E. C. Interferons: rationale for clinical trials in neoplastic disease. Ann Intern Med. 1979 Sep;91(3):472–479. doi: 10.7326/0003-4819-91-3-472. [DOI] [PubMed] [Google Scholar]
- Foon K. A., Smalley R. V., Riggs C. W., Gale R. P. The role of immunotherapy in acute myelogenous leukemia. Arch Intern Med. 1983 Sep;143(9):1726–1731. [PubMed] [Google Scholar]
- Garaci E., Mastino A., Jezzi T., Riccardi C., Favalli C. Effect of in vivo administration of prostaglandins and interferon on natural killer activity and on B-16 melanoma growth in mice. Cell Immunol. 1987 Apr 15;106(1):43–52. doi: 10.1016/0008-8749(87)90148-1. [DOI] [PubMed] [Google Scholar]
- Golub S. H., D'Amore P., Rainey M. Systemic administration of human leukocyte interferon to melanoma patients. II. Cellular events associated with changes in natural killer cytotoxicity. J Natl Cancer Inst. 1982 May;68(5):711–717. [PubMed] [Google Scholar]
- Hackett J., Jr, Tutt M., Lipscomb M., Bennett M., Koo G., Kumar V. Origin and differentiation of natural killer cells. II. Functional and morphologic studies of purified NK-1.1+ cells. J Immunol. 1986 Apr 15;136(8):3124–3131. [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R., Djeu J. Y., Holden H. T., Jett J., Lang N. P., Rubinstein M., Pestka S. Role of interferon in regulation of cytotoxicity by natural killer cells and macrophages. Ann N Y Acad Sci. 1980;350:63–71. doi: 10.1111/j.1749-6632.1980.tb20608.x. [DOI] [PubMed] [Google Scholar]
- Kalland T. Generation of natural killer cells from bone marrow precursors in vitro. Immunology. 1986 Apr;57(4):493–498. [PMC free article] [PubMed] [Google Scholar]
- Lala P. K., Santer V., Libenson H., Parhar R. S. Changes in the host natural killer cell population in mice during tumor development. 1. Kinetics and in vivo significance. Cell Immunol. 1985 Jul;93(2):250–264. doi: 10.1016/0008-8749(85)90132-7. [DOI] [PubMed] [Google Scholar]
- Parhar R. S., Lala P. K. Changes in the host natural killer cell population in mice during tumor development. 2. The mechanism of suppression of NK activity. Cell Immunol. 1985 Jul;93(2):265–279. doi: 10.1016/0008-8749(85)90133-9. [DOI] [PubMed] [Google Scholar]
- Quesada J. R., Reuben J., Manning J. T., Hersh E. M., Gutterman J. U. Alpha interferon for induction of remission in hairy-cell leukemia. N Engl J Med. 1984 Jan 5;310(1):15–18. doi: 10.1056/NEJM198401053100104. [DOI] [PubMed] [Google Scholar]
- Saito T., Welker R. D., Fukui H., Herberman R. B., Chirigos M. A. Development of hyporesponsiveness to augmentation of natural killer cell activity after multiple doses of maleic anhydride divinyl ether: association with decreased numbers of large granular lymphocytes. Cell Immunol. 1985 Feb;90(2):577–589. doi: 10.1016/0008-8749(85)90222-9. [DOI] [PubMed] [Google Scholar]
- Serrate S. A., Schulof R. S., Leondaridis L., Goldstein A. L., Sztein M. B. Modulation of human natural killer cell cytotoxic activity, lymphokine production, and interleukin 2 receptor expression by thymic hormones. J Immunol. 1987 Oct 1;139(7):2338–2343. [PubMed] [Google Scholar]
- Spina C. A., Fahey J. L., Durkos-Smith D., Dorey F., Sarna G. Suppression of natural killer cell cytotoxicity in the peripheral blood of patients receiving interferon therapy. J Biol Response Mod. 1983;2(5):458–469. [PubMed] [Google Scholar]


