Abstract
Congenital diaphragmatic hernia (CDH) is an often fatal birth defect that is commonly associated with pulmonary hypoplasia and cardiac malformations. Some investigators hypothesize that this constellation of defects results from genetic or environmental triggers that disrupt mesenchymal cell function in not only the primordial diaphragm but also the thoracic organs. The alternative hypothesis is that the displacement of the abdominal viscera in the chest secondarily perturbs the development of the heart and lungs. Recently, loss-of-function mutations in the gene encoding FOG-2, a transcriptional co-regulator, have been linked to CDH and pulmonary hypoplasia in humans and mice. Here we show that mutagenesis of the gene for GATA-4, a transcription factor known to functionally interact with FOG-2, predisposes inbred mice to a similar set of birth defects. Analysis of wild-type mouse embryos demonstrated co-expression of Gata4 and Fog2 in mesenchymal cells of the developing diaphragm, lungs, and heart. A significant fraction of C57Bl/6 mice heterozygous for a Gata4 deletion mutation died within one day of birth. Developmental defects in the heterozygotes included midline diaphragmatic hernias, dilated distal airways, and cardiac malformations. Heterozygotes had any combination of these defects or none. In chimeric mice, Gata4−/− cells retained the capacity to contribute to cells in the diaphragmatic central tendon and lung mesenchyme, indicating that GATA-4 is not required for differentiation of these lineages. We conclude that GATA-4, like its co-regulator FOG-2, is required for proper mesenchymal cell function in the developing diaphragm, lungs, and heart.
Keywords: birth defect, diaphragm, pulmonary hypoplasia, transcription factor
Introduction
Congenital diaphragmatic hernia (CDH) is a severe developmental anomaly that affects 1 per 3000 live births and accounts for 1-2% of infant mortality (Pober et al., 2005; Colvin et al., 2005; Yang et al., 2006). The hallmark of the disorder is a defect in the muscular or tendinous portion of the diaphragm. CDH is thought to result from abnormal embryonic development of the diaphragmatic substratum, but the molecular pathogenesis of this disorder is poorly understood (Greer et al., 2000b; Babiuk and Greer, 2002).
Primary defects in lung morphogenesis and cardiovascular malformations often accompany CDH in animal models and patients (Migliazza et al., 1999; Losty et al., 1999; Graziano, 2005). This association has given rise to the mesenchymal hit hypothesis, which posits that genetic or environmental triggers of CDH disrupt the function of mesenchymal cells in not only the primordial diaphragm but also the developing lungs and heart (Keijzer et al., 2000). A corollary, the smooth muscle hypothesis, proposes that disruption of mesenchymal progenitors of smooth muscle in the pulmonary vasculature and airways leads to pulmonary hypertension, airway hyperreactivity, and other pulmonary complications commonly encountered in patients with CDH (Jesudason, 2006). Consequently, a major goal of CDH research is to identify genes critical for early mesenchymal cell function in the diaphragm, lungs, and cardiovascular system.
Recent studies, including analysis of recurring chromosomal rearrangements in patients with CDH (Lurie, 2003), suggest that transcription factor haploinsufficiency may cause or contribute to this disorder. Chromosome 8q22-23 abnormalities have been associated with CDH (Wilson et al., 1983; Temple et al., 1994; Howe et al., 1996), and mutation of one of the genes in this region, FOG2, has been shown to cause diaphragmatic defects and primary pulmonary hypoplasia in humans and mice (Ackerman et al., 2005). FOG2 encodes a transcriptional co-regulator that is expressed by mesenchymal cells in the diaphragm, lung, and heart and by somatic cells in the testis (Svensson et al., 2000; Tevosian et al., 2000; Ketola et al., 2002; Ackerman et al., 2005). Another recurring chromosomal abnormality in CDH is microdeletion of 8p23.1 (Pecile et al., 1990; Faivre et al., 1998; Borys and Taxy, 2004; Shimokawa et al., 2005; Barber et al., 2005; López et al., 2006). One of the genes in the critical region of 8p23.1 is GATA4, which encodes a transcription factor that physically interacts with FOG-2 during morphogenesis of the heart and testis (Crispino et al., 2001; Tevosian et al., 2002). It is therefore plausible that GATA4 haploinsufficiency contributes to the pathogenesis of CDH, particularly in patients with concomitant diaphragm and heart defects, as loss-of-function mutations in GATA4 have been linked to cardiac malformations in humans (Garg et al., 2003; Okubo et al., 2004; Nemer et al., 2006) and mice (Kuo et al., 1997; Molkentin et al., 1997; Crispino et al., 2001; Watt et al., 2004; Pu et al., 2004; Zeisberg et al., 2005; Xin et al., 2006).
Here we show that C57Bl/6 mice heterozygous for a mutant allele of Gata4 are predisposed to CDH and primary lung abnormalities. We propose that GATA-4, working in concert with FOG-2 or other transcription factors, regulates mesenchymal cell function in the developing diaphragm and lungs. Our findings support the premise that GATA-4 mutation contributes to the pathogenesis of CDH and related developmental anomalies in man.
Materials and Methods
Experimental mice
Procedures involving mice were approved by institutional committees for laboratory animal care and were conducted in accordance with NIH and EU guidelines for the care and use of experimental animals. C57Bl/6 and Rosa26 (C57Bl/6, Gpi-1b) (Friedrich and Soriano, 1991) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Gata4+/Δex2 mice, which harbor a deletion in exon 2 of the gene, were generated and genotyped as described elsewhere (Pu et al., 2004; Zeisberg et al., 2005). These mice were backcrossed with C57Bl/6 mice for a minimum of 7 generations. Nkx2-5+/− mice were prepared and genotyped as described previously (Tanaka et al., 2002) and were backcrossed with C57BL/6 mice for a minimum of 12 generations. Chimeric mice were prepared by injection of XYGata4−/− ES cells (CCE, 129/Sv//Ev, Gpi-1c) ES cells (Soudais et al., 1995; Kuo et al., 1997) into Rosa26 embryos as described (Narita et al., 1997a). These nullizygous ES cells harbor a neomycin resistance cassette in exon 2 of the gene. Chimeras were initially identified by GPI-1 isoenzyme analysis of tail tissue (Narita et al., 1997a). XIST RT-PCR analysis of tail or hind limb tissue was used to distinguish chimeras derived from XX versus XY host blastocysts (Natoli et al., 2004). Only chimeric mice derived from XY hosts were subjected to further analysis.
Tissue isolation and histological analyses
Late gestation fetuses were harvested, fixed overnight in 4% paraformaldehyde in PBS, and embedded in paraffin for routine histology. In some cases, pregnant females were injected intraperitoneally with 2 mg bromodeoxyuridine (BrdU) 15 hr before embryo harvest. Alternatively, unfixed fetuses were embedded directly in Tissue-Tek OCT compound (Sakura Finetek, Torrence, CA) for preparation of cryosections. Thoraces of newborn pups were isolated by decapitation and transection at the level of the liver. Skin and soft tissue were removed, and the intact thorax was fixed for 1-2 days in 4% paraformaldehyde ± 1% glutaraldehyde in PBS at 4°C. Diaphragm, heart, and lungs were then dissected and processed for morphometric and ultrastructural analyses. Paraffin sections (5-6 μm) were stained with hematoxylin and eosin (H&E) or with Masson’s trichrome to visualize extracellular matrix (ECM).
To detect β-galactosidase (β-gal) expression, frozen tissue sections (10 μm) were fixed with 0.2% gluteraldehyde for 5 min, permeabilized with 100 mM potassium phosphate pH 7.4, 0.02% NP-40 and 0.01% sodium deoxycholate for 5-15 min, and then incubated in 0.5 mg/ml X-gal (Promega) with 10 mM K3[Fe(CN)6], 10 mM K4[Fe(CN)6], 100 mM potassium phosphate pH 7.4, 0.02% NP-40 and 0.01% sodium deoxycholate at 30°C overnight (Narita et al., 1997a). The sections were then counterstained with eosin.
Morphometric analysis
Air space size was estimated from the mean chord length of the airspace (Ray et al., 1997). Images of H&E stained lung tissue from postnatal day 1 mice were acquired at 400x magnification using an Olympus BX60 microscope and a Zeiss Axiocam digital camera and superimposed on a grid (Ray et al., 1997). The length of the lines overlying air space air measured using Scion® Image software and then averaged to obtain the mean chord length. Fields containing large airways and vessels were excluded from the chord length measurements. The number of bronchioles and arteries per mm2 were quantified as described elsewhere (Mäki et al., 2005).
Electron microscopy
Excised tissue was processed for electron microscopy as described (Narita et al., 1997a). Briefly, paraformaldehyde/glutaraldehyde-fixed tissue was treated with OsO4, and then embedded in resin. Sections (1 μm) were cut and stained with methylene blue for preliminary light microscopic evaluation. Ultrathin sections were cut, stained with uranyl acetate and lead citrate, and then examined in a JEM 1010 transmission electron microscope.
Immunostaining
Paraformaldehyde-fixed, paraffin-embedded tissue sections were processed for immunoperoxidase staining using previously described methods (Bielinska et al., 2005; Jacobsen et al., 2005). The following primary antibodies were employed: 1) goat anti-mouse GATA-4 IgG (sc-1237, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), 1:200 dilution; 2) goat anti-mouse FOG-2 (sc-9264, Santa Cruz Biotechnology, Inc.), 1:100 dilution; 3) rabbit anti-mouse smooth muscle α-actin (AnaSpec, Inc., San Jose, CA), 1:1000 dilution; 4) rabbit anti-mouse Clara cell secretory protein CC10 (sc-25555, Santa Cruz Biotechnology, Inc.), 1:100 dilution; 5) goat anti-mouse surfactant protein C (sc-7706, Santa Cruz Biotechnology, Inc.), 1:200 dilution; 6) goat anti-phosphohistone H3 (sc-12927, Santa Cruz Biotechnology, Inc.), 1:200 dilution; and 7) mouse anti-BrdU (sc-32323, Santa Cruz Biotechnology, Inc.), 1:200 dilution. Secondary antibodies employed for immunoperoxidase staining were: donkey anti-goat biotinylated IgG (Jackson Immunoresearch, West Grove, PA) 1:1000 dilution; donkey anti-mouse biotinylated IgG (Jackson Immunoresearch), 1:2000 dilution; goat anti-rabbit biotinylated IgG (NEF-813, NEN Life Science, Boston MA), 1:2000 dilution. The avidin-biotin immunoperoxidase system (Vectastain Elite ABC Kit, Vector Laboratories, Inc., Burlingame, CA) and diaminobenzidine (Sigma-Aldrich Corp., St. Louis, MO) were used to visualize the bound antibody; slides were then counterstained with hematoxylin.
