Abstract
Neuronal precursors reside in the subventricular zone (SVZ) of adult mammals. This region is composed of a network of chains of migrating neuroblasts ensheathed by astrocytes and juxtaposed by clusters of immature precursors (type C cells). Here we show that after antimitotic treatment with cytosine-β-d-arabinofuranoside, neuroblasts and type C cells are eliminated but some astrocytes remain. Remarkably, the SVZ network rapidly regenerates. Soon after cytosine-β-d-arabinofuranoside treatment astrocytes divide. Two days later, type C cells reappear, followed at 4.5 days by migrating neuroblasts. By 10 days the SVZ network is fully regenerated, and the orientation and organization of chains of migrating neuroblasts resemble that of normal mice. This regeneration reveals an unexpected plasticity in the adult central nervous system and should provide a model system to study the early stages of neurogenesis in the adult brain.
The adult mammalian brain has long been considered incapable of regeneration. After injury, neurons in the central nervous system do not spontaneously re-establish their connections. However, recent advances have been made in identifying factors and cells that can enhance axonal regeneration. An unanticipated form of neuroplasticity in the adult mammalian brain is the continued production of new neurons in certain brain regions. Proliferating cells persist throughout adult life along the length of the lateral wall of the lateral ventricles. This germinal region, called the subventricular zone (SVZ), generates new neurons destined for the olfactory bulb (1–3). The SVZ is organized as an extensive network of chains of migrating neuroblasts (type A cells) (3) that travel through glial tubes formed by the processes of slowly proliferating SVZ astrocytes (type B cells). Clusters of rapidly dividing immature precursors (type C cells) are scattered along the network of migrating chains (4). A single layer of differentiated epithelial cells, known as ependymal cells, separates the SVZ from the lateral ventricles.
The newly generated neuroblasts migrate tangentially through the SVZ to join a restricted path called the rostral migratory stream (RMS) that leads to the olfactory bulb (3). In the olfactory bulb, the new neurons differentiate into granule and periglomerular neurons (1–3). The neuroblasts migrate as long chains by means of homotypic interactions without radial glial or axonal guides (chain migration) (5–7). The network of chains of migrating neuroblasts in the SVZ can be revealed by immunostaining for polysialylated neural cell adhesion molecule (PSA-NCAM) (3).
Here we show that after elimination of all immature precursors (type C cells) and migrating neuroblasts in this region with the antimitotic drug cytosine-β-d-arabinofuranoside (Ara-C), the entire SVZ network regenerates very rapidly. Immediately after treatment, only type B cells and ependymal cells remain. Type C cells and then type A cells reappear sequentially 2 and 4 days after treatment. Interestingly, only type B cells divide after Ara-C treatment. Furthermore, some type B cells contact the ventricle and have a unique single cilium that is characteristic of neural precursors. This work demonstrates the regeneration of a central nervous system germinal layer in the adult mammalian brain after complete elimination of young neurons and their immediate precursors. This regeneration should provide a model system to investigate the early stages of neurogenesis in the adult brain.
MATERIALS AND METHODS
Infusions.
Ara-C (2%, Sigma) in vehicle (0.9% saline) or vehicle alone was infused onto the surface of the brain of adult CD-1 male mice (2–3 months) with a mini-osmotic pump (Alzet, Palo Alto, CA, model 1007D; flow rate 0.5 μl/hr, 7 d). Cannulas were implanted onto the surface of the brain at A/P 0, L 1.1 mm relative to bregma. This treatment produced no lesion on the ventricular cavities or in the SVZ. After 6 days of infusion, mice were killed immediately or at the indicated survivals after pump removal (n = 3 for each survival).
Immunohistochemistry.
Whole mounts of the lateral wall of the lateral ventricle were dissected and immunostained as described (3) for PSA-NCAM (G. Rougon, University of Marseilles, France) or TuJ1 (Babco, Richmond, CA). BrdUrd (Sigma) was injected i.p. (100 μl, 10 mg/ml) 1 hr before whole-mount dissection (n = 3–4 per time point). Whole mounts were fixed overnight in 3% paraformaldehyde, washed in phosphate buffer (PB), and sequentially incubated in 100% methanol and 100% acetone (30 min each at −20°C), then 3% hydrogen peroxide in methanol for 3–4 hr at room temperature. Whole mounts were rehydrated in methanol/distilled water, washed three times in PB/0.5% Triton-X, incubated in 2 N HCl at 37°C for 10 min, washed, blocked in 10% horse serum in PB/0.5% Triton-X for 2 hr, incubated for 48–72 hr with 1:200 anti-BrdUrd antibodies (Dako) in blocking solution and revealed with secondary antibodies, ABC reaction (Vector Laboratories), and 0.02% diaminobenzidine (Sigma), and 0.01% H2O2 (Fisher). The distribution and number of BrdUrd-positive cells were analyzed in whole-mount preparations of the lateral wall of the lateral ventricle by using a computerized mapping system (8). Postembedding immunostaining for glial fibrillary acidic protein (GFAP) [1:300 Dako (polyclonal)] was performed in 50-nm ultrathin sections from brains perfused with 3% paraformaldehyde/1% glutaraldehyde and embedded in araldite (Durcupan, Fluka) as described (9).
