Abstract
Gap junctions are regarded as the primary pathway underlying propagation of Ca2+ waves between astrocytes, although signaling through extracellular space may also contribute. Results obtained from astrocytes cultured from sibling Cx43 knockout (KO) and wild-type (WT) mice in six litters showed that Ca2+ waves propagated more slowly in Cx43 KO than in WT astrocytes; however, because this difference in velocity was only seen in conditions where cell confluence was higher in WT than KO astrocytes, it is attributable to differences in plating density. By contrast, density-independent differences were observed in the amplitudes of the Ca2+ responses (15% smaller in KO astrocytes) and efficacy of spread (to 14% fewer cells in KO astrocytes). Blockade of purinergic receptors with suramin reduced the velocities of the waves by 40% in WT and KO astrocytes and reduced the amplitudes by 20% and 6%, respectively. In the presence of heptanol, Ca2+ waves spread to only 30% of the cells, with a 70% reduced velocity and 30% reduced amplitude. It is concluded that the propagation of Ca2+ waves between astrocytes from Cx43 KO mice is not so greatly affected as expected by deletion of the major gap junction protein between these cells. The residual 5% coupling contributed by the additional connexins (Cx40, Cx45, and Cx46) expressed in KO astrocytes still suffices to provide a more substantial portion of Ca2+ wave propagation than does signaling through extracellular purinergic pathways. These studies demonstrate that, even with severely reduced junctional conductance, Cx43 KO astrocytes are capable of performing long-range Ca2+ wave signaling, perhaps preserving one mechanism critical to neural function.
Keywords: gap junctions, intercellular communication, connexins, ATP receptors, suramin, heptanol
INTRODUCTION
The participation of astrocytes in brain function as an integrated and a coordinated syncytium is believed to rely on the presence of intercellular gap junction channels, which can provide a pathway through which signaling molecules, such as cAMP, Ca2+ and IP3 spread between cells (see Saez et al., 1993). Gap junctions are constituted by two hemichannels each formed of six connexin subunits (Bennett et al., 1991). Of the 14 connexins so far identified in mammals, connexin43 (Cx43) is believed to be the major protein constituent of gap junctions in astrocytes (Dermietzel et al., 1989, 1991; Giaume et al., 1991; Nagy et al., 1992), although recent studies indicate a minor contribution of other connexins (Cx40, Cx45, and Cx46) to junctional communication in these cells (Dermietzel, 1996; Spray, 1996; Spray et al., 1998).
Among the several proposed functions of gap junctions, such as the dissipation and homeostasis of K+ ions (Kuffler and Nichols, 1966), control of cell proliferation (Naus et al., 1996), regulation of cell volume (Kimelberg and Kettenmann, 1990; Bender et al., 1993), and the propagation of intercellular Ca2+ waves between astrocytes, the last has been considered the mechanism by which cooperative cell activity is coordinated (Sanderson, 1996). Evidence implicating gap junctions in the spread of calcium waves has included studies on Cx43 transfected C6 glioma cells in which expression of Cx43 protein resulted in the ability of the cells to exhibit dye coupling and to propagate Ca2+ waves (Zhu et al., 1991; Charles et al., 1992) and the demonstration that treatment of cells with compounds that reduce gap junction conductance (halothane and octanol) inhibited intercellular wave propagation (Saez et al., 1989). Whereas it is well established that gap junctions participate in Ca2+ wave propagation in astrocytes and other cell types, an extracellular signaling component was initially shown to mediate Ca2+ signaling in mast cells (Osipchuk and Cahalan, 1992) and has been recently proposed to be necessary for the maximal propagation of astrocytic calcium waves in confluent cultures (Hassinger et al. 1996).
This paper describing the properties of mechanically induced calcium waves between Cx43 knockout astrocytes shows that gap junction channels contribute substantially to the propagation of calcium waves between these glial cells. The results revealed that the residual 5% coupling contributed by Cx40, Cx45, and Cx46 between knockout astrocytes is sufficient to provide a substantial portion of calcium wave propagation, which spreads with similar velocity, amplitude, and to a similar number of cells as do such waves in wild-type astrocytes.
MATERIAL AND METHODS
Cell Culture
Purified primary astrocyte cell cultures were obtained from whole brain tissue that was dissected from neonatal mice (GJA1M1 strain, obtained from Jackson Laboratories, Bar Harbor, ME). After trypsinization (0.1% trypsin at 37°C), the dissociated cells were plated in imaging dishes (Glass bottomed microwells, model 15, MakTek Co.) containing Dulbecco’s essential culture medium supplemented with 45% (vol/vol) Ham’s F12, 10% (vol/vol) fetal calf serum, penicillin (50 μg/ml), streptomycin (50 μg/ml), and glutamine (2 mM), buffered to pH 7.3 with 21 mM sodium bicarbonate. Cells were maintained in 5% CO2, 95% air atmosphere at 37°C, and 100% humidity. About 90% of the cells were immunopositive for glial acidic fibrillary protein (GFAP). Astrocyte cultures derived from brains of wild-type (WT) and Cx43 knockout (KO) littermates were established in parallel. Confocal microscopy experiments were performed on low, medium, and high density cultures of astrocytes.