In situ DNA 3′-end labeling
Apoptosis was quantified in the embryonic diaphragm using an in situ thymidine deoxyribose-mediated deoxy-UTP nick end labeling (TUNEL) DNA 3′-end labeling kit (Oncor, Gaithersburg, MD). Paraformaldehyde-fixed paraffin sections were rehydrated through an alcohol series. The permeability of the cell membranes was increased by incubating the sections in 400 μg proteinase K (Roche Molecular Biochemicals, Mannheim, Germany) in 200 ml PBS for 15 min. Endogenous peroxidase activity was inhibited by quenching the samples for 5 min in 5% hydrogen peroxide. DNA fragmentation was identified by applying terminal transferase enzyme with digoxigenin-labeled nucleotides to the samples and incubating for 1 h under coverslips. Antidigoxigenin antibody was used to recognize the digoxigenin-labeled nucleotide chains attached to the 3′-ends of sample DNA. A color reaction was produced with diaminobenzidine in the presence of 0.03% hydrogen peroxide. The tissue sections were counterstained with hematoxylin.
RT-PCR
Lung tissue was homogenized in TRIzol (Invitrogen, Carlsbad, CA). Purified RNA (200 ng) was subjected to RT-PCR using a TITANIUM™ one-step kit (BD Biosciences, Palo Alto, CA), oligo(dT) primers for the reverse transcriptase reaction, and the PCR primers were as follows: 1) wild-type Gata4-specific; forward 5′-gaagctgcagcctacggcagt-3′, reverse 5′-gcgcatgtcttcactgctgc-3′, 707 bp; 2) Gata4Δ-specific; forward 5′-tgtcattcttcgctggagcc-3′, reverse 5′-gcgcatgtcttcactgctgc-3′, 761 bp; Gata6; forward 5′-gcaatgcatgcggcttctac-3′, reverse 5′-ctcttggtagcaccagctca-3′, 554 bp (Johnsen et al., 2006). Gapdh primers are published elsewhere (Xin et al., 2006). Agarose gel electrophoresis (1.2%) in the presence of ethidium bromide demonstrated a single band of the expected size for each of the PCR primer pairs. Authenticity of the PCR products was confirmed by direct DNA sequencing.
Results
Gata4 is expressed in early mesenchymal cells of the diaphragm, lungs, and great vessels
We delineated the developmental expression of GATA-4 protein and mRNA in the diaphragm, lungs, and cardiovascular system of wild-type mice. Between embryonic day 11.5 (E11.5) and E15.5, intense GATA-4 immunoreactivity was evident in tissues critical for diaphragm formation (Greer et al., 2000b), including the septum transversum (and its derivative the central tendon), pleuroperitoneal folds (PPF), and esophageal mesenchyme (Fig 1A,B). During this same period of development, GATA-4 was observed in mesodermal derivatives in the lung (mesothelium, sub-mesothelial mesenchyme, and to a lesser extent sub-epithelial mesenchyme; Fig 1A,D). This expression was most pronounced in the middle and accessory lobes of the right lung. GATA-4 was also observed in hepatic sinusoidal cells (Fig 1B) and cardiac cell types, including cardiomyocytes, valvuloseptal tissue, and epicardial cells (Fig 1A and data not shown). In each of these tissues the expression pattern of Gata4 overlapped with that of Fog2 (Fig 1C,E).
Fig 1.

Immunohistochemical staining of diaphragm and lung in the embryonic and newborn mouse. Paraformaldehyde-fixed, paraffin-embedded tissue sections from either E12.5 (A-E) or P1 (F-I) wild-type mice were subjected to immunoperoxidase staining for GATA-4 (A,B,D,F,G,H), FOG-2 (C,E), or α-smooth muscle actin (α-SMA) (I) and then counterstained with hematoxylin. Nuclear GATA-4 antigen was seen in tissues that fuse to form the diaphragm, including esophageal mesenchyme (A, arrowhead), pleuroperitoneal folds, and the central tendon, a derivative of the septum transversum. GATA-4 was also detected in cardiomyocytes, hepatic sinusoidal cells, pulmonary mesenchymal cells (A, arrows) and mesothelial cells of the visceral pleura. At E12.5 GATA-4 co-localized with FOG-2 in mesenchymal cells in the central tendon (B,C) and in the terminal acinar buds of the right middle and accessory lobes of the lung (D,E). At P1 some GATA-4 expression persisted in cells of the central tendon (F), especially cells lining the surface of the tendon, but little GATA-4 was evident in the muscular diaphragm (G). In the newborn lung, GATA-4 was observed in vascular smooth muscle cells (red arrows) but not airway smooth muscle cells (yellow arrows) (H). Control staining with α-SMA documented expression in both vascular and airway smooth muscle (I). Abbreviations: ct, central tendon of the diaphragm; dv, ductus venosus; e, esophagus; ht, heart; ivc, inferior vena cava; li, liver; lu, lung; ppf, pleuroperitoneal fold; s, sinusoidal cell of liver; vp, visceral pleura. Bars, 75 μm (A-E), 25 μm (F-I).
Expression of GATA-4 in the diaphragm and lungs decreased at later stages of development. By postnatal day 1 (P1), only ~50% of the cells in the central tendon exhibited GATA-4 immunoreactivity (principally those lining the surface of the tendon; Fig 1F), and the vast majority of cells in the muscular diaphragm lacked expression of this factor (Fig 1G). In the lungs of E18.5-P1 mice, GATA-4 mRNA and protein were observed in the walls of large and medium arteries (Fig 1H; 2B,E) and in mesothelial cells (Fig 2H), but there was little or no expression of Gata4 in airway epithelial cells (Fig 1H), respiratory smooth muscle (Fig 1H,I), small arteries (Fig 2E), or other pulmonary interstitial cells. By comparison, transcripts encoding GATA-6, a transcription factor implicated in differentiation of pulmonary epithelium and vascular smooth muscle (Keijzer et al., 2001; Yang et al., 2002), were detected in distal airway epithelial cells (Fig 2C, F, I), large pulmonary arteries (Fig 2C), small pulmonary arteries (Fig 2F), and mesothelial cells.
Fig 2.

Expression of GATA-4 and GATA-4 transcripts in late gestation mouse lung. Serial cryosections from a wild-type mouse (E18.5) were stained with H&E (A,D,G) or subjected to in situ hybridization for GATA-4 (B,E,H) or GATA-6 (C,F,I). Dark-field views are shown in B, C, E, F, H & I. The arrows in panels D-F highlight respiratory epithelium. The arrowheads in panels G-I indicate the pleural surface of the right lung. Note that GATA-4 is expressed in the walls of large arteries (B) and in mesothelium (H, arrowheads) but not small arteries (E) or respiratory epithelium. In contrast, GATA-6 is expressed in both large (C) and small arteries (F) as well as distal airway epithelium (F,I) and mesothelium (I). Abbreviations: br, bronchus; la, large artery; sa, small artery. Bars, 50 μm.
Thus, GATA-4, like its co-regulator FOG-2, is expressed in mesenchymal and mesothelial cells of the diaphragm and lungs during the embryonic development of these organs. This pattern of expression implies that GATA-4 could play a functional role in organogenesis of not only the heart (Crispino et al., 2001; Watt et al., 2004; Pu et al., 2004; Zeisberg et al., 2005; Xin et al., 2006) but also the diaphragm and lungs.
Neonatal deaths among C57Bl/6 mice heterozygous for a Gata4 deletion mutation
Gata4 null mice derived from either heterozygote matings (Kuo et al., 1997; Molkentin et al., 1997) or through diploid/tetraploid complementation of Gata4−/− ES cells (Narita et al., 1997b; Watt et al., 2004) die by E10, so insights into diaphragm or lung development cannot be ascertained from these animals. We found that ~40% of C57Bl/6 mice harboring a single copy of a Gata4 mutant allele died within one day of birth, irrespective of gender (Fig 3). This allele, termed Gata4Δex2 (Pu et al., 2004), has a large deletion in exon 2 of the gene, which removes the normal translation start site and N-terminal activation domain. The allele was generated using Cre-lox technology and lacks a neomycin resistance cassette or other plasmid sequences that might affect expression of neighboring genes.
Fig 3.
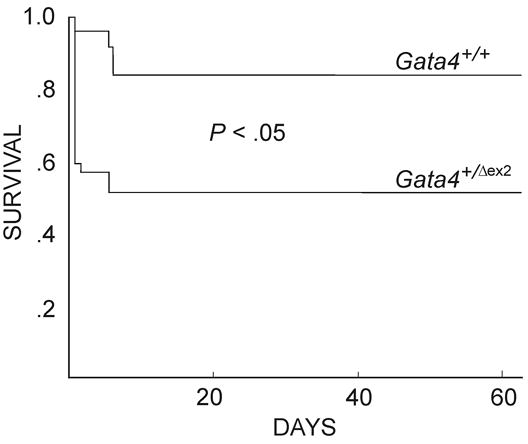
Neonatal mortality among C57Bl/6 Gata4 heterozygotes. Gata4+/Δex2 males were mated with either Gata4+/Δex2 or wild-type females and survival of the resultant offspring monitored. A total of 42 offspring from 8 matings were genotyped. Kaplan-Meier survival curves were prepared using Statview® software. Gata4+/Δex2 mice had an inferior overall survival rate due to early neonatal deaths (P < 0.05, Mantel-Cox Rank test). Gender did not impact survival (data not shown). Neonatal deaths among Gata4+/+ mice were presumed to reflect maternal neglect, as autopsy of these animals showed no evidence of birth defects.
Among newborn offspring of Gata4+/Δex2 matings, the ratio of wild type mice to heterozygotes did not deviate from the expected Mendelian ratio of 1:2. Thus, Gata4 heterozygosity did not appear to be associated with a high rate of intrauterine demise on this genetic background. Pathological analysis of Gata4+/Δex2 mice, including healthy-appearing adults, revealed developmental anomalies in the diaphragm, lungs, and heart. The prevalence of these birth defects in 14 consecutive heterozygotes is summarized in Fig 4. Overall, ~70% of the heterozygotes in this series had a defect in at least one of these organs. We conclude that on the C57Bl/6 background Gata4Δex2 heterozygosity is associated with congenital anomalies of the diaphragm, lungs, and heart, which may occur in isolation or in combination. This paper focuses on anomalies of the diaphragm and lungs in these mice. Cardiac anomalies, including a range of potentially fatal lesions in the newborn period, such as ventricular septal and atrioventricular canal defects, will be discussed in a separate report.
Fig 4.

Phenotypic variability among Gata4+/Δex2 mice. Fourteen successive heterozygotes, ranging in age from P1 to P100, were analyzed. A shaded box indicates the presence of a developmental defect in the diaphragm (e.g., herniation or fusion of diaphragm to the liver), lungs (e.g., distal airway dilatation or delayed airway epithelial cell differentiation), or heart. We observed incomplete penetrance and variable expressivity of Gata4 heterozygosity on this genetic background. Abbreviations: a, secundum atrial septal defect (ASD); av, atrioventricular canal (AV-canal); v, ventricular septal defect (VSD).