Electron Microscopy (EM) Analysis.
Brains were processed for EM as described (4). The SVZs of Ara-C-treated mice were serially sectioned and analyzed at the electron microscope. Two hundred serial 70-nm ultrathin sections containing the lateral wall of the lateral ventricle were obtained from one mouse each for 0-, 1-, 2-, or 14-day time points after pump removal. The same 250- to 300-μm segment of SVZ was photographed in every fifth section. Cell types were identified in multiple sections and their contours were drawn onto acetate sheets. Twenty-two levels were digitized for each animal as described (4). One level of each reconstruction is presented in Fig. 3C. The number of different cell types in the entire dorsoventral extent of the SVZ was determined at the EM in two ultrathin sections for each animal (n = 3 per time point). Total cell counts obtained by single-section analysis are comparable to those in three-dimensional reconstructions, indicating that our counts are not biased by cell size (4). Cells with only small fragments of cytoplasm or nucleus in a given section were classed as unidentified.
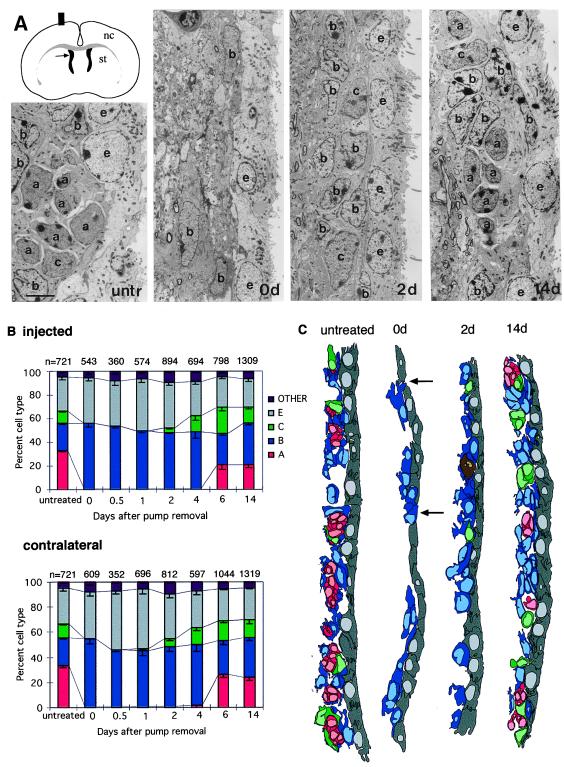
Figure 3.
EM analysis of regenerating SVZ. (A) Schematic of coronal section of brain with location of the SVZ adjacent to the lateral ventricles (arrow) and electron micrographs of SVZ (injected hemisphere) of untreated mice, and 0, 2, and 14 days after Ara-C treatment. Untreated (untr) SVZ with a chain of migrating neuroblasts (type A cells, a) cut in cross section and ensheathed by astrocytes (type B cells, b). Type C cell (c). Ependymal cells (e) line the lateral ventricle. Immediately after the termination of Ara-C treatment (0d), SVZ contains only scattered astrocytes (b) next to the ependymal cell layer (e). At 2 days after Ara-C treatment type C cells (c) reappear in the SVZ. By 14 days after Ara-C treatment SVZ organization is similar to untreated animals. (Scale bar = 5 μm.) (B) Sequence of appearance of SVZ cell types at different survivals after Ara-C treatment in injected and contralateral hemispheres. Cell counts were done at the EM as indicated in Materials and Methods. At 0 days after Ara-C treatment, the majority of cells corresponded to type B cells (blue), and ependymal (gray) with small number of other (microglia, pyknotic, neurons, tanycytes, and unidentified) cells embedded in the SVZ; type A (red) and C (green) cells are absent immediately after the termination of Ara-C treatment. Type C cells are present 2–14 days after Ara-C in the injected hemisphere. At 6 and 14 days, type A cells are present. In the contralateral hemisphere type C and A cells are first present 1 and 4 days, respectively, after Ara-C treatment. n indicates the total number of cells counted at each survival. Three to five animals were analyzed for each time point. (C) One of 22 sections from a serial reconstruction of the SVZ (see Materials and Methods) in untreated and Ara-C treated mice at 0, 2, and 14 days after pump removal. The region reconstructed is indicated by an arrow in the schematic in A. Arrows at 0d show type B cells in contact with the lateral ventricle. A mitosis in the 2-day reconstruction is indicated in brown. Type A cells (red), B (blue), C (green), and ependymal (gray).
[3H]Thymidine Autoradiography.