Confocal Microscopy
Intracellular calcium measurements
Astrocytes plated on glass-bottomed microwells were loaded with Indo-1-AM (10 μM; Molecular Probes, Eugene, OR) at 37°C for 45 min, after which they were rinsed and used for confocal microscopy. Intracellular Ca2+ was measured in loaded astrocytes bathed in a solution containing 140 mM NaCl, 4 mM KCl, 2 mM CaCl2, and 5 mM HEPES (pH 7.3). The ratio of Indo-1 fluorescence intensity emitted at two wavelengths (390–440 nm and >440 nm) was imaged using UV laser excitation at 351 nm. Ratio images were continuously acquired at 0.5 or 1 Hz after background and shading correction using a Nikon real time confocal microscope (RCM 8000) with UV large pinhole and Nikon 40× water immersion objective (N.A. 1.15; working distance 0.2 mm). Indo-1 fluorescence ratio images were continuously acquired before and 1–2 min after the induction of intercellular calcium waves (see below). The ratiometric images were saved on an optical magnetic disk recorder as the average of 16 or 32 frames and then played back for measurements of changes in calcium level using Polygon-Star software (Nikon). The gray levels (number of pixels per area) within the regions of interest (circular spots with radii of 6.41 μm, containing about 200 pixels) were averaged and then used for analysis.
Velocity, amplitude, and efficacy of calcium wave spread
Calcium waves between cultures of Indo-1-AM loaded astrocytes were evoked by mechanical stimulation of one cell in the confocal field (171 × 128 μm; 21,888 μm2) using a glass pipette with a 1–2 μm outer diameter. The velocities of calcium waves were calculated as the distance (μm) between the stimulated and the non-stimulated cells divided by the time interval (s) between the half-maximal calcium increases within the stimulated and responding cells. Half-maximal calcium increases were obtained from sigmoidal curves fitted to the ascending phase of each Indo-1 fluorescence ratio increase using Origin 3.01 software (see Fig. 1D).
Fig. 1.

Analysis of properties of calcium waves spreading between cultured astrocytes. A,B: A three-dimensional representation of the images of Indo-1-AM loaded astrocytes acquired with a Nikon RCM-8000 confocal microscope. The positions (in μm) and the temporal changes in fluorescence ratio of loaded astrocytes are shown before (A) and after (B) applying a mechanical stimulus (at time 14 sec) to one cell (located at position 0 μm within the confocal field). C: The time course of Indo-1 fluorescence ratio changes observed in the stimulated (cell A) and responding cells (cells B,D,E,H, and I) that are shown in part (B). D: Sigmoidal curves with equation in inset fitted to the ascending portions of Indo-1 fluorescence ratio changes and the half maximal (EC50) values calculated using Origin 3.0 software. The time interval (tE-tA) between the two half-maximal (EC50) responses of the stimulated (cell A) and a responding cell (cell E) was used to calculate the velocity of calcium wave propagation between the two cells.
Amplitudes of calcium waves were considered to be the maximal increments in intracellular calcium observed in responding cells, calculated for each cell as the value of Indo-1 fluorescence ratio rise at the peak of the response divided by the basal fluorescence ratio value acquired before the induction of the calcium wave.
The efficacy of calcium spread between glial cells is reported here as the proportion of cells responding with an intracellular calcium increase during the propagation of the wave in relation to the total number of cells within the field of view.
Contribution of Extracellular Signaling and Intercellular Communication to the Propagation of Calcium Waves
The contribution of ATP-mediated calcium waves between cultured astrocytes was evaluated by exposing sibling cultures of WT and Cx43 KO astrocytes to 50 μM suramin (a purinergic P2 antagonist, see King et al., 1996; Bolego et al., 1997) and comparing the velocities, amplitudes, and efficacies of calcium spread with those of untreated cultures.
Heptanol, a potent gap junction channel blocker in astrocytes (Dermietzel et al., 1991), was bath applied (3 mM final concentration) to astrocytes cultured from both WT and Cx43 KO siblings in order to measure the effects of gap junction blockade on calcium wave spread between these glial cells.
RESULTS
Properties of Calcium Waves Between WT and Between Cx43 KO Astrocytes
The properties of calcium spread between cultured whole brain astrocytes derived from six litters of WT and Cx43 KO mice were analyzed in terms of velocity, amplitude, and efficacy of the spread.
When one cell in the confocal field was mechanically stimulated, there was a rapid increase in Indo-1 fluorescence ratio, indicating the rise in intracellular calcium levels. Within a few seconds after stimulation of one cell, the majority of the cells present in the confocal field displayed increases in intracellular calcium levels (see Fig. 1). The velocity with which this phenomenon propagated from the stimulated cell to the neighbors was variable, ranging from 2.1 to 53.5 μm/sec for WT astrocytes and from 0.1 to 47 μm/sec for Cx43 KO cells. The amplitudes of intracellular calcium changes in the responding cells also varied, from 1.03- to 3.34-fold from basal levels in WT and from 1.01- to 2.08-fold in Cx43 KO astrocytes. This variability in velocities and amplitudes of the wave was not correlated with the distance of responding cells from the stimulated one, i.e., the velocity and amplitude of calcium wave spreading to the first order cells (located at about 20 μm from the stimulated cell) was as variable as the spread to the third order astrocytes (located at about 65 μm from the stimulus) (Fig. 2). These results indicate that, within the limits of the confocal field (171 × 121 μm), the spread of calcium waves between WT and between Cx43 KO astrocytes was non-uniform.