Expression of normal and variant Gata4 transcripts in the heterozygotes
To confirm a reduction in Gata4 expression in the heterozygotes, we performed semi-quantitative RT-PCR on samples of embryonic lung using PCR primers that were specific for the wild-type allele. The level of wild-type Gata4 mRNA in the lungs of E14.5 Gata4+/Δex2 mice was 51% that of littermate controls (P < 0.05, two-tailed t-test), whereas the level of Gata6 mRNA in the lungs of heterozygotes was similar to that of controls (Fig 5A,B).
Fig 5.
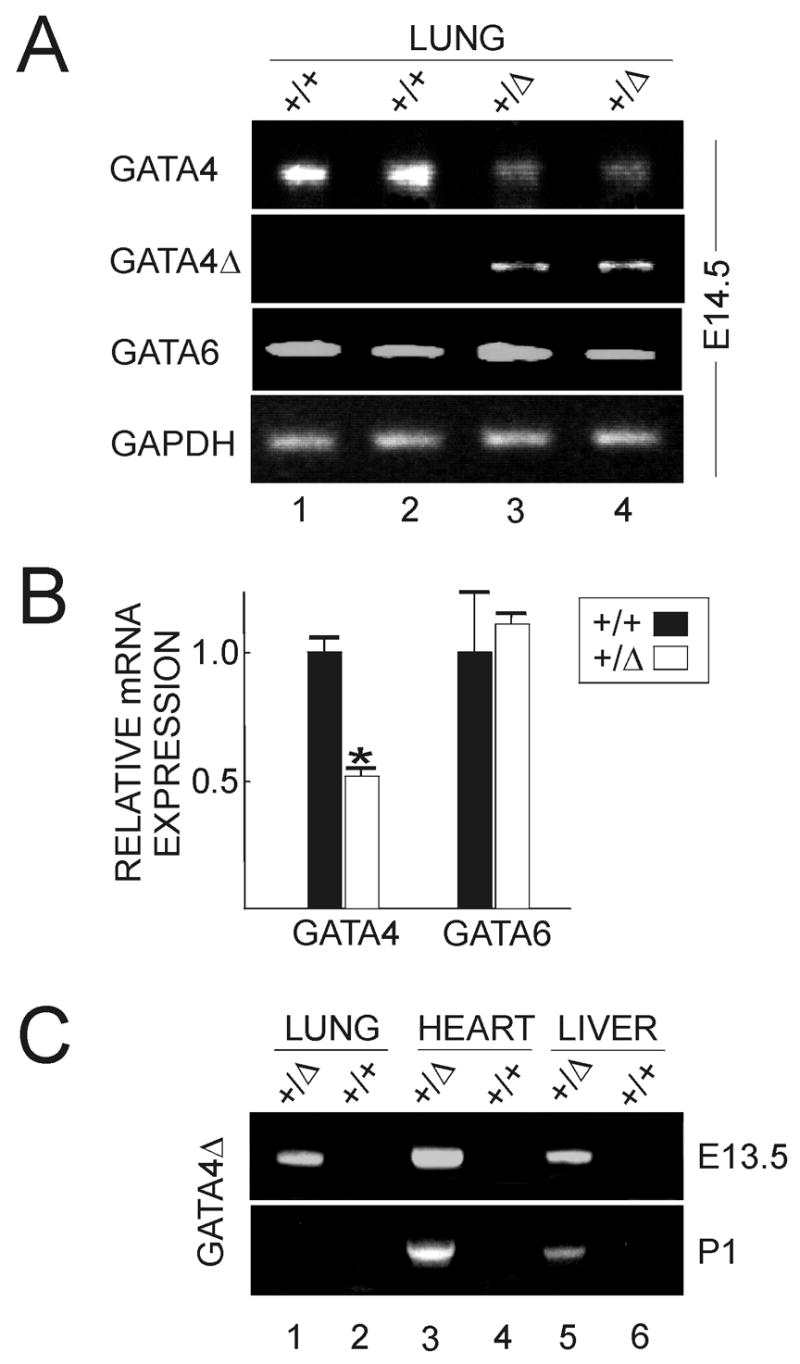
Expression of normal and variant Gata4 transcripts in the heterozygotes. (A) RT-PCR was performed using RNA isolated from E14.5 lungs of Gata4+/+ (+/+) and Gata4+/Δex2 (+/Δ) mice. Duplicates are shown. The PCR primers were specific for wild-type GATA4, Gata4Δex2 (GATA4Δ), GATA6, or GAPDH. (B) Relative mRNA expression normalized to GAPDH in wild-type (filled bars; n = 3) and Gata4+/Δex2 (open bars, n = 3) embryos is shown. The asterisk indicates a significant downregulation of Gata4 mRNA in the heterozygotes (P < 0.05, two-tailed t-test). (C) RT-PCR for the GATA4Δ transcript was performed using RNA from lungs, heart, or liver of Gata4+/+ (+/+) and Gata4+/Δex2 (+/Δ) mice (ages E13.5 and P1).
Using RT-PCR, we found that the Gata4Δex2 allele was transcribed in embryonic (E13.5-E14.5) but not newborn lung (Fig 5A,C). In two other tissues, the heart and liver, expression of Gata4Δex2 mRNA was evident in both embryonic and newborn mice (Fig 5C). If translated using an alternative ATG initiation codon (Arceci et al., 1993), this transcript could give rise to a truncated protein that lacks the N-terminal activation domain but retains both zinc fingers. Such a protein might act as a hypomorph or in a dominant-negative manner. Therefore, the phenotype of the heterozygotes may reflect insufficient amounts of wild-type GATA-4, the production of a mutant protein, or both.
Diaphragmatic defects in Gata4+/Δex2 mice
Diaphragmatic hernias were observed in neonatal and adult Gata4 heterozygotes. The hernias arose in the ventral midline and allowed abdominal viscera (liver and gallbladder) to protrude into the thoracic cavity (Fig 6A). Each hernia was covered by a sac that was continuous with the diaphragm. Portions of the hernia sac near but not adherent to the liver contained a mixture of hepatocytes and connective tissue cells [Fig 6A, proximal hernia sac (phs)], whereas the more peripheral aspects of the sac were composed exclusively of connective tissue [Fig 6A, distal hernia sac (dhs)]. Slit3−/− mice display similar hernia sac histopathology, which indicates a failure of the liver and central tendon to separate during development, a process normally completed before birth (Yuan et al., 2003; Liu et al., 2003). In one newborn heterozygote, herniation was associated with ischemic changes in the median lobe of the liver, which were visible on both gross inspection (Fig 6B) and histological sections (Fig 6B, inset). In all newborn wild-type mice, the liver and central tendon were separated by peritoneal reflections, and trichrome staining revealed well-organized collagen bundles in the central tendon (Fig 6C, left). On the other hand, some Gata4+/Δex2 mice exhibited abnormal fusion of the central tendon to the liver surface, and trichrome staining revealed disorganized collagen bundles (Fig 6C, right).
Fig 6.
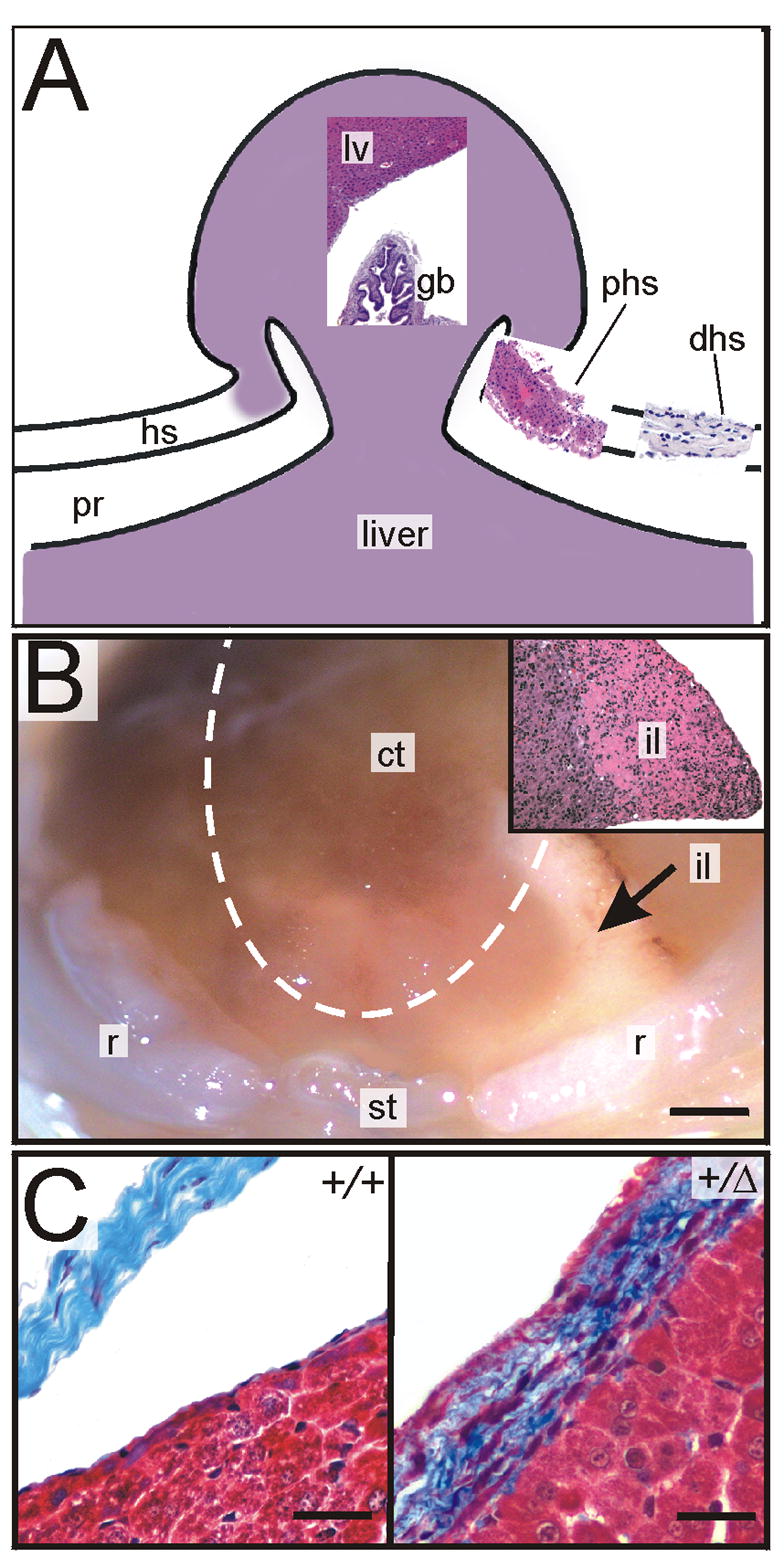
Diaphragmatic defects in Gata4+/Δex2 mice. (A) Diagram of a diaphragmatic hernia in an adult Gata4+/Δex2 mouse. The photographs show H&E-stained sections from different regions in the herniated tissue. Both liver (median lobe) and gallbladder were found in the hernia sac. The portion of the hernia sac adjacent to liver contained a mixture of hepatocytes and connective tissue, indicating incomplete separation of the diaphragm and liver. The distal portion of the hernia sac was composed exclusively of connective tissue and was continuous with the amuscular diaphragm. (B) Stereomicroscopic view of the liver through the translucent diaphragm of a P1 Gata4+/Δex2 mouse. The dashed line shows the boundary between the central tendon and muscular diaphragm. The left lateral aspect of the median lobe of the liver is ischemic due to incarceration in a retrosternal diaphragmatic hernia (arrow). The inset shows an H&E-stained section of the ischemic liver tissue. Note the extensive parenchymal necrosis. (C) Left panel. Trichrome-stained diaphragm from a P1 Gata4+/+ (+/+) mouse, illustrating separation of the central tendon from the liver. Right panel. Trichrome-stained diaphragm from a P1 Gata4+/Δex2 (+/Δ) mouse. Note fusion of the diaphragm and liver and the presence of disorganized fibrils in the central tendon. Abbreviations: ct, central tendon; gb, gallbladder; hs, hernia sac; il, ischemic liver (median lobe); lv, liver (normal); phs, proximal hernia sac; pr, peritoneal recess; r, rib; st, sternum. Bars, 1 mm (B), 50 μm (C).