For ultrastructural identification of cell types undergoing division, mice were injected i.p. with 50 μl of 6.7 Ci/mmol [3H]thymidine 1 hr before sacrifice, and the brains were processed for autoradiography and EM analysis as described (4). Three animals were analyzed for each survival. Samples for EM analysis were from the lateral wall of the lateral ventricle throughout the anterior horn.
RESULTS
Complete Elimination of the SVZ Network of Chains After Ara-C Treatment.
A network of chains of migrating neuroblasts extends throughout the lateral wall of the lateral ventricle of adult mice (3) (Fig. 1A) and can be visualized by immunostaining whole mounts of this wall for PSA-NCAM or neuron-specific βIII-tubulin (TuJ1) (3), both markers of type A cells (4, 5, 10–12). We tested the effectiveness of different antimitotic treatments in eliminating the network of chains by immunostaining whole-mount preparations for PSA-NCAM. Infusion of the antimitotic Ara-C unilaterally onto the surface of the brain for 6 days with an osmotic mini-pump (Fig. 1B) resulted in the complete elimination of PSA-NCAM-positive chains in the SVZ (Fig. 1 C and D). No TuJ1-positive chains were detected in whole mounts (not shown), and no type A cells were detected in serial section analysis in transverse sections at the EM level (see below). Extraneous type A cells also were not detected in the striatum, confirming that type A cells were eliminated and not simply down-regulating the expression of PSA-NCAM. Furthermore, after Ara-C treatment there was a marked degeneration of the cellular organization of the SVZ (see below). In controls infused with saline, the network of PSA-NCAM-positive chains was similar to that of untreated mice (Fig. 1A).
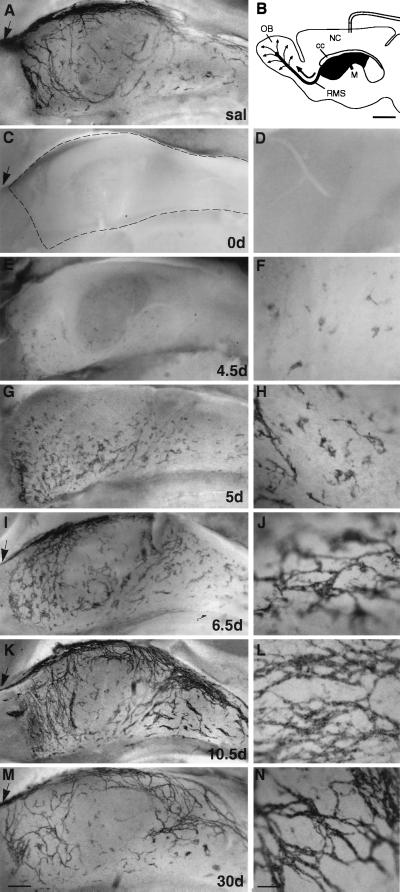
Figure 1.
Elimination and regeneration of the network of PSA-NCAM-positive chains in the SVZ. Mini-osmotic pumps containing Ara-C were implanted onto the surface of the right hemisphere as shown in B (scale bar: 1 mm). Low-magnification photomicrographs (A, C, E, G, I, K, and M; scale bar: 200 μm) are from the portion of the wall of the lateral ventricle indicated by dark gray in the schematic. (D, F, H, J, L, and N) Higher magnification (scale bar: 35 μm) for each of the time points. Dotted line in C outlines the borders of the lateral ventricle. Arrows indicate the RMS. (A) SVZ network of PSA-NCAM-positive chains in saline-infused mice. (C–N) Whole-mount PSA-NCAM immunostaining at different survivals after Ara-C treatment in the injected hemisphere. 0 days after Ara-C treatment, no PSA-NCAM immunostaining is found in SVZ (C and D). Individual PSA-NCAM-positive cells and small clusters first appear at 4.5 days scattered over the entire wall of the lateral ventricle (E and F). At 5 days the clusters are larger, and some short chains begin to form (G and H). By 6.5 days, many chains are now present and form a simple disorganized network (I and J). At 10 days a largely normal network is visible (K and L), although chains are more abundant than in saline-infused animals. At 30 days, the SVZ network appears normal (M and N). NC, neocortex; OB, olfactory bulb; cc, corpus callosum; M, Foramen of Monro.
The SVZ Regenerates After Ara-C.