Fig. 2.
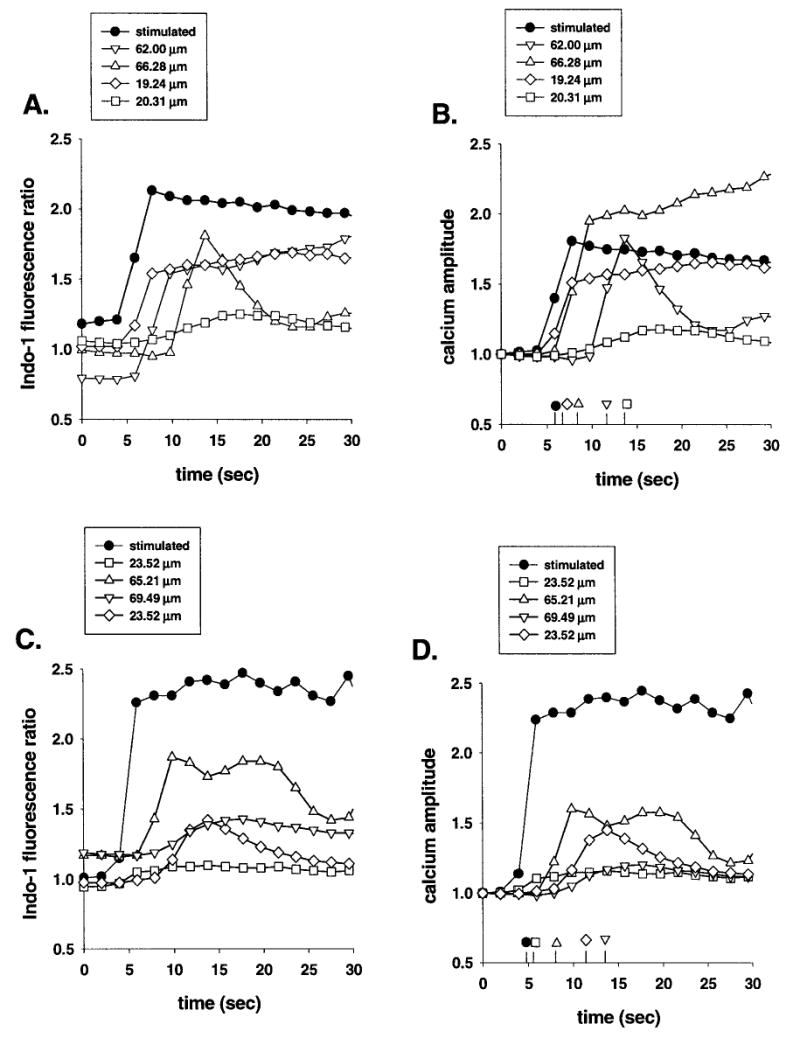
Propagation of calcium waves between cultured wild-type (A,B) and between Cx43 knockout (C,D) astrocytes. Indo-1 fluorescence ratio (A,C) and intracellular calcium amplitude changes (B,D) recorded from cells located at about 20 μm (squares and diamond symbols) and at 65 μm (up and down triangles) away from a mechanically stimulated cell (black circle). Note that in both cultures of WT and KO astrocytes, the two cells located at about 20 μm or the ones at 65 μm apart from the stimulated cell display different intracellular calcium level changes as well as different latencies of responses, indicating that calcium waves propagate from the point of stimulation without uniform velocities and amplitudes over distance.
The analysis of the overall data obtained from astrocytes of the six litters of WT and Cx43 KO mice revealed that the deletion of Cx43 gene by homologous recombination resulted in decreased velocity, amplitude, and efficacy of calcium wave spread (see last rows of Table 1). However, when the properties of calcium waves between individual paired sibling WT and Cx43 KO astrocytes are compared (Table 1), a totally different picture emerges, indicating that the properties of calcium waves between astrocytes are only slightly altered by Cx43 deletion.
TABLE 1.