Among Gata4 heterozygotes the overall incidence of diaphragmatic defects (frank herniation or aberrant fusion of central tendon to liver documented on histological sections) was 6/21 (29%). Overt herniation was seen in 3/21 (14%). No diaphragmatic defects were observed on gross or histological inspection of Gata4+/+ littermate controls (n = 15; P < 0.05, two-population proportions test). Nor were diaphragmatic hernias observed on gross inspection of a larger number of unrelated wild-type C57Bl/6 mice (n = 54; ages P116-P766; P < 0.05) or C57Bl/6 mice heterozygous deficient in another cardiac transcription factor, Nkx2-5 (n = 87; ages P140-P373; P < 0.01).
Reduced expression of Gata4 has been associated with increased apoptosis and impaired cellular proliferation in the heart and other tissues (Suzuki and Evans, 2004; Aries et al., 2004). TUNEL staining of E13.5 embryos showed increased apoptosis in the amuscular diaphragm of Gata4+/Δex2 mice compared to littermate controls (Fig 7B vs. A). To determine whether Gata4Δex2 heterozygosity was associated with decreased cellular proliferation, we performed BrdU labeling. At E13.5 there was no difference in BrdU incorporation in the amuscular diaphragm in Gata4+/ex2 versus wild-type embryos (Fig 7D vs. C). Similar results were obtained when proliferation was measured by staining for the M-phase specific marker phosphohistone H3 (Fig 7F vs. E).
Fig 7.
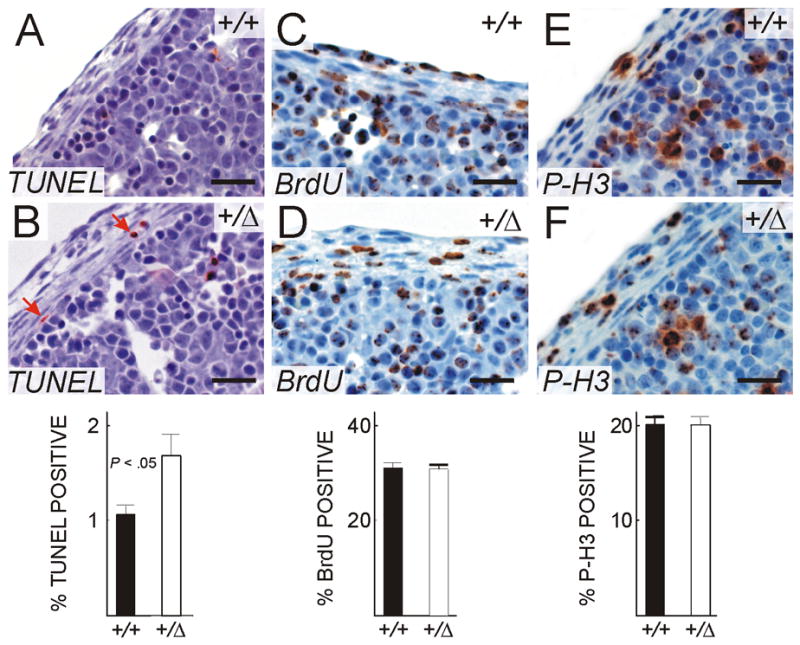
Impact of Gata4 heterozygosity on apoptosis and cellular proliferation in the embryonic diaphragm. Paraformaldehyde-fixed, paraffin-embedded tissue sections from BrdU-labeled E13.5 Gata4+/+ (A,D) or Gata4+/Δex2 (B,E) mice were subjected to TUNEL staining (A,B) or immunoperoxidase staining for BrdU (C,D) or phosphohistone H3 (E,F). The frequencies of TUNEL-, BrdU-, and phosphohistone H3-positive cells in Gata4+/Δex2 (open bars; n = 4) mice versus littermate controls (filled bars; n = 3) are shown in the bar graphs. Note the statistically significant increase in apoptosis in the diaphragm of Gata4+/Δex2 embryos. Bars, 30 μm.
We conclude that heterozygosity for the Gata4 deletion mutation predisposes ex2 C57Bl/6 mice to develop diaphragmatic hernias in the ventral midline. Gata4 heterozygosity does not impair cell proliferation but leads to increased apoptosis of cells in the amuscular diaphragm, which may contribute to the development of diaphragmatic hernias.
Pulmonary abnormalities in Gata4+/Δex2 mice
On gross inspection the lungs of Gata4+/Δex2 mice (ages E16.5-P60) resembled control lungs in overall dimensions and lobation pattern. Internally, however, dilated distal airways and patches of thickened mesenchyme were observed in the lungs of some newborn heterozygotes (Fig 8A vs. B). Airway dilatation was most pronounced in the accessory and middle lobes of the right lung (i.e., sites where mesenchymal Gata4 expression is especially pronounced). Measurement of mean chord length (Ray et al., 1997), a gauge of airway diameter, confirmed a significant increase in saccule size in the Gata4+/Δex2 vs. control mice (Table 1). The overall incidence of distal airway dilatation among Gata4 heterozygotes (ages P1-P8) was 7/21 (33%). No dilatation was observed on histological inspection of Gata4+/+ controls (n = 10; P < 0.05). The number of proximal airways was similar in Gata4+/Δex2 and Gata4+/+ mice, but there was a statistically insignificant decrease in the number of arteries/mm2 in the heterozygotes (Table 1).
Fig 8.
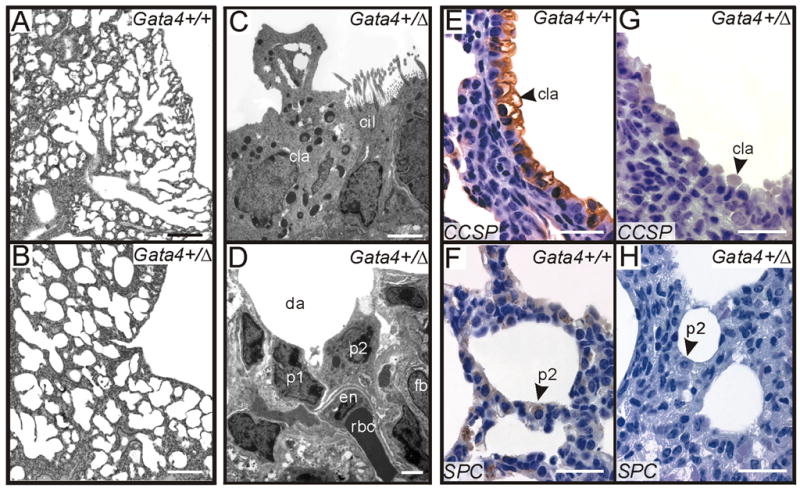
Abnormalities in the lungs of Gata4+/Δex2 mice. (A,B) Paraformaldehyde-fixed, paraffin-embedded sections of lung from a P1 Gata4+/+ (A) or Gata4+/Δex2 (B) mouse were stained with H&E. Note the enlarged distal airspaces in the Gata4+/Δex2 lung tissue. Neither a heart malformation nor diaphragm defect was evident in this Gata4+/Δex2 mouse, suggesting that airway dilatation was not a secondary phenomenon. (C,D) Transmission electron microscopy of proximal (C) and distal (D) airway epithelium from Gata4+/Δex2 mice. The proximal airway epithelium contains ciliated cells and Clara cells, the latter recognizable by the typical dome-shaped apical cytoplasmic protrusions into the airway lumen. The lumen of distal airway is dilated; cells with the hallmark features of type 1 pneumocytes, type 2 pneumocytes, and endothelial cells are evident. (E-H) Paraformaldehyde-fixed, paraffin-embedded sections of lung from a P1 Gata4+/+ mouse (C,D) or P1 Gata4+/Δex2 (E,F) mouse were subjected to immunoperoxidase staining for CCSP (E,G) or SPC (F,H). Note the reduced expression of both the proximal and distal epithelial markers in the Gata4+/Δex2 lung tissue. Lung specimens in panels C, D, G, & H were from the same heterozygote. Abbreviations: cil, ciliated airway cell; cla, Clara cell; p1, da, dilated (distal) airspace; en, endothelial cell; fb, fibroblast; p1, type 1 pneumocyte; p2, type 2 pneumocyte; rbc, red blood cell. Bars, (A,B) 300 μm, (C,D), 3 μm. (E-H) 30 μm
Table 1.
Morphometric analysis of wild-type and Gata4+/Δex2 lungs at P1
| Wild-type (n = 6) | Gata4+/Δex2 (n = 6) | |
|---|---|---|
| Mean chord length (μm) | 20 ± 2.8 | 29 ± 3.1* |
| # bronchioles/mm2 | 8.8 + 2.1 | 8.2 + 1.6 |
| # arteries/mm2 | 16.9 + 1.7 | 14.7 + 2.4 |
P < 0.05, Student’s t-test
To determine the impact of Gata4Δex2 heterozygosity on the differentiation of endodermal and mesodermal lineages, we performed electron microscopy and immunostaining on lungs from newborn Gata4+/Δex2 mice and wild-type controls. Ultrastructural analysis showed that proximal airways in Gata4+/Δex2 lungs were lined by normal appearing Clara cells and ciliated cells (Fig 8C), and the dilated distal airways were lined by cells with the typical features of mature type 1 and type 2 pneumocytes (Fig 8D). However, immunohistochemistry revealed reduced or delayed expression of both Clara cell secretory protein (CCSP; Fig 8E vs. G) and the type 2 pneumocyte marker, surfactant protein C (SPC; Fig 8F vs. H) in some P1 mice. This impaired expression of airway markers was evident in only 2/20 (10%) of Gata4+/Δex2 mice and was limited to those with the most severe distal airway dilatation.