Remarkably, after such extensive ablation, the SVZ regenerated and young migrating neurons reappeared. We followed the time course of SVZ regeneration by immunostaining whole-mount preparations for PSA-NCAM at different survivals after pump removal (Fig. 1). No PSA-NCAM-immunopositive cells were encountered from 0 to 4 days. Isolated PSA-NCAM-positive cells and small cell clusters appeared scattered throughout the entire SVZ of the injected hemisphere 4.5 days after pump removal (Fig. 1 E and F). The clusters of PSA-NCAM-positive cells initially appeared away from the infusion site, suggesting that some Ara-C may remain present in the cannula or tissue close to the site of implantation. In the contralateral hemisphere, the reappearance of PSA-NCAM-positive cells was uniform throughout the wall. The number and size of the cell clusters increased dramatically 1 day later (Fig. 1 G and H). By 6.5 days after pump removal, the PSA-NCAM-positive clusters were interconnected by short chains (Fig. 1 I and J). This web, however, was patchy and did not show the longitudinal arrangement found in the SVZ of untreated animals (3). By 10, 14, and 30 days after pump removal, the network of chains was fully reconstituted and resembled that of untreated mice (Fig. 1 K-N). Furthermore, by this time, the RMS also was repopulated with PSA-NCAM-positive cells (arrows in Fig. 1 I, K, and M), indicating that migration of neuroblasts into the olfactory bulb had resumed. In the contralateral hemisphere a similar pattern of reappearance was observed, but was accelerated by 12–24 hr (not shown). The reappearance of PSA-NCAM-positive cells occurred rapidly throughout the SVZ, suggesting that neuronal precursors are numerous and widely distributed.
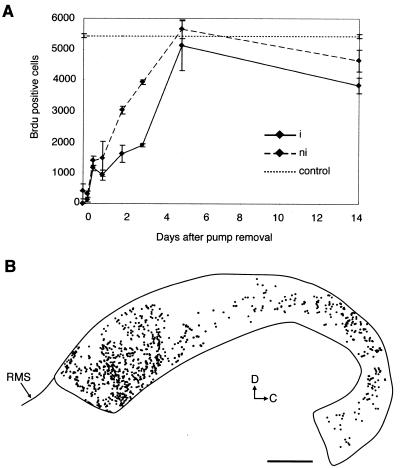
To determine the time course of the reappearance of dividing cells in the regenerating SVZ, we injected BrdUrd, a marker of DNA synthesis, 1 hr before dissection 0, 0.25, 0.5, 1, 2, 3, 5, or 14 days after pump removal and analyzed the distribution and number of BrdUrd-positive nuclei in whole-mount preparations. No proliferating cells were observed in the SVZ immediately after termination of Ara-C treatment in the injected hemisphere (Fig. 2A). BrdUrd-positive cells appeared 6–12 hr after pump removal and were scattered throughout the SVZ (Fig. 2B). The number of BrdUrd-positive cells increased until 5 days when approximately normal levels were observed. However, 14 days after pump removal, the number of BrdUrd-positive cells in the injected side was 30% lower than in untreated animals. In the contralateral hemisphere, a few BrdUrd-labeled cells were present immediately after pump removal. On this side, BrdUrd-labeled cells increased in number with a similar time course to the injected side, but accelerated by 12 hr (Fig. 2A).
Figure 2.
Elimination and appearance of BrdUrd-labeled SVZ cells after Ara-C treatment. (A) Number of BrdUrd-positive nuclei counted in whole mounts of the lateral wall of the lateral ventricle at different survivals after pump removal in infused (i, solid line) and contralateral (ni, dashed line) hemispheres and in untreated (dotted line) mice. Error bars show the SE. (B) Distribution of BrdUrd-positive nuclei in whole-mount preparation of injected hemisphere 0.5 d after pump removal. BrdUrd-positive cells are more prevalent in the anterior horn where the SVZ is thicker, but reappear throughout the SVZ. BrdUrd-positive nuclei initially appear away from the infusion site (white area in the dorsal SVZ), suggesting that more cells were killed in that region or that some Ara-C remains in the tissue. (Scale bar = 500 μm.)
Type B Cells and Ependymal Cells Remain After Ara-C.
We used EM to analyze the composition of the SVZ immediately after termination of Ara-C treatment. The SVZ of Ara-C-treated mice comprised only a few, scattered cells (Fig. 3A, 0d) in contrast to the untreated SVZ (Fig. 3A, untr). Immediately after the termination of Ara-C treatment, no type A or C cells were present in the injected hemisphere. Fifty-five percent of the cells remaining after Ara-C treatment corresponded to type B cells and 37% to ependymal cells. The ultrastructure of type B cells remaining after Ara-C was very similar to that previously described (4) with multiple processes that intercalate between other cells, intermediate filaments, and dense bodies in their cytoplasm and gap junctions. After termination of Ara-C treatment type B cells appeared more electron dense and had thicker bundles of intermediate filaments. Type B cells express GFAP (4) in both normal and Ara-C-treated mice. Ependymal cells, which line the lateral ventricle, had similar ultrastructural characteristics before (4) and after Ara-C treatment.