Properties of calcium waves between astrocytesa
| Litters | Velocity (μm/sec) | Probability | Amplitude (fold) | Probability | Efficacy (number of cells) | N | |
|---|---|---|---|---|---|---|---|
| A | WTH | 20.02 ± 1.22 | 0.0326 | 1.52 ± 0.04 | 0.595 | 0.98 ± 0.02 | 98 |
| KOM | 16.50 ± 1.22 | 1.48 ± 0.04 | 1.00 ± 0.00 | 62 | |||
| B | WTM | 15.10 ± 1.22 | 0.0192 | 1.67 ± 0.01 | 0.0061 | 0.95 ± 0.03 | 29 |
| KOL | 10.80 ± 1.55 | 1.38 ± 0.05 | 0.75 ± 0.10 | 24 | |||
| C | WTM | 18.10 ± 1.13 | 0.0001 | 2.16 ± 0.06 | 0.0007 | 0.91 ± 0.07 | 89 |
| KOL | 10.60 ± 1.16 | 1.74 ± 0.08 | 0.89 ± 0.05 | 35 | |||
| D | WTH | 19.86 ± 1.54 | 0.9236 | 2.22 ± 0.05 | 0.0001 | 0.92 ± 0.08 | 29 |
| KOH | 20.08 ± 1.75 | 1.87 ± 0.06 | 1.00 ± 0.00 | 24 | |||
| E | WTM | 11.75 ± 1.18 | 0.6417 | 1.61 ± 0.05 | 0.4625 | 1.00 ± 0.00 | 14 |
| KOM | 15.60 ± 3.71 | 1.54 ± 0.09 | 0.53 ± 0.28 | 11 | |||
| F | WTM | 14.37 ± 1.87 | 0.3500 | 1.54 ± 0.10 | 0.6847 | 1.00 ± 0.00 | 15 |
| KOM | 15.52 ± 3.05 | 1.60 ± 0.11 | 0.88 ± 0.01 | 14 | |||
| A–F | WT | 18.80 ± 0.74 | 0.0001 | 1.81 ± 0.04 | 0.0001 | 0.94 ± 0.03 | 216 |
| KO | 12.1 ± 0.79 | 1.54 ± 0.03 | 0.80 ± 0.05 | 146 | |||
Velocities, amplitudes and efficacies of calcium wave spread between cells was obtained from six sibling pairs of wild type (WT) and Cx43 knockout (KO) (litters A–F). The probability values obtained from one way analysis of variance used to compare the responses of WT and KO cells of each litters are shown only for the velocities and amplitudes of calcium waves. The uppercase H (high), M (medium), and L (low) refer to cell culture densities. The bottom rows show the calcium wave property values calculated for data combined for all six littermate WT and KO astrocytes. N corresponds to the number of cells from which the velocity and amplitude data (mean ± standard error) were obtained.
The velocity of calcium wave propagation calculated for all the six litters of WT astrocytes (18.80 ± 0.74 μm/sec) was significantly higher than between Cx43 KO astrocytes (12.1 ± 0.79 μm/s). However, comparison of sibling littermates showed that the propagation velocities were significantly reduced in only three out of six litters (A–C but not D–F; Table 1) in Cx43 KO astrocytes. Because Cx43 KO astrocytes from litters A, B, and C were evaluated in lower density than their WT siblings, the observed decreases in velocity may be due to this difference in cell culture density. This hypothesis is supported by the fact that in cultures of cells from WT and KO littermates (litters D, E, and F), which were cultured at comparable degrees of confluence, calcium waves travelled between WT and between KO astrocytes with similar velocities (Table 1).
The analysis of the overall data from the six littermates also indicated that the calcium waves travelling through the Cx43 KO astrocytic network had a significantly larger amplitude attenuation than the waves spreading through WT cells (Table 1). In responding WT astrocytes, intracellular calcium rose 1.81 ± 0.04 fold during the spread of calcium waves while a 1.54 ± 0.03 fold increment of intracellular Ca2+ occurred in Cx43 KO astrocytes (Table 1). However, such attenuation in calcium wave amplitude was observed to be statistically significant in only three of six sibling littermates (litters B, C, and D but not A, E, and F in Table 1). In contrast to the velocities of calcium waves discussed above, density of cell cultures did not account for the differences in the amplitude attenuation between litters. Such behavior may be related to differences involving other steps responsible for the generation of intracellular calcium responses.
The analysis of data from astrocytes from WT and Cx43 KO siblings surprisingly showed that the efficacy of calcium spread (measured as the proportion of responding cells relative to the number of cells present in the confocal field) was not statistically different in any of the six litters studied (Table 1). Although analysis performed combining data from all six litters revealed a significant decrease in responding cells in KO cultures, the difference in efficacy of calcium spread between WT and between Cx43 KO astrocytes was not as high as expected for the deletion of gene expressing the major protein forming the astrocytic gap junctions. In cultures of WT astrocytes, 94% of the cells present in the confocal microscope field participated in the propagation of calcium waves, while 80% of the cells from Cx43 KO mice displayed intracellular calcium changes after mechanical stimulation of one cell (Table 1).
Contribution of Extracellular Signaling and Intercellular Communication to the Propagation of Calcium Waves
It has been reported that extracellular signals contribute to the propagation of calcium waves between astrocytes (Enkvist and McCarthy, 1992; Hassinger et al., 1996) and in some other cell types, an ATP-dependent and gap junction-independent signaling mechanism has been identified as an extracellular component for the process of intercellular calcium spread (Osipchuk and Cahalan, 1992; Schlosser et al., 1996; Cao et al., 1997).