Expression of smooth muscle α-actin (α-SMA) in pulmonary arteries and airway smooth muscle was similar in wild type mice (Fig 9A) and Gata4 heterozygotes (Fig 9C). On the other hand, ectopic expression of α-SMA was evident in mesothelial and submesothelial cells lining the right middle and accessory lobes of the heterozygotes (Fig 9B vs. D). This aberrant α-SMA expression, which was observed in 10% of heterozygotes, might reflect trans-differentiation of mesothelial cells to myofibroblasts or de-repression of the α-SMA gene. Intriguingly, ectopic α-SMA expression has been observed (Shikama et al., 2003) in the pleura of mice bearing a mutant of p300, a known cofactor of GATA-4 (Dai and Markham, 2001).
Fig 9.

Ectopic expression of α-SMA in the lungs of Gata4+/Δex2 mice. Sections of paraformaldehyde-fixed, paraffin-embedded lungs from P1 Gata4+/+ (A,B) or Gata4+/Δex2 (C,D) mice were subjected to immunoperoxidase staining for α-smooth muscle actin (α-SMA) followed by hematoxylin counterstaining. Proximal airways are shown in (A,C), while distal airways and pleura are shown in (B,D). In both Gata4+/+ and Gata4+/Δex2 lungs, α-SMA is seen in airway smooth muscle (yellow arrowheads) and vascular smooth muscle (black or white arrows). Robust expression of α-SMA was observed in mesothelial cells and subjacent mesenchymal cells in Gata4+/Δex2 but not Gata4+/+ lungs (compare red arrows in B vs. D). Abbreviations: da, dilated (distal) airway; pa, proximal airway. Bars, 50 μm.
In summary, Gata4+/Δex2 mice are predisposed to distal airway dilatation and specific alterations in pulmonary gene expression, affecting both endoderm and mesoderm derivatives. The presence of ectopic α-SMA expression in Gata4+/Δex2 lungs suggests that GATA-4 might directly or indirectly function as a repressor of certain genes. Since some Gata4 heterozygotes exhibited lung defects in the absence of CDH (Fig 5), we infer that the pulmonary abnormalities are not merely secondary to diaphragmatic defects.
Absence of GATA-4 does not block mesenchymal cell differentiation in the diaphragm or lung
As noted above, Gata4−/− embryos exhibit severe malformations on both pure (129 or C57Bl/6) and mixed (129:C67Bl/6) genetic backgrounds (Kuo et al., 1997; Molkentin et al., 1997; Narita et al., 1997b; Watt et al., 2004). We therefore wondered whether Gata4−/− progenitors might exhibit a block in early mesenchymal cell differentiation regardless of genetic background. To test this possibility, we generated chimeric mouse embryos by injecting Gata4−/− ES cells (129/Sv//Ev) into Rosa26 (C57BL/6) embryos. Both copies of the Gata4 gene in these ES cells bear a neomycin resistance cassette, which abrogates mRNA expression (Soudais et al., 1995; Kuo et al., 1997). Rosa26 mice bear a ubiquitously expressed β-gal transgene that facilitates lineage tracing (Friedrich and Soriano, 1991).
Nullizygous ES cells retained the capacity to contribute to the central tendon of E14.5 chimeras, as demonstrated by X-gal staining (Fig 10A). In situ hybridization of adjacent tissue sections verified that these central tendon cells lacked expression of Gata4 (Fig 10B) but maintained expression of another mesenchymal marker, Gata6 (Fig 10C). Similarly, Gata4−/− ES cells contributed to mesenchymal cells and mesothelial cells in the lungs of E15.5 chimeras (data not shown).
Fig 10.
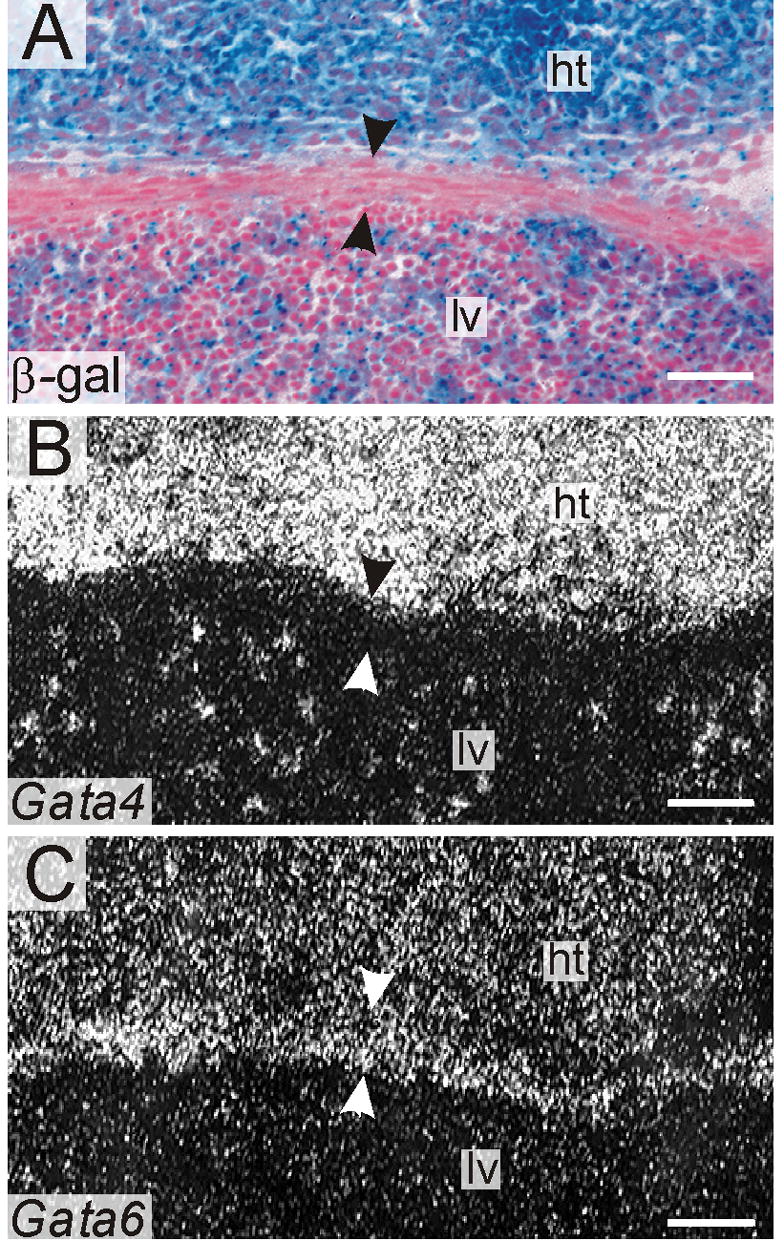
Gata4−/− cells retain the capacity to differentiate into mesenchymal cells in the diaphragm. Adjacent cryosections through an E14.5 Gata4−/− Rosa26 chimera were stained with X-gal (A) or subjected to in situ hybridization for Gata4 (B) and Gata6 (C). Dark-field views are shown in B & C. Note the presence of β-gal negative (Gata4−/−) cells in the central tendon (A, arrowheads). These cells lack Gata4 mRNA (B, arrowheads) but express abundant Gata6 mRNA (C, arrowheads). Control experiments (not shown) documented β-gal and Gata4 mRNA in the central tendon of age-matched non-chimeric mice. Abbreviations: ht, heart; liver, lv. Bars, 100 μm
We did not observe diaphragmatic hernias or overt lung anomalies among chimeric embryos (n = 7, 25-60% chimerism by GPI isozyme analysis). High percentage Gata4−/− chimeras do not survive to term (Narita et al., 1997a; Narita et al., 1997b; Watt et al., 2004), precluding an analysis of the impact of GATA-4 absence on postnatal diaphragm or lung development. Based on analysis of fetal chimeras, we conclude that GATA-4 is not essential for differentiation of mesenchymal cells in the diaphragm or lungs.
Discussion
Despite advances in antenatal diagnosis, surgical techniques, and medical management, the morbidity and mortality associated with CDH remain substantial (Colvin et al., 2005; Smith et al., 2005). Approximately half of the patients die in the neonatal period, and survivors often have longstanding cardiopulmonary dysfunction (West and Wilson, 2005). Although theoretically promising, fetal surgery has not been shown to improve outcome for infants with this condition. Consequently, a major focus of ongoing CDH research is on disease prevention, which mandates a better understanding of its pathogenesis.
As with other developmental defects, our ability to characterize the molecular basis of CDH is dependent on animal models, and the most widely used is exposure of fetal rodents to nitrofen (Greer et al., 2000a). Diaphragmatic hernias have been observed in several genetically engineered mouse strains, including those with germline homozygous mutations of Wt1 (Kreidberg et al., 1993; Moore et al., 1998; Moore et al., 1999), Slit3 (Yuan et al., 2003; Liu et al., 2003), the Slit receptor Robo1 (Xian et al., 2001), Fog2 (Ackerman et al., 2005), or Lox (Mäki et al., 2002; Hornstra et al., 2003; Mäki et al., 2005). More recently, conditional mutagenesis of Coup-TFII in the foregut mesentery and PPF has been shown to cause CDH in mice (You et al., 2005). Supporting the mesenchymal hit hypothesis, some of these mouse strains predisposed to CDH have attendant defects in the lungs or heart.
We describe here a novel mouse model of CDH based on heterozygosity of a Gata4 deletion mutation. Like other genes implicated in CDH, Gata4 is expressed in the embryonic structures that fuse to form the diaphragm. Gata4+/Δex2 mice develop midline diaphragmatic hernias resembling those seen in Slit3−/− mice (Yuan et al., 2003; Liu et al., 2003). Increased apoptosis in the diaphragmatic substratum of the heterozygotes (Fig 7) may compromise tensile strength and contribute to hernia formation. The type of diaphragmatic hernia seen in the Gata4+/Δex2 mouse differs from the posterolateral (Bochdalek) hernia typically seen in patients with CDH. These differences in anatomic pathology might be species-specific. Modeling studies suggest that diaphragm morphogenesis in rodents is dominated by the PPF and may not be strictly applicable to man (Fisher and Bodenstein, 2006).