Type B cells were reduced in number in Ara-C-treated mice as compared with untreated mice, suggesting that some of these cells underwent division and were killed by the Ara-C treatment. Neurons (1.5%), tanycytes (0.4%), microglia (1.2%), and pyknotic cells (1.0%) were also present in the SVZ immediately after termination of Ara-C treatment. Four percent of the cells could not be identified. To confirm that the cells classified as unidentified did not correspond to a different cell type, we serially reconstructed at the EM level the dorsal SVZ in the anterior horn, a region where many type A cells reappear during regeneration. Forty-one cells were studied serially immediately after pump removal; 24 corresponded to type B cells and 17 to ependymal cells. No other cells were present. This finding indicates that unidentified cells in single-section EM analysis correspond to fragments of type B or ependymal cells. One of 22 levels of this reconstruction is shown in Fig. 3C, 0d. Interestingly, we noticed that shortly after the termination of Ara-C treatment some type B cells extended processes between ependymal cells and contacted the lateral ventricle (Fig. 3C, 0d).
Type C and Then Type A Cells Reappear After Ara-C.
The whole-mount PSA-NCAM immunostaining suggests that migrating neuroblasts reappear 4.5 days after pump removal (Fig. 1). To determine the sequence of reappearance of the different cell types, we analyzed the SVZ composition 0.5, 1, 2, 4, 6, and 14 days after pump removal at the EM (Fig. 3B). This analysis was done in ultrathin sections that encompassed the entire dorsal-ventral extent of the lateral wall of the lateral ventricle and was performed independently of the light microscope analysis and blindly as to survival time. Type C cells first appeared 2 days after pump removal on the injected side. By 4 days, type C cells accounted for 14% of cells in this region. Their proportion increased steadily until day 6, but declined again by 14 days to normal levels. Type A cells were present 6 days after pump removal. By 14 days the proportion of the different cell types and the organization of the SVZ was similar to that of control animals, with chains of migrating cells ensheathed by the processes of type B cells and clusters of type C cells scattered along the chains (4) (Fig. 3 A and C, 14d). In the contralateral hemisphere, the order of reappearance was equivalent to that of the injected side except that type C cells first appeared at 1 day and type A cells 4 days after pump removal (Fig. 3B, 14d). The RMS, which links the SVZ and the olfactory bulb, contains only migrating type A cells and astrocytes (type B cells) in the untreated animal (6). Immediately after the termination of Ara-C treatment, only type B cells were present in the RMS, consistent with the interpretation that neuroblasts had been eliminated. By 4–5 days after pump removal, type A cells reappeared in the RMS. These ultrastructural observations correlate tightly with the PSA-NCAM light microscope analysis.
Type B and Then Type C Cells Divide After Ara-C Treatment.
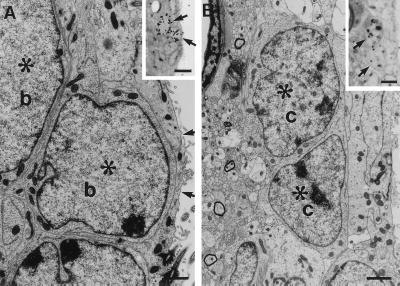
The above results show that after termination of Ara-C treatment, ependymal cells, tanycytes, and type B cells remain. To identify which cells divided at different survivals after Ara-C treatment, we injected mice with [3H]thymidine 1 hr before sacrifice (0, 0.5, 1, 2, and 3 days after pump removal) and identified the labeled cells at the EM. At 0 days, no 3H-thymidine-labeled cells were encountered in the injected hemisphere, consistent with the BrdUrd analysis. At 0.5 day and 1 day, 99 3H-thymidine-labeled cells were identified at the EM in the injected hemisphere: 97 corresponded to type B cells and two to microglia (Fig. 4A and Table 1). None corresponded to ependymal cells or tanycytes. Some of the dividing cells were very close to the ependymal layer (Fig. 4A), but all corresponded to type B cells. Two days after pump removal, labeled type C cells were present (Table 1). By 3 days, the majority of proliferating cells were type C cells (Fig. 4B and Table 1). In the contralateral hemisphere, 81 3H-thymidine-labeled cells were studied at the EM level at 0 and 0.5 days after pump removal. These cells all corresponded to type B cells (Table 1). In the contralateral hemisphere, 3H-thymidine-labeled type C cells appeared 1 day after Ara-C treatment (Table 1), 1 day earlier than in the injected hemisphere.
Figure 4.
Type B cells divide after Ara-C treatment. Twelve hours after Ara-C treatment [3H]thymidine was injected i.p. (A) In [3H]thymidine injected mice killed 1 hr after injection, type B cells (b) were observed with EM (scale bar: 2.2 μm); upper inset in A shows two cells (arrows) with silver grains overlying their nuclei (see Table 1). (Scale bar: 28 μm.) Note how close one of the two labeled cells (∗) is to the lateral ventricle. The arrows point to the thin process of an adjacent ependymal cell that separates the labeled cell from the ventricle. (B) Three days after termination of Ara-C treatment-labeled type C cells are present. The inset shows two cells (arrows) with silver grains overlying their nuclei. These same cells at the EM level (∗). (Scale bar: 1.6 μm; Inset: 13 μm.)
Table 1.