An extracellular, ATP-dependent, signaling component in the propagation of calcium waves is here reported to be present in mouse astrocytes. Suramin, a purinergic receptor antagonist (King et al., 1996; Centemeri et al., 1997; Reetz et al., 1997), reduced both the velocity and the amplitude but did not affect the efficacy of calcium wave spread between WT and Cx43 KO astrocytes (Table 2). The addition of 50 μM suramin to cultures of WT astrocytes reduced the velocity of calcium waves from 17.22 ± 1.25 μm/sec to 10.66 ± 0.86 μm/sec and reduced the amplitude of the calcium response from 2.02 ± 0.06 fold to 1.61 ± 0.04 fold. In cultured Cx43 KO astrocytes, the velocity of calcium waves was reduced from 15.56 ± 2.31 μm/sec to 8.92 ± 0.91 μm/sec after blocking the purinergic receptors with suramin; under the same circumstances, intracellular calcium increments were attenuated from 1.72 ± 0.05 to 1.66 ± 0.10-fold. Blockade of this extracellular component, however, did not affect the efficacy of calcium wave spread; 98% of WT and 83% of Cx43 KO astrocytes present in the confocal fields participated in the propagation of the waves after the addition of suramin.
TABLE 2.
Effects of suramin and heptanol on the properties of calcium waves between astrocytesa
| Litters | Velocity (μm/sec) | Probability | Amplitude (folds) | Probability | Efficacy (number of cells) | N |
|---|---|---|---|---|---|---|
| 17.22 ± 1.25 | 0.0001 | 2.02 ± 0.06 | 0.0001 | 0.94 ± 0.06 | 43 | |
| 10.66 ± 0.86 | 1.61 ± 0.04 | 0.86 ± 0.07 | 103 | |||
| 15.56 ± 2.31 | 0.0062 | 1.72 ± 0.05 | 0.046 | 0.83 ± 0.10 | 25 | |
| 8.92 ± 0.91 | 1.66 ± 0.10 | 0.92 ± 0.03 | 89 | |||
| 19.86 ± 1.54 | 0.0001 | 2.22 ± 0.05 | 0.0001 | 0.94 ± 0.067 | 29 | |
| 5.02 ± 1.20 | 1.30 ± 0.13 | 0.25 ± 0.10 | 15 | |||
| 18.40 ± 1.59 | 0.019 | 1.77 ± 0.06 | 0.0001 | 0.95 ± 0.03 | 38 | |
| 9.90 ± 2.52 | 1.22 ± 0.09 | 0.38 ± 0.21 | 11 |
Velocities, amplitudes, and efficacies of calcium waves spread between cells were measured from siblings in three litters (D, E, F) of wild type (WT) and Cx43 knockout (KO) mice. The cells from littermates D–F are the same as shown in Table 1. The probability values obtained from one way analysis of variance used to compare the effects of the two treatments are shown only for the velocities and amplitudes of calcium waves. The lowercase c, s, and h correspond to experiments performed under control conditions and after suramin and heptanol treatments, respectively. N corresponds to the number of cells from which the velocity and amplitude data (mean ± standard error) were obtained.
Heptanol, which has been shown to reversibly block astrocyte gap junction channels (Dermietzel et al., 1991), strongly affected the efficacy of calcium wave spread, reducing by 75% the number of cells involved in calcium wave propagation between WT as well as between Cx43 KO astrocytes (Table 2). The remaining active population of WT cells propagated the waves at a velocity of 5.02 ± 1.2 μm/sec with an attenuated amplitude (1.30 ± 0.13-fold increment in Ca2+ level), values much lower than those observed under control conditions (19.86 ± 1.54 μm/sec and 2.22 ± 0.05 fold, respectively) (Table 2, Fig. 3). In Cx43 KO astrocytes, the velocity of calcium waves between responding cells was reduced from 18.40 ± 1.59 to 9.90 ± 2.52 μm/s and intracellular Ca2+ increments decreased from 1.77 ± 0.06- to 1.22 ± 0.09-fold in the presence of heptanol (Table 2. Fig. 3).
Fig. 3.

Heptanol blockade of calcium waves spreading between WT and between Cx43 KO astrocytes. Three-dimensional representation of images acquired in the confocal microscope from Indo-1-AM loaded WT (A,B) and KO (C,D) astrocytes in the absence (A,C) and in the presence (B,D) of 3 mM heptanol. In both WT and KO astrocytes the spread of calcium waves shown to occur over a distance of about 100 μm from the stimulated cell (located at position 0 μm) was prevented by heptanol.
DISCUSSION
Astrocytes are strongly coupled to each other by gap junction channels (Dermietzel et al., 1989, 1991; Giaume et al., 1991; Nagy et al., 1992) that provide the intercellular pathway by which ions (Kuffler and Nicholls, 1966), metabolites (Tabernero et al., 1996; Giaume et al., 1997) and signaling molecules (e.g., IP3, cAMP, Ca2+: see Saez et al., 1993; Charles et al., 1991, 1992; Giaume and McCarthy, 1996) are spread throughout the astrocytic syncytium. With regard to the various functions that have been proposed for gap junctions between astrocytes, the spread of intercellular Ca2+ waves has been hypothesized to provide a mechanism by which cooperative cell activity is coordinated (Sanderson, 1996). Because conditions that increase or decrease gap junction conductance or expression have been found to increase or decrease the number of cells through which Ca2+ waves propagate (Enkvist and McCarthy, 1992; Charles et al., 1992), it has been postulated that the number of open gap junction channels dictates the rate and amount of second messenger diffusion, thereby determining velocity, amplitude, and efficacy of the propagated Ca2+ waves.