In addition to CHD, Gata4+/Δex2 mice are predisposed to primary pulmonary developmental defects, including dilated distal airspaces, impaired (or delayed) expression of airway epithelial markers, and ectopic expression of α-SMA in mesothelium. Proper lung development requires coordinated interactions between endoderm-derived airway epithelial cells and mesodermal derivatives (mesothelium, submesothelial mesenchyme, subepithelial mesenchyme, etc.) (Shannon and Hyatt, 2004; Borok et al., 2006; White et al., 2006). Since pulmonary expression of GATA-4 is restricted to mesodermal derivatives, the airway defects in Gata4 heterozygotes are presumably the indirect effects of perturbed signaling from mesenchyme or mesothelium to endoderm. Similar abnormalities in airway development have been observed in mice deficient in certain ECM proteins, including elastin (Wendel et al., 2000), lysyl oxidase (Mäki et al., 2005), fibulin-4 (McLaughlin et al., 2006), fibulin-5 (Yanagisawa et al., 2002), laminin α5 (Nguyen et al., 2005), and laminin γ2 (Nguyen et al., 2006), and in mice deficient in the transcription factors Foxf1 (Mahlapuu et al., 2001; Kalinichenko et al., 2004), FOG-2 (Ackerman et al., 2005), and p300 (Shikama et al., 2003). In several of these cases, lung abnormalities were more pronounced in the middle and accessory lobes of the right lung.
Phenotypic similarities between Fog2−/− (Ackerman et al., 2005) and Gata4+/Δex2 mice suggest that the main activity affected by FOG-2 deficiency in the diaphragm and lungs is GATA-4 rather than another GATA factor. Supporting this premise, a recent abstract (Ackerman et al., 2006) reported primary defects in lung development in mice harboring a Gata4 missense mutation that disrupts GATA4-FOG interactions; the pulmonary phenotype of these Gata4 missense mutants resembled that of Fog2−/− mice. Traditionally GATA-6, a factor expressed in pulmonary epithelium, has been viewed as the sole member of the GATA family required for lung morphogenesis (Keijzer et al., 2001; Yang et al., 2002); it is now apparent that GATA-4 also influences pulmonary development through its expression in mesodermal cells.
Our observations connecting murine Gata4 mutations to CDH and related developmental anomalies are consistent with other lines of evidence. First, microdeletions spanning the human GATA4 gene on 8p23.1 have been linked to cases of CHD, including cases associated with cardiac malformations typical of GATA4 haploinsufficiency (Faivre et al., 1998; Devriendt et al., 1999; Shimokawa et al., 2005; Barber et al., 2005). In fact, most reported cases of CDH in del(8)(p22.1-pter) had an accompanying cardiac malformation, especially atrioventricular canal defects (Lurie, 2003). Indeed, it has been suggested that deletion 8p23.1 should be considered whenever the combination of diaphragmatic hernia and AV canal is encountered (Faivre et al., 1998). Second, the expression and activity of GATA-4 are influenced by retinoids (Arceci et al., 1993; Kostetskii et al., 1999; Clabby et al., 2003; Ghatpande et al., 2006), and perturbed retinoid metabolism has been linked to malformations of the diaphragm, lungs, and heart (reviewed in Greer et al., 2003; Gallot et al., 2005; Montedonico et al., 2006). Ultimate proof that GATA-4 is involved in the pathogenesis of human CDH awaits the identification of patients with diaphragmatic hernias and small deletions or point mutations within the GATA4 gene. Given the established role of GATA-4 in heart and testis development, such patients with CDH might be predicted to have concomitant cardiac malformations (Pehlivan et al., 1999; Garg et al., 2003; Okubo et al., 2004) or male-to-female sex reversal (Meacham et al., 1991; Maaswinkel-Mooij and Stokvis-Brantsma, 1992; Manouvrier-Hanu et al., 2000; Killeen et al., 2002; Kent et al., 2004).
Our findings, coupled with those of other investigators (Kreidberg et al., 1993; Ackerman et al., 2005; You et al., 2005), suggest that proper development of the diaphragm requires the concerted action of several transcription factors, including GATA-4, FOG-2, COUP-TFII and WT-1. We speculate that these factors regulate a set of genes critical for mesenchymal cell function in the diaphragmatic substratum as well as the lungs and heart. Preliminary analysis of Fog2−/− mouse embryos suggests that the hepatocyte growth factor gene may be one of the targets (Ackerman et al., 2005). Whatever the target genes are, we note that they may be abnormally expressed in some but not all affected animals or even within the cells or tissues of the same animal, as shown, for example, by the random ectopic expression of α-SMA in regions of the pleura of some Gata4 heterozygotes. The molecular mechanism of stochastic gene expression is unknown but could underlie the ostensibly random combination of diaphragm, lung and heart defects in mutant animals and human patients.
Earlier studies of Gata4 heterozygous deficient mice failed to detect CDH or any other obvious birth defect (Kuo et al., 1997; Molkentin et al., 1997; Watt et al., 2004), although Gata4 heterozygotes have been shown to have increased sensitivity to doxorubicin-induced cardiotoxicity (Aries et al., 2004). Differences in genetic background probably impact the development of birth defects in Gata4 heterozygotes, since previous studies employed mixed backgrounds (Kuo et al., 1997; Molkentin et al., 1997) rather than a C57Bl/6 “congenic” strain. The Gata4 loss-of-function allele used here (Gata4Δex2) differs from that used in other studies (Kuo et al., 1997; Molkentin et al., 1997; Watt et al., 2004; Aries et al., 2004), so allele-specific effects might account for some of the findings.
We postulate that epigenetic influences and stochastic events contribute to the phenotypic variability in the Gata4Δex2 heterozygotes on the C57Bl/6 background (Cook et al., 1998; Magee et al., 2003). This incomplete penetrance may be exploited for future mechanistic studies; C57Bl/6 Gata4+/Δex2 mice may serve as a “sensitized” strain that is useful for probing the effects of vitamin A deficiency, teratogens, and modifier genes on the development of CDH and related anomalies.
Acknowledgments
We thank Karen Hutton in the DDRCC Histology Core for her assistance. We thank Brian Hackett for helpful discussions. This research was supported by NIH HL61006 & DK52574, MOD FY02-203, Mallinckrodt Foundation, Finnish Pediatric Research Foundation, and the Juselius Foundation. PYJ is a Scholar of the Child Health Research Center of Excellence in Developmental Biology at Washington University School of Medicine (K12-HD001487).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ackerman KG, Herron BJ, Vargas SO, Huang H, Tevosian SG, Kochilas L, Rao C, Pober BR, Babiuk RP, Epstein JA, Greer JJ, Beier DR. Fog2 is required for normal diaphragm and lung development in mice and humans. PLoS Genet. 2005;1:58–65. doi: 10.1371/journal.pgen.0010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ackerman KG, Wang J, Fujiwara Y, Luo LL, Wait A, Orkin SH, Beier DR. Gata4 is necessary for normal pulmonary lobar development; Pediatric Academic Societies' Meeting; San Francisco. 2006. [Google Scholar]
- Arceci RJ, King AAJ, Simon MC, Orkin SH, Wilson DB. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermal derivatives and heart. Mol Cell Biol. 1993;13:2235–2246. doi: 10.1128/mcb.13.4.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aries A, Paradis P, Lefebvre C, Schwartz RJ, Nemer M. Essential role of GATA-4 in cell survival and drug-induced cardiotoxicity. Proc Natl Acad Sci U S A. 2004;101:6975–6980. doi: 10.1073/pnas.0401833101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiuk RP, Greer JJ. Diaphragm defects occur in a CDH hernia model independently of myogenesis and lung formation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1310–L1314. doi: 10.1152/ajplung.00257.2002. [DOI] [PubMed] [Google Scholar]
- Barber JC, Maloney V, Hollox EJ, Stuke-Sontheimer A, du BG, Daumiller E, Klein-Vogler U, Dufke A, Armour JA, Liehr T. Duplications and copy number variants of 8p23.1 are cytogenetically indistinguishable but distinct at the molecular level. Eur J Hum Genet. 2005;13:1131–1136. doi: 10.1038/sj.ejhg.5201475. [DOI] [PubMed] [Google Scholar]
- Bielinska M, Genova E, Boime I, Parviainen H, Kiiveri S, Leppaluoto J, Rahman N, Heikinheimo M, Wilson DB. Gonadotropin-induced adrenocortical neoplasia in NU/J nude mice. Endocrinol. 2005;146:3975–3984. doi: 10.1210/en.2004-1643. [DOI] [PubMed] [Google Scholar]
- Borok Z, Li C, Liebler J, Aghamohammadi N, Londhe VA, Minoo P. Developmental pathways and specification of intrapulmonary stem cells. Pediatr Res. 2006;59:84R–93R. doi: 10.1203/01.pdr.0000203563.37626.77. [DOI] [PubMed] [Google Scholar]
- Borys D, Taxy JB. Congenital diaphragmatic hernia and chromosomal anomalies: autopsy study. Pediatr Dev Pathol. 2004;7:35–38. doi: 10.1007/s10024-003-2133-7. [DOI] [PubMed] [Google Scholar]
- Clabby ML, Robison TA, Quigley HF, Wilson DB, Kelly DP. Retinoid X receptor alpha represses GATA-4-mediated transcription via a retinoid-dependent interaction with the cardiac-enriched repressor FOG-2. J Biol Chem. 2003;278:5760–5767. doi: 10.1074/jbc.M208173200. [DOI] [PubMed] [Google Scholar]
- Colvin J, Bower C, Dickinson JE, Sokol J. Outcomes of congenital diaphragmatic hernia: a population-based study in Western Australia. Pediatrics. 2005;116:e356–e363. doi: 10.1542/peds.2004-2845. [DOI] [PubMed] [Google Scholar]