Cell types incorporating [3H]thymidine at different survivals after Ara-C treatment
| Survival, days | Type E (ependymal) | Type B (astrocyte) | Type C (precursor) | Type A (neuroblast) | Microglia | Mitoses | n cells | |
|---|---|---|---|---|---|---|---|---|
| Injected | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.5 | 0 | 97.6% (82) | 0 | 0 | 2.4% (2) | 0 | 84 | |
| 1 | 0 | 100% (15) | 0 | 0 | 0 | 0 | 15 | |
| 2 | 0 | 91.7% (33) | 8.3% (3) | 0 | 0 | 0 | 36 | |
| 3 | 0 | 22.7% (5) | 72.7% (16) | 0 | 4.5% (1) | 0 | 22 | |
| Contralateral | 0 | 0 | 100% (11) | 0 | 0 | 0 | 0 | 11 |
| 0.5 | 0 | 100% (70) | 0 | 0 | 0 | 0 | 70 | |
| 1 | 0 | 70.8% (17) | 29.2% (7) | 0 | 0 | 24 | ||
| 2 | 0 | 67.5% (27) | 30% (12) | 0 | 2.5% (1) | 0 | 40 | |
| 3 | 0 | 35.3% (18) | 60.8% (31) | 0 | 0 | 3.9% (2) | 51 |
Percentage of each cell type labeled with [3H]thymidine of total labeled cells. Pumps were removed and [3H]thymidine injected 1 h before killing at the survivals indicated.
Type B Cells Contain a Specialized Cilium.
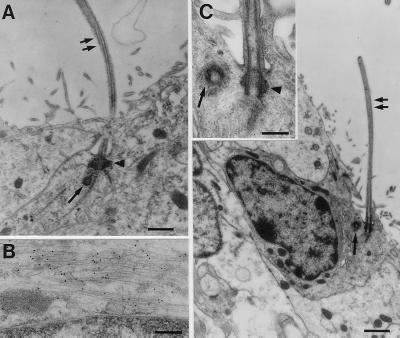
After Ara-C treatment, some type B cells were observed touching the lateral ventricle (Fig. 3C, 0D). Interestingly, some of the extensions of type B cells touching the ventricle had a single cilium with a centriole at its base (Fig. 5A). This single cilium lacked a central pair of microtubules. Such cilia are typical of early neuroepithelial cells. In contrast, ependymal cells had multiple cilia (40–50 per cell) that are six times longer than the single cilium of type B cells. Their cilia had a characteristic internal structure of 9+1. We performed immunocytochemistry staining at the EM level to determine whether the cells with a single cilium expressed GFAP, a marker of SVZ astrocytes. Cells touching the lateral ventricle containing a single cilium were also GFAP-positive (Fig. 5B). The many contorted processes of type B cells made it impossible to determine how many of the type B cells after Ara-C treatment contacted the ventricle or had a cilium. On close examination in untreated mice, a small number of type B cells also were found that had thin processes touching the lateral ventricle. Some of these processes also contained a characteristic single cilium (Fig. 5C).
Figure 5.
(A) Some GFAP-positive type B cells contacting the ventricle exhibit a single cilium. After Ara-C treatment, type B cells contacting the ventricle extend a single cilium (double arrow). Characteristic basal bodies (arrowhead) and centriole (single arrow) are found at its base. (Scale bar: 0.25 μm.) (B) Postembedding immunostaining for GFAP revealed that this same cell has GFAP-positive intermediate filaments. The black dots overlying the thin intermediate filaments are gold particles. (Scale bar: 0.6 μm.) (C) In untreated mice, type B cells touching the ventricle with a single cilium (double arrow) also are found. (Scale bar: 1 μm.) (Inset) The characteristic basal bodies (arrowhead) and centriole (single arrow) associated with the base of the cilium. (Scale bar: 0.3 μm.)
DISCUSSION
The regeneration of the SVZ network of chains described here reveals an unexpected plasticity in the adult mammalian brain. Although neural tissue is capable of regeneration after injury in adult amphibians (e.g., retina) (13) and lizards (14), such processes are unprecedented in the adult mammalian brain. The remarkable fidelity with which the network of chains is re-established in the adult rodent SVZ suggests that directional cues for chain migration remain even after ablation of all chains in the SVZ. Directional migration appears to be, at least initially, extrinsic to the chains of migrating cells.
The rapid regeneration and pattern of reappearance of PSA-NCAM-positive clusters after Ara-C treatment suggest that neuronal precursors are not rare or concentrated at specific locations. Earlier work has suggested that rapidly dividing cells, those likely to be killed by antimitotic agents, are not the stem cells for the new neurons (15). Our results are consistent with this conclusion. Type A cells, which divide relatively frequently (2, 4, 16), and type C cells, which divide very actively (4), are readily killed by the Ara-C treatment, yet neurogenesis resumed. This finding suggests that the stem cells were not killed and were able to regenerate the SVZ neuroblasts. Alternatively the stem cells may divide but be resistant to Ara-C treatment.