The major gap junction protein expressed between astrocytes is connexin 43 (Cx43), as evidenced by immunocytochemistry, Northern and Western blot analyses, and by electrophysiological recordings (Giaume et al., 1991; Dermietzel et al., 1991; Nagy et al., 1992). However, recent studies have indicated that these glia express minor amounts of other gap junction proteins, including Cx40, Cx45, and Cx46 (Dermietzel, 1996; Spray et al., 1998). Electrophysiological and dye coupling studies on astrocytes obtained from Cx43-null (Cx43 knockout) mice have demonstrated that these cells are coupled with respect to electrical current and Lucifer Yellow diffusion (Spray, 1996); comparing levels of macroscopic conductance with amplitudes of single channel conductances, it has been calculated that, on average, cultured wild-type astrocytes express about 200 functional channels, whereas in astrocytes from Cx43 KO mice this functional channel number is about 12 (Spray et al., 1998).
This paper reports the properties (velocity, amplitude, and efficacy) of mechanically induced calcium waves between astrocytes cultured from wild-type (WT) and Cx43 knockout (KO) mice. The relative contributions of extracellular signals (Enkvist and McCarthy, 1992; Hassinger et al., 1996) and of the other gap junction channels present in the Cx43 KO cells to the propagation of calcium waves were evaluated in experiments where suramin and heptanol were used to block purinergic P2 receptors and gap junction channels, respectively.
It is shown here that the propagation of calcium waves between astrocytes was diminished in cultures from Cx43 KO mice, but the parameters of propagation (see Table 1) were not so greatly affected as would be expected to result from deletion of the major gap junction protein expressed in this tissue. A recent study by Naus et al. (1997) reported a more substantial, though also incomplete, deficit in Ca2+ wave propagation (efficacy in that study was reduced by 54%) in cultured astrocytes from Cx43 KO mice.
Two possible mechanisms could account for the observed results: the safety factor for Ca2+ wave propagation may be so high that the residual junctional permeability present in Cx43 KO astrocytes is sufficient to fulfill this function, and/or an extracellular signaling mechanism (as was first demonstrated in mast cells: Osipchuk and Cahalan, 1992, and now shown in astrocytes: Hassinger et al., 1996) may substantially contribute to Ca2+ wave propagation in astrocytes. Because gap junction blockade by heptanol had a much greater impact on the properties of calcium wave spread between Cx43 KO astrocytes than did blockade of purinergic receptors by suramin (see Table 2), it is strongly suggested that the residual junctional channels present in the Cx43 KO astrocytes provide the necessary and sufficient requirement for the propagation of Ca2+ waves between astrocytes. Thus, although an extracellular route for Ca2+ wave spread has been demonstrated by the finding that Ca2+ waves can leap small boundaries in cultures (Enkvist and McCarthy, 1992; Hassinger et al., 1996), this extracellular route for intercellular communication appears to be less critical under the experimental conditions described here than does the direct route of signal transfer through gap junction channels. This is consistent with the view that phospholipase C (PLC) activity, IP3 formation, intracellular Ca2+ stores and gap junctional communication comprise the critical steps for the initiation and propagation of these Ca2+ waves in cultured astrocytes (Venace et al., 1997).
The currently accepted mechanistic model for propagation of mechanically induced intercellular Ca2+ waves is based on diffusion of IP3 through gap junctions (see Sanderson, 1996). In this model, mechanical stimulation generates intracellular IP3 through the activation of PLC, which then diffuses across cell boundaries, leading to the release of Ca2+ from intracellular stores via IP3 receptors (IP3R); although IP3 concentration would decline as a function of distance from the source cell, regenerative responses at concentrations above threshold for Ca2+ mobilization are explained by local amplification through feedback of Ca2+ concentration on IP3 release (Sanderson, 1995; Sneyd et al., 1995). Thus, the velocity, amplitude and number of cells recruited into the Ca2+ wave would all be dependent on the concentration of IP3 liberated from intracellular stores by the initial stimulus.
It is shown here that astrocytes cultured from both wild-type mouse and Cx43 KO siblings exhibited Ca2+ waves that propagated at about 12 μm/s with little attenuation and to almost all throughout the field of view. Although conduction velocity, amplitude, and number of responding cells indicated decreased intercellular communication in the Cx43 KO astrocytes, studies presented here have been limited to relatively small cell populations. More substantial differences would be expected to occur at the fringes of the area, where propagation fails, examination of which will require low power microscope objectives with high numerical aperture and high UV transparency.