- Cook DL, Gerber AN, Tapscott SJ. Modeling stochastic gene expression: implications for haploinsufficiency. Proc Natl Acad Sci U S A. 1998;95:15641–15646. doi: 10.1073/pnas.95.26.15641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispino JD, Lodish MB, Thurberg BL, Litovsky SH, Collins T, Molkentin JD, Orkin SH. Proper coronary vascular development and heart morphogenesis depend on interaction of GATA-4 with FOG cofactors. Genes Dev. 2001;15:839–844. doi: 10.1101/gad.875201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai YS, Markham BE. p300 functions as a coactivator of transcription factor GATA-4. J Biol Chem. 2001;276:37178–37185. doi: 10.1074/jbc.M103731200. [DOI] [PubMed] [Google Scholar]
- Devriendt K, Matthijs G, Van Dael R, Gewillig M, Eyskens B, Hjalgrim H, Dolmer B, McGaughran JBr, Marynen P, Fryns JP, Vermeesch JR. Delineation of the critical deletion region for congenital heart defects, on chromosome 8p23.1. Am J Hum Genet. 1999;64:1119–1126. doi: 10.1086/302330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre L, Morichon-Delvallez N, Viot G, Narcy F, Loison S, Mandelbrot L, Aubry MC, Raclin V, Edery P, Munnich A, Vekemans M. Prenatal diagnosis of an 8p23.1 deletion in a fetus with a diaphragmatic hernia and review of the literature. Prenat Diagn. 1998;18:1055–1060. doi: 10.1002/(sici)1097-0223(1998100)18:10<1055::aid-pd405>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Fisher JC, Bodenstein L. Computer simulation analysis of normal and abnormal development of the mammalian diaphragm. Theor Biol Med Model. 2006;3:9. doi: 10.1186/1742-4682-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich G, Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991;5:1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Gallot D, Marceau G, Coste K, Hadden H, Robert-Gnansia E, Laurichesse H, Dechelotte PJ, Labbe A, Dastugue B, Lemery D, Sapin V. Congenital diaphragmatic hernia: a retinoid-signaling pathway disruption during lung development? Birth Defects Res A Clin Mol Teratol. 2005;73:523–531. doi: 10.1002/bdra.20151. [DOI] [PubMed] [Google Scholar]
- Garg V, Kathiriya IS, Barnes R, Schluterman MK, King IN, Butler CA, Rothrock CR, Eapen RS, Hirayama-Yamada K, Joo K, Matsuoka R, Cohen JC, Srivastava D. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:443–447. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- Ghatpande S, Brand T, Zile M, Evans T. Bmp2 and Gata4 function additively to rescue heart tube development in the absence of retinoids. Dev Dyn. 2006;235:2030–2039. doi: 10.1002/dvdy.20836. [DOI] [PubMed] [Google Scholar]
- Graziano JN. Cardiac anomalies in patients with congenital diaphragmatic hernia and their prognosis: a report from the Congenital Diaphragmatic Hernia Study Group. J Pediatr Surg. 2005;40:1045–1049. doi: 10.1016/j.jpedsurg.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Allan DW, Babiuk RP, Lemke RP. Recent advances in understanding the pathogenesis of nitrofen-induced congenital diaphragmatic hernia. Pediatr Pulmonol. 2000a;29:394–399. doi: 10.1002/(sici)1099-0496(200005)29:5<394::aid-ppul9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Babiuk RP, Thebaud B. Etiology of congenital diaphragmatic hernia: the retinoid hypothesis. Pediatr Res. 2003;53:726–730. doi: 10.1203/01.PDR.0000062660.12769.E6. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Cote D, Allan DW, Zhang W, Babiuk RP, Ly L, Lemke RP, Bagnall K. Structure of the primordial diaphragm and defects associated with nitrofen-induced CDH. J Appl Physiol. 2000b;89:2123–2129. doi: 10.1152/jappl.2000.89.6.2123. [DOI] [PubMed] [Google Scholar]
- Hornstra IK, Birge S, Starcher B, Bailey AJ, Mecham RP, Shapiro SD. Lysyl oxidase is required for vascular and diaphragmatic development in mice. J Biol Chem. 2003;278:14387–14393. doi: 10.1074/jbc.M210144200. [DOI] [PubMed] [Google Scholar]
- Howe DT, Kilby MD, Sirry H, Barker GM, Roberts E, Davison EV, Mchugo J, Whittle MJ. Structural chromosome anomalies in congenital diaphragmatic hernia. Prenat Diagn. 1996;16:1003–1009. doi: 10.1002/(SICI)1097-0223(199611)16:11<1003::AID-PD995>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Jacobsen CM, Mannisto S, Porter-Tinge S, Genova E, Parviainen H, Heikinheimo M, Adameyko II, Tevosian SG, Wilson DB. GATA-4:FOG interactions regulate gastric epithelial development in the mouse. Dev Dyn. 2005;234:355–362. doi: 10.1002/dvdy.20552. [DOI] [PubMed] [Google Scholar]
- Jesudason EC. Small lungs and suspect smooth muscle: congenital diaphragmatic hernia and the smooth muscle hypothesis. J Pediatr Surg. 2006;41:431–435. doi: 10.1016/j.jpedsurg.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Johnsen IK, Slawik M, Shapiro I, Hartmann MF, Wudy SA, Looyenga BD, Hammer GD, Reincke M, Beuschlein F. Gonadectomy in mice of the inbred strain CE/J induces proliferation of sub-capsular adrenal cells expressing gonadal marker genes. J Endocrinol. 2006;190:47–57. doi: 10.1677/joe.1.06750. [DOI] [PubMed] [Google Scholar]
- Kalinichenko VV, Gusarova GA, Kim IM, Shin B, Yoder HM, Clark J, Sapozhnikov AM, Whitsett JA, Costa RH. Foxf1 haploinsufficiency reduces Notch-2 signaling during mouse lung development. Am J Physiol Lung Cell Mol Physiol. 2004;286:L521–L530. doi: 10.1152/ajplung.00212.2003. [DOI] [PubMed] [Google Scholar]
- Keijzer R, Liu J, Deimling J, Tibboel D, Post M. Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol. 2000;156:1299–1306. doi: 10.1016/S0002-9440(10)65000-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keijzer R, van Tuyl M, Meijers C, Post M, Tibboel D, Grosveld F, Koutsourakis M. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development. 2001;128:503–511. doi: 10.1242/dev.128.4.503. [DOI] [PubMed] [Google Scholar]
- Kent A, Simpson E, Ellwood D, Silink M. 46,XY sex-reversal (Swyer syndrome) and congenital diaphragmatic hernia. Am J Med Genet A. 2004;131:103–105. doi: 10.1002/ajmg.a.30298. [DOI] [PubMed] [Google Scholar]
- Ketola I, Anttonen M, Vaskivuo T, Tapanainen JS, Toppari J, Heikinheimo M. Developmental expression and spermatogenic stage specificity of transcription factors GATA-1 and GATA-4 and their cofactors FOG-1 and FOG-2 in the mouse testis. Eur J Endocrinol. 2002;147:397–406. doi: 10.1530/eje.0.1470397. [DOI] [PubMed] [Google Scholar]
- Killeen OG, Kelehan P, Reardon W. Double vagina with sex reversal, congenital diaphragmatic hernia, pulmonary and cardiac malformations--another case of Meacham syndrome. Clin Dysmorphol. 2002;11:25–28. doi: 10.1097/00019605-200201000-00005. [DOI] [PubMed] [Google Scholar]
- Kostetskii I, Jiang Y, Kostetskaia E, Yuan S, Evans T, Zile M. Retinoid signaling required for normal heart development regulates GATA-4 in a pathway distinct from cardiomyocyte differentiation. Dev Biol. 1999;206:206–218. doi: 10.1006/dbio.1998.9139. [DOI] [PubMed] [Google Scholar]
- Kreidberg JA, Sariola H, Loring JM, Maeda M, Pelletia J, Housman J, Jaenesch R. WT-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anadappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Liu J, Zhang L, Wang D, Shen H, Jiang M, Mei P, Hayden PS, Sedor JR, Hu H. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice. Mech Dev. 2003;120:1059–1070. doi: 10.1016/s0925-4773(03)00161-8. [DOI] [PubMed] [Google Scholar]
- López I, Bafalliu JA, Bernabé MC, García F, Costa M, Guillén-Navarro E. Prenatal diagnosis of de novo deletions of 8p23.1 or 15q26.1 in two fetuses with diaphragmatic hernia and congenital heart defects. Prenat Diagn. 2006;26:577–580. doi: 10.1002/pd.1468. [DOI] [PubMed] [Google Scholar]
- Losty PD, Connell MG, Freese R, Laval S, Okoye BO, Smith A, Kluth D, Lloyd DA. Cardiovascular malformations in experimental congenital diaphragmatic hernia. J Pediatr Surg. 1999;34:1203–1207. doi: 10.1016/s0022-3468(99)90152-5. [DOI] [PubMed] [Google Scholar]
- Lurie IW. Where to look for the genes related to diaphragmatic hernia? Genet Couns. 2003;14:75–93. [PubMed] [Google Scholar]
- Maaswinkel-Mooij PD, Stokvis-Brantsma WH. Phenotypically normal girl with male pseudohermaphroditism, hypoplastic left ventricle, lung aplasia, horseshoe kidney, and diaphragmatic hernia. Am J Med Genet. 1992;42:647–648. doi: 10.1002/ajmg.1320420503. [DOI] [PubMed] [Google Scholar]
- Magee JA, Abdulkadir SA, Milbrandt J. Haploinsufficiency at the Nkx3.1 locus. A paradigm for stochastic, dosage-sensitive gene regulation during tumor initiation. Cancer Cell. 2003;3:273–283. doi: 10.1016/s1535-6108(03)00047-3. [DOI] [PubMed] [Google Scholar]
- Mahlapuu M, Enerback S, Carlsson P. Haploinsufficiency of the forkhead gene Foxf1, a target for sonic hedgehog signaling, causes lung and foregut malformations. Development. 2001;128:2397–2406. doi: 10.1242/dev.128.12.2397. [DOI] [PubMed] [Google Scholar]
- Mäki JM, Räsänen J, Tikkanen H, Sormunen R, Mäkikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Mäki JM, Sormunen R, Lippo S, Kaarteenaho-Wiik R, Soininen R, Myllyharju J. Lysyl oxidase is essential for normal development and function of the respiratory system and for the integrity of elastic and collagen fibers in various tissues. Am J Pathol. 2005;167:927–936. doi: 10.1016/S0002-9440(10)61183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manouvrier-Hanu S, Besson R, Cousin L, Jeanpierre C, Kacet N, Cartigny M, Devisme L, Storme L, De MB, Lequien P. Sex reversal and diaphragmatic hernia in phenotypicaly female sibs with normal XY chromosomes. J Med Genet. 2000;37:315–318. doi: 10.1136/jmg.37.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin PJ, Chen Q, Horiguchi M, Starcher BC, Stanton JB, Broekelmann TJ, Marmorstein AD, McKay B, Mecham R, Nakamura T, Marmorstein LY. Targeted disruption of fibulin-4 abolishes elastogenesis and causes perinatal lethality in mice. Mol Cell Biol. 2006;26:1700–1709. doi: 10.1128/MCB.26.5.1700-1709.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meacham LR, Winn KJ, Culler FL, Parks JS. Double vagina, cardiac, pulmonary, and other genital malformations with 46,XY karyotype. Am J Med Genet. 1991;41:478–481. doi: 10.1002/ajmg.1320410420. [DOI] [PubMed] [Google Scholar]