In a separate publication, we show that SVZ astrocytes are neural stem cells in the adult SVZ that generate new neurons destined for the olfactory bulb: Type C cells arise from dividing type B cells and in turn generate type A cells (17). This finding is in contrast to another recent publication that suggests that ependymal cells are the neural stem cells in vivo (18). The results presented here, studying the order of reappearance of different SVZ cell types and their proliferation after Ara-C treatment, are consistent with our finding that type B cells are the in vivo stem cells. We did not observe 3H-thymidine-labeled ependymal cells at any of the survival times studied. After Ara-C treatment, only type B cells divide. Between 1 and 2 days after pump removal, dividing type C cells appear, followed 2 days later by type A cells. We cannot discard the possibility that ependymal cells may transdifferentiate into type B or C cells without mitosis from the data presented here. We did not, however, observe ependymal cells retracting their cilia or with morphologies intermediate between ependymal and type B or C cells. Furthermore many type C cells arise from proliferating type B cells (17).
Both after Ara-C treatment and in the normal brain, type B cells were observed touching the lateral ventricle. The ultrastructure of these cells was different from that of ependymal cells. Some type B cells contacting the ventricle extended a single cilium lacking the central pair of microtubules. This single cilium is quite distinct from those of the multiciliated ependymal cells, which have a central pair of microtubules and are much longer. Neuronal precursors in the embryonic neuroepithelium (19, 20) and in primary precursors of new neurons in adult avian brain (21) also extend a single cilium. The role of the single cilium and whether all type B cells possess such a cilium are as yet unknown. Perhaps contact of type B cells with the ventricular fluid may be required for neurogenesis. Further work on the nature of the cilium may provide markers for early neuroepithelial cells and clues about the cell biology of neurogenesis.
In one animal we counted all PSA-NCAM-positive cells present at 4.5 days in a whole mount. PSA-NCAM-positive cells (1,086 type A cells) were present in this animal, and similar density of PSA-NCAM-positive cells was observed in other animals. Based on the proportions presented in Fig. 3B, type C cells were 8.5 times more abundant at 4 days, indicating that by this time between 5,000 and 10,000 cells had been regenerated. This finding suggests that the precursors for new neurons are widely distributed and that cell generation is rapid. Interestingly, the new neuroblasts did not reappear homogeneously, but were found in discrete foci throughout the SVZ. One possibility is that only a subset of SVZ cells that remain after Ara-C treatment may be neurogenic. Alternatively, SVZ cells that become neurogenic may inhibit neighboring equipotent cells from following this path. Lateral inhibition of neurogenesis is a prevalent mechanism in development in which equipotent cells in a field are inhibited from following a particular fate. Notch signaling has been implicated in such processes (22). It will be interesting to test whether similar signaling is at work during the regeneration of the SVZ after Ara-C treatment and whether this mechanism controls the number of neurogenic events per unit volume of SVZ.
Cell-cell interactions likely play an important role in neurogenesis in the adult SVZ. In vitro, contact with astrocytes supports neurogenesis of SVZ cells (23). In vivo, migrating neuroblasts (type A cells) travel as chains through glial tunnels formed by SVZ astrocytes (type B cells) (4). The elimination of type A and C cells by Ara-C treatment disrupts these cell-cell interactions. Neurogenesis may be induced when type B cells, which are separated by the chains of type A cells before treatment, come into contact with one another as the chains are eliminated. Alternatively, migrating neuroblasts may release factors that inhibit neuronal production. Such mechanisms also may be at work in untreated animals when type A cells become depleted by migration or cell death. Thus, as glial tunnels become devoid of cells, either through cell death or emigration, neurogenesis may be activated. The molecular signals underlying neurogenesis in the adult brain remain unknown, but the model of regeneration described here should allow investigation into these mechanisms.
The finding described here, that the SVZ network of adult mice fully regenerates after antimitotic treatment, may have important clinical implications. New neurons are produced in the dentate gyrus of adult primates and humans (24, 25). Neuronal precursors also have been cultured from human brain tissue excised during surgery (26). The SVZ network of PSA-NCAM-positive chains of new neurons is present in adult primates (F.D. and A.A.-B., unpublished observation). Interestingly, the capacity to regenerate the SVZ network in adult mice was observed even after a 2-week treatment with Ara-C (not shown). After 2 weeks a further reduction in the number of type B cells was observed, suggesting that, with longer treatments, it may be possible to eventually deplete the population of stem cells. However, our current results suggest that stem cells may be numerous and relatively resilient to complete elimination. Chemotherapeutic agents, such as Ara-C, used to treat cancer will likely also kill dividing neuronal precursors in the adult human brain. The regeneration described here suggests that although the neuroblasts and their immediate precursors are temporarily eliminated, they can be regenerated. Such plasticity indicates that perhaps these cells also can be manipulated to differentiate into multiple cell types.