Gap junctions in other systems are permeable to both Ca2+ and IP3 (Saez et al., 1989; Christ et al., 1992; Sanderson, 1995; Charles et al., 1991, 1992), and the permeability of junctions in Cx43 KO astrocytes to an anion of similar size (Lucifer Yellow) suggests that the residual channels permit IP3 diffusion. Whether Ca2+ or other second messenger molecules further contribute to this phenomenon is unresolved, although the relatively short range of diffusion expected for Ca2+ ions within cytoplasm (diffusion distance of 0.08 μm, compared with 13 μm for IP3; see Kasai and Petersen, 1994) would be expected to result in such rapid attenuation of the signal as to render it useless unless continuously amplified through Ca2+-induced Ca2+ release.
Cx43 KO mice die at birth due to cardiac hyperplasia obstructing the right ventricular outflow tract; however, their brains are grossly normal in appearance and exhibit no profound difference from WT animals in cortical lamination (Dermietzel, 1996). These observations suggest either that gap junctions between astrocytes play no role in brain development or organization or that the residually expressed connexins are sufficient for this task. The studies presented here demonstrate that, even with severely reduced junctional conductance (Spray et al., 1998), Cx43 KO astrocytes are capable of performing long-range Ca2+ wave signaling, perhaps preserving one mechanism critical to neural function.
Acknowledgments
The authors are grateful to Ms. Delia Vieira for culturing astrocytes and to Dr. Yang Gao for the GFAP staining mentioned in the Material and Methods section. This work was supported by NIH-NS07512 and NS34931 to DCS.
Footnotes
Contract grant sponsor: NIH; Contract grant numbers: NS07512 and NS34931.
References
- Bender AS, Neary JT, Norenberg MD. Role of phosphoinositide hydrolysis in astrocyte volume regulation. Neurochemistry. 1993;6:1506–1514. doi: 10.1111/j.1471-4159.1993.tb13646.x. [DOI] [PubMed] [Google Scholar]
- Bennett MVL, Barrio LC, Bargiello TA, Spray DC, Hertzberg EL, Saez JC. Gap junctions: New tools, new answers, new questions. Neuron. 1991;6:305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bolego C, Ceruti S, Brambilla R, Puglisi L, Cattabeni F, Burnstock G, Abbracchio MP. Characterization of signaling pathways involved in ATP and basic fibroblast growth factor-induced astrogliosis. Br J Pharmacol. 1997;121:1698–1699. doi: 10.1038/sj.bjp.0701294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao D, Lin G, Westphale EM, Beyer EC, Steinberg TH. Mechanisms for the coordination of intercellular calcium signaling in insulin-secreting cells. J Cell Sci. 1997;110:497–504. doi: 10.1242/jcs.110.4.497. [DOI] [PubMed] [Google Scholar]
- Centemeri C, Bolego C, Abbracchio MP, Cattabeni F, Puglisi L, Nicosia S. Characterization of the Ca2+ responses evoked by ATP and other nucleotides in mammalian brain astrocytes. Br J Pharmacol. 1997;121:1700–1706. doi: 10.1038/sj.bjp.0701293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: Calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron. 1991;6:983–992. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- Charles AC, Naus CC, Kidder GM, Dirksen ER, Sanderson MJ. Intercellular calcium signaling via gap junctions in glioma cells. J Cell Biol. 1992;118:195–201. doi: 10.1083/jcb.118.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ GJ, Moreno AP, Melman A, Spray DC. Gap junction-mediated intercellular diffusion of Ca2+ in cultured human corporal smooth muscle cells. Am J Physiol. 1992;263:C373–C383. doi: 10.1152/ajpcell.1992.263.2.C373. [DOI] [PubMed] [Google Scholar]
- Dermietzel, R. (1996) Molecular diversity and plasticity of gap junctions in the nervous system. In: Gap Junctions in the Nervous System D.C. Spray and R. Dermietzel, eds. R.G. Landes Company, Georgetown, TX, pp. 13–38.