- Migliazza L, Otten C, Xia H, Rodriguez JI, ez-Pardo JA, Tovar JA. Cardiovascular malformations in congenital diaphragmatic hernia: human and experimental studies. J Pediatr Surg. 1999;34:1352–1358. doi: 10.1016/s0022-3468(99)90010-6. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Montedonico S, Nakazawa N, Puri P. Retinoic acid rescues lung hypoplasia in nitrofen-induced hypoplastic foetal rat lung explants. Pediatr Surg Int. 2006;22:2–8. doi: 10.1007/s00383-005-1571-x. [DOI] [PubMed] [Google Scholar]
- Moore AW, McInnes L, Kreidberg J, Hastie ND, Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999;126:1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- Moore AW, Schedl A, McInnes L, Doyle M, Hecksher-Sorensen J, Hastie ND. YAC transgenic analysis reveals Wilms' tumour 1 gene activity in the proliferating coelomic epithelium, developing diaphragm and limb. Mech Dev. 1998;79:169–184. doi: 10.1016/s0925-4773(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson D. Cardiomyocyte differentiation by GATA-4 deficient embryonic stem cells. Development. 1997a;122:3755–3764. doi: 10.1242/dev.124.19.3755. [DOI] [PubMed] [Google Scholar]
- Narita N, Bielinska M, Wilson DB. Wild-type endoderm abrogates the ventral developmental defects associated with GATA-4 deficiency in the mouse. Dev Biol. 1997b;189:270–274. doi: 10.1006/dbio.1997.8684. [DOI] [PubMed] [Google Scholar]
- Natoli TA, Alberta JA, Bortvin A, Taglienti ME, Menke DB, Loring J, Jaenisch R, Page DC, Housman DE, Kreidberg JA. Wt1 functions in the development of germ cells in addition to somatic cell lineages of the testis. Dev Biol. 2004;268:429–440. doi: 10.1016/j.ydbio.2003.12.033. [DOI] [PubMed] [Google Scholar]
- Nemer G, Fadlalah F, Usta J, Nemer M, Dbaibo G, Obeid M, Bitar F. A novel mutation in the GATA4 gene in patients with Tetralogy of Fallot. Hum Mutat. 2006;27:293–294. doi: 10.1002/humu.9410. [DOI] [PubMed] [Google Scholar]
- Nguyen NM, Kelley DG, Schlueter JA, Meyer MJ, Senior RM, Miner JH. Epithelial laminin alpha5 is necessary for distal epithelial cell maturation, VEGF production, and alveolization in the developing murine lung. Dev Biol. 2005;282:111–125. doi: 10.1016/j.ydbio.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Nguyen NM, Pulkkinen L, Schlueter JA, Meneguzzi G, Uitto J, Senior RM. Lung development in laminin gamma2 deficiency: abnormal tracheal hemidesmosomes with normal branching morphogenesis and epithelial differentiation. Respir Res. 2006;7:28. doi: 10.1186/1465-9921-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo A, Miyoshi O, Baba K, Takagi M, Tsukamoto K, Kinoshita A, Yoshiura K, Kishino T, Ohta T, Niikawa N, Matsumoto N. A novel GATA4 mutation completely segregated with atrial septal defect in a large Japanese family. J Med Genet. 2004;41:e97. doi: 10.1136/jmg.2004.018895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecile V, Petroni MG, Fertz MC, Filippi G. Deficiency of distal 8p-: report of two cases and review of the literature. Clinical Genetics. 1990;38:271–278. doi: 10.1111/j.1399-0004.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Pehlivan T, Pober BR, Brueckner M, Garrett S, Slaugh R, Van RR, Wilson DB, Watson MS, Hing AV. GATA4 haploinsufficiency in patients with interstitial deletion of chromosome region 8p23.1 and congenital heart disease. Am J Med Genet. 1999;83:201–206. [PubMed] [Google Scholar]
- Pober BR, Lin A, Russell M, Ackerman KG, Chakravorty S, Strauss B, Westgate MN, Wilson J, Donahoe PK, Holmes LB. Infants with Bochdalek diaphragmatic hernia: sibling precurrence and monozygotic twin discordance in a hospital-based malformation surveillance program. Am J Med Genet A. 2005;138:81–88. doi: 10.1002/ajmg.a.30904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu WT, Ishiwata T, Juraszek AL, Ma Q, Izumo S. GATA4 is a dosage-sensitive regulator of cardiac morphogenesis. Dev Biol. 2004;275:235–244. doi: 10.1016/j.ydbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Ray P, Tang W, Wang P, Homer R, Kuhn C, III, Flavell RA, Elias JA. Regulated overexpression of interleukin 11 in the lung. Use to dissociate development-dependent and -independent phenotypes. J Clin Invest. 1997;100:2501–2511. doi: 10.1172/JCI119792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JM, Hyatt BA. Epithelial-mesenchymal interactions in the developing lung. Annu Rev Physiol. 2004;66:625–645. doi: 10.1146/annurev.physiol.66.032102.135749. [DOI] [PubMed] [Google Scholar]
- Shikama N, Lutz W, Kretzschmar R, Sauter N, Roth JF, Marino S, Wittwer J, Scheidweiler A, Eckner R. Essential function of p300 acetyltransferase activity in heart, lung and small intestine formation. EMBO J. 2003;22:5175–5185. doi: 10.1093/emboj/cdg502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimokawa O, Miyake N, Yoshimura T, Sosonkina N, Harada N, Mizuguchi T, Kondoh S, Kishino T, Ohta T, Remco V, Takashima T, Kinoshita A, Yoshiura K, Niikawa N, Matsumoto N. Molecular characterization of del(8)(p23.1p23.1) in a case of congenital diaphragmatic hernia. Am J Med Genet A. 2005;136:49–51. doi: 10.1002/ajmg.a.30778. [DOI] [PubMed] [Google Scholar]
- Smith NP, Jesudason EC, Featherstone NC, Corbett HJ, Losty PD. Recent advances in congenital diaphragmatic hernia. Arch Dis Child. 2005;90:426–428. doi: 10.1136/adc.2003.045765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soudais C, Bielinska M, Heikinheimo M, MacArthur CA, Narita N, Saffitz JE, Simon MC, Leiden JM, Wilson DB. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development. 1995;121:3877–3888. doi: 10.1242/dev.121.11.3877. [DOI] [PubMed] [Google Scholar]
- Suzuki YJ, Evans T. Regulation of cardiac myocyte apoptosis by the GATA-4 transcription factor. Life Sci. 2004;74:1829–1838. doi: 10.1016/j.lfs.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Svensson EC, Huggins GS, Lin H, Clendenin C, Jiang F, Tufts R, Dardik FB, Leiden JM. A syndrome of tricuspid atresia in mice with a targeted mutation of the gene encoding Fog-2. Nat Genet. 2000;25:353–356. doi: 10.1038/77146. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Berul CI, Ishii M, Jay PY, Wakimoto H, Douglas P, Yamasaki N, Kawamoto T, Gehrmann J, Maguire CT, Schinke M, Seidman CE, Seidman JG, Kurachi Y, Izumo S. A mouse model of congenital heart disease: cardiac arrhythmias and atrial septal defect caused by haploinsufficiency of the cardiac transcription factor Csx/Nkx2.5. Cold Spring Harb Symp Quant Biol. 2002;67:317–325. doi: 10.1101/sqb.2002.67.317. [DOI] [PubMed] [Google Scholar]
- Temple IK, Barber JC, James RS, Burge D. Diaphragmatic herniae and translocations involving 8q22 in two patients. J Med Genet. 1994;31:735–737. doi: 10.1136/jmg.31.9.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tevosian SG, Albrecht KH, Crispino JD, Fujiwara Y, Eicher EM, Orkin SH. Gonadal differentiation, sex determination and normal Sry expression in mice require direct interaction between transcription partners GATA4 and FOG2. Development. 2002;129:4627–4634. doi: 10.1242/dev.129.19.4627. [DOI] [PubMed] [Google Scholar]
- Tevosian SG, Deconinck AE, Tanaka M, Schinke M, Litovsky SH, Izumo S, Fujiwara Y, Orkin SH. FOG-2, a cofactor for GATA transcription factors, is essential for heart morphogenesis and development of coronary vessels from epicardium. Cell. 2000;101:729–739. doi: 10.1016/s0092-8674(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Watt AJ, Battle MA, Li J, Duncan SA. GATA4 is essential for formation of the proepicardium and regulates cardiogenesis. Proc Natl Acad Sci U S A. 2004;101:12573–12578. doi: 10.1073/pnas.0400752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY. Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol. 2000;23:320–326. doi: 10.1165/ajrcmb.23.3.3906. [DOI] [PubMed] [Google Scholar]
- West SD, Wilson JM. Follow up of infants with congenital diaphragmatic hernia. Semin Perinatol. 2005;29:129–133. doi: 10.1053/j.semperi.2005.04.007. [DOI] [PubMed] [Google Scholar]
- White AC, Xu J, Yin Y, Smith C, Schmid G, Ornitz DM. FGF9 and SHH signaling coordinate lung growth and development through regulation of distinct mesenchymal domains. Development. 2006;133:1507–1517. doi: 10.1242/dev.02313. [DOI] [PubMed] [Google Scholar]
- Wilson WG, Wyandt HE, Shah H. Interstitial deletion of 8q. Occurrence in a patient with multiple exostoses and unusual facies. Am J Dis Child. 1983;137:444–448. [PubMed] [Google Scholar]
- Xian J, Clark KJ, Fordham R, Pannell R, Rabbitts TH, Rabbitts PH. Inadequate lung development and bronchial hyperplasia in mice with a targeted deletion in the Dutt1/Robo1 gene. Proc Natl Acad Sci U S A. 2001;98:15062–15066. doi: 10.1073/pnas.251407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Davis CA, Molkentin JD, Lien CL, Duncan SA, Richardson JA, Olson EN. A threshold of GATA4 and GATA6 expression is required for cardiovascular development. Proc Natl Acad Sci U S A. 2006;103:11189–11194. doi: 10.1073/pnas.0604604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development. 2002;129:2233–2246. doi: 10.1242/dev.129.9.2233. [DOI] [PubMed] [Google Scholar]
- Yang W, Carmichael SL, Harris JA, Shaw GM. Epidemiologic characteristics of congenital diaphragmatic hernia among 2.5 million california births, 1989-1997. Birth Defects Res A Clin Mol Teratol. 2006;76:170–174. doi: 10.1002/bdra.20230. [DOI] [PubMed] [Google Scholar]
- You LR, Takamoto N, Yu CT, Tanaka T, Kodama T, DeMayo FJ, Tsai SY, Tsai MJ. Mouse lacking COUP-TFII as an animal model of Bochdalek-type congenital diaphragmatic hernia. Proc Natl Acad Sci U S A. 2005;102:16351–16356. doi: 10.1073/pnas.0507832102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Rao Y, Babiuk RP, Greer JJ, Wu JY, Ornitz DM. A genetic model for a central (septum transversum) congenital diaphragmatic hernia in mice lacking Slit3. Proc Natl Acad Sci U S A. 2003;100:5217–5222. doi: 10.1073/pnas.0730709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]