In vitro studies have suggested that neural stem cells reside within the adult mammalian SVZ (15). One property of stem cells is their ability to regenerate tissue (27–29). The regeneration of the SVZ we describe here provides evidence for the presence and activation of neural stem cells in vivo and suggests that these cells are widely distributed in the SVZ.
Acknowledgments
We thank F. Nottebohm, C. Scharff, and H. Wichterle for critical and insightful reading of this manuscript and A. Mateo for technical assistance. We thank G. Rougon for the kind gift of anti-Men-B antibodies. This work was supported by National Institutes of Health Grants HD32116 and NS28378. F.D. is a Baker Fellow.
ABBREVIATIONS
- SVZ
subventricular zone
- Ara-C
cytosine-β-d-arabinofuranoside
- RMS
rostral migratory stream
- PSA-NCAM
polysialylated neural cell adhesion molecule
- GFAP
glial fibrillary acidic protein
- EM
electron microscopy
References
- 1.Altman J. J Comp Neurol. 1969;137:433–458. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- 2.Lois C, Alvarez-Buylla A. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- 3.Doetsch F, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1996;93:14895–14900. doi: 10.1073/pnas.93.25.14895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doetsch F, Garcia-Verdugo J M, Alvarez-Buylla A. J Neurosci. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousselot P, Lois C, Alvarez-Buylla A. J Comp Neurol. 1995;351:51–61. doi: 10.1002/cne.903510106. [DOI] [PubMed] [Google Scholar]
- 6.Lois C, Garcia-Verdugo J M, Alvarez-Buylla A. Science. 1996;271:978–981. doi: 10.1126/science.271.5251.978. [DOI] [PubMed] [Google Scholar]
- 7.Wichterle H, Garcia-Verdugo J M, Alvarez-Buylla A. Neuron. 1997;18:779–791. doi: 10.1016/s0896-6273(00)80317-7. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez-Buylla A, Vicario D S. J Neurosci Methods. 1988;25:165–173. doi: 10.1016/0165-0270(88)90155-0. [DOI] [PubMed] [Google Scholar]
- 9.Blasco-Ibáñez J M, Martinez-Guijarro F J, Freund T F. Eur J Neurosci. 1998;10:1784–1795. doi: 10.1046/j.1460-9568.1998.00190.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonfanti L, Theodosis D T. Neuroscience. 1994;62:291–305. doi: 10.1016/0306-4522(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 11.Menezes J R L, Luskin M B. J Neurosci. 1994;14:5399–5416. doi: 10.1523/JNEUROSCI.14-09-05399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jankovski A, Sotelo C. J Comp Neurol. 1996;371:376–396. doi: 10.1002/(SICI)1096-9861(19960729)371:3<376::AID-CNE3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 13.Reh T A, Levine E M. J Neurobiol. 1998;36:206–220. [PubMed] [Google Scholar]
- 14.Font E, Desfilis E, Perez-Canellas M M, Alcantara S, García-Verdugo J M. Brain Res. 1997;754:245–259. doi: 10.1016/s0006-8993(97)00085-1. [DOI] [PubMed] [Google Scholar]
- 15.Morshead C M, Reynolds B A, Craig C G, McBurney M W, Staines W A, Morassutti D, Weiss S, Van der Kooy D. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 16.Menezes J R L, Smith C M, Nelson K C, Luskin M B. Mol Cell Neurosci. 1995;6:496–508. doi: 10.1006/mcne.1995.0002. [DOI] [PubMed] [Google Scholar]
- 17.Doetsch F, Caille I, Lim D A, Garcia-Verdugo J M, Alvarez-Buylla A. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 18.Johansson C B, Momma S, Clarke D L, Risling M, Lendahl U, Frisén J. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 19.Sotelo J R, Trujillo-Cenóz O. Z Zellforsch. 1958;49:1–12. doi: 10.1007/BF00335059. [DOI] [PubMed] [Google Scholar]
- 20.Stensaas L J, Stensaas S S. Z Zellforsch. 1968;91:341–365. doi: 10.1007/BF00440763. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez-Buylla A, García-Verdugo J M, Mateo A, Merchant-Larios H. J Neurosci. 1998;18:1020–1037. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis J. Semin Cell Dev Biol. 1998;9:583–589. doi: 10.1006/scdb.1998.0266. [DOI] [PubMed] [Google Scholar]
- 23.Lim D A, Alvarez-Buylla A. Proc Natl Acad Sci USA. 1999;96:7526–7531. doi: 10.1073/pnas.96.13.7526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gould E, Tanapat P, McEwen B S, Flugge G, Fuchs E. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eriksson P S, Perfilieva E, Bjork-Eriksson T, Alborn A, Nordborg C, Peterson D A, Gage F H. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 26.Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser R A R, Goldman S A. Cereb Cortex. 1994;6:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- 27.Hall P A, Watt F M. Development (Cambridge, UK) 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- 28.Potten C S, Loeffler M. Development (Cambridge, UK) 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 29.Morrison S J, Shah N M, Anderson D J. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]