- Dermietzel R, Hertzberg EL, Kessler JA, Spray DC. Gap junctions between cultured astrocytes: Immunocytochemical, molecular, and electrophysiological analysis. J Neurosci. 1991;11:1421–1432. doi: 10.1523/JNEUROSCI.11-05-01421.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R, Traub O, Hwang TK, Beyer E, Bennett MVL, Spray DC, Willecke K. Differential expression of three gap junction proteins in developing and mature brain tissues. Proc Natl Acad Sci USA. 1989;86:10148–10152. doi: 10.1073/pnas.86.24.10148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkvist MOK, McCarthy KD. Activation of protein kinase C blocks astroglial gap junction communication and inhibits the spread of calcium waves. J Neurochem. 1992;59:519–526. doi: 10.1111/j.1471-4159.1992.tb09401.x. [DOI] [PubMed] [Google Scholar]
- Giaume C, Fromaget C, El Aoumari A, Cordier J, Glowinski J, Gros D. Gap junctions in cultured astrocytes: Single-channel currents and characterization of channel-forming protein. Neuron. 1991;6:133–143. doi: 10.1016/0896-6273(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–325. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- Giaume C, Tabernero A, Medina JM. Metabolic trafficking through astrocytic gap junctions. Glia. 1997;21:114–123. [PubMed] [Google Scholar]
- Hassinger TD, Gutherie PB, Atkinson PB, Bennett MVL, Kater SB. An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA. 1996;93:13268–13273. doi: 10.1073/pnas.93.23.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Petersen OH. Spatial dynamics of second messengers: IP3 and cAMP as long-range and associative messengers. Trends Neurosci. 1994;17:95–100. doi: 10.1016/0166-2236(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Kimelberg HK, Kettenmann H. Swelling-induced changes in electrophysiological properties of cultured astrocytes and oligodendrocytes. I Effects on membrane potentials, input impedance and cell-cell coupling. Brain Res. 1990;529:255–261. doi: 10.1016/0006-8993(90)90835-y. [DOI] [PubMed] [Google Scholar]
- King BF, Neary JT, Zhu Q, Wang S, Norenberg MD, Burnstock G. P2 purinergic receptors in cortical astrocytes: expression, calcium-imaging and signaling studies. Neuroscience. 1996;74:1187–1196. doi: 10.1016/0306-4522(96)00209-6. [DOI] [PubMed] [Google Scholar]
- Kuffler SW, Nichols JG. The physiology of neuroglial cells. Ergeb Physiol. 1966;57:1–90. [PubMed] [Google Scholar]
- Nagy JI, Yamamoto T, Sawchuk MA, Nance DM, Hertzberg EL. Quantitative immunohistochemical and biochemical correlates of connexin 43 localization in rat brain. Glia. 1992;5:1–9. doi: 10.1002/glia.440050102. [DOI] [PubMed] [Google Scholar]
- Naus, C.C.G., Becherger, J.F., and Bond, S.L. (1996) Effect of gap junctional communication on glioma cell function. In: Gap Junctions in the Nervous System D.C. Spray and R. Dermietzel, eds. R.G. Landes, Georgetown, TX, pp. 193–202.
- Naus CCG, Bechberger JF, Zhang Y, Venance L, Yamasaki H, Juneja SC, Kidder GM, Giaume C. Altered gap junctional communication, intercellular signaling, and growth in cultured astrocytes deficient in connexin43. J Neurosci Res. 1997;49:528–540. doi: 10.1002/(SICI)1097-4547(19970901)49:5<528::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated byATP receptors in mast cells. Nature. 1992;359:241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- Reetz G, Wiesinger H, Reiser G. ATP-induced oscillation of cytosolic Ca2+ activity in cultured astrocytes from rat brain are modulated by medium osmolarity indicating a control of [Ca2+]i oscillations by cell volume. Neurochem Res. 1997;22:621–628. doi: 10.1023/a:1022430305491. [DOI] [PubMed] [Google Scholar]
- Sáez JC, Connor JA, Spray DC, Bennett MVL. hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci USA. 1989;86:2708–2712. doi: 10.1073/pnas.86.8.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez JC, Berthoud VM, Moreno AP, Spray DC. Gap junctions: Multiplicity of controls in differentiated and undifferentiated cells and possible functional implications. Adv Second Messenger Phosphoprotein Res. 1993;27:163–198. [PubMed] [Google Scholar]
- Sanderson, M.J. (1995) Intercellular calcium waves mediated by inositol triphosphate. In: Calcium Waves, Gradients and Oscillations G.R. Bock and K.Akrill, eds. Wiley, Chicester, UK, pp. 175–194. [PubMed]
- Sanderson MJ. Intercellular waves of communication. News Physiol Sci. 1996;11:262–269. [Google Scholar]
- Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA. 1996;93:9948–9953. doi: 10.1073/pnas.93.18.9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneyd JB, Wetton AC, Charles AC, Sanderson MJ. Intercellular calcium waves mediated by the diffusion of inositol triphosphate: A two-dimensional model. Am J Physiol. 1995;268:C1537–C1545. doi: 10.1152/ajpcell.1995.268.6.C1537. (Cell Physiol., 37) [DOI] [PubMed] [Google Scholar]
- Spray, D.C. (1996) Physiological properties of gap junction channels in the nervous system. In: Gap Junctions in the Nervous System D.C. Spray and R. Dermietzel, eds. R.G Landes Company, Georgetown, TX, pp. 39–59.
- Spray, D.C., Vink, M.J., Scemes, E., Suadicani, S.O., Fishman, G.I., and Dermietzel, R. (1998) Characteristics of Coupling in Cardiac Myocytes and CNS Astrocytes Cultured From Wildtype and Cx43-Null Mice. IOS Press, Netherlands, pp. 281–285.
- Tabernero A, Giaume C, Medina JM. Endothelin-1 regulates glucose utilization in cultured astrocytes by controlling intercellular communication through gap junctions. Glia. 1996;16:187–195. doi: 10.1002/(SICI)1098-1136(199603)16:3<187::AID-GLIA1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Venace L, Stella N, Glowinski J, Giaume C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J Neurosci. 1997;17:1981–1992. doi: 10.1523/JNEUROSCI.17-06-01981.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Caveney S, Kidder GM, Naus CC. Transfection of C6 glioma cells with connexin 43 cDNA: Analysis of expression, intercellular coupling, and cell proliferation. Proc Natl Acad Sci USA. 1991;88:1883–1887. doi: 10.1073/pnas.88.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]


