Abstract
Previously, we reported that bilateral excitotoxic lesions of the basolateral nucleus of the amygdala (BLA) block the enhancing effects of posttraining systemic or intrahippocampal glucocorticoid administration on memory for inhibitory avoidance training. The present study further examined the basis of this permissive influence of the BLA on hippocampal memory functioning. Immediate posttraining unilateral infusions of the specific glucocorticoid receptor agonist RU 28362 (11β,17β-dihydroxy-6,21-dimethyl-17α-pregna-4,6-trien-20-yn-3-one; 3.0, 10.0, or 30.0 ng in 0.5 μl) administered into the dorsal hippocampus of male Sprague–Dawley rats induced dose-dependent enhancement of 48-h inhibitory avoidance retention. Infusions of the β-adrenoceptor antagonist atenolol (0.5 μg in 0.2 μl) into the ipsilateral, but not the contralateral, BLA 10 min prior to training blocked the hippocampal glucocorticoid effects on memory consolidation. Infusions of the muscarinic cholinergic antagonist atropine (0.5 μg in 0.2 μl) into either the ipsilateral or contralateral BLA before training did not block the hippocampal glucocorticoid effects. These findings provide further evidence that β-adrenergic activity in the BLA is essential in enabling glucocorticoid-induced modulation of memory consolidation and are consistent with the hypothesis that the BLA regulates the strength of memory consolidation in other brain structures. The ipsilateral nature of the BLA–hippocampus interaction indicates that BLA influences on hippocampal memory processes are mediated through neural pathways rather than by influences by means of the activation of peripheral stress responses.
Keywords: cognition, emotional arousal, memory consolidation, norepinephrine, RU 28362
There is considerable evidence that the basolateral nucleus of the amygdala (BLA) is critically involved in regulating the consolidation of different forms of memory, including explicit/declarative memory. Many findings indicate that this amygdala nucleus mediates the memory-modulatory effects of adrenal stress hormones released by emotional arousal (1, 2). Findings of several recent studies from our laboratory indicate that posttraining infusions of the specific glucocorticoid receptor (GR or type II) agonist RU 28362 administered into the BLA induce dose-dependent enhancement of inhibitory avoidance retention (3) and that excitotoxic lesions of the BLA induced before training block the memory-enhancing effects of systemically administered glucocorticoids (4) as well as the memory-impairing effects induced by adrenalectomy (5, 6). BLA lesions also block the memory-enhancing effects of the GR agonist administered directly into the hippocampus (7). These findings suggest that BLA neuronal activity modulates stress-induced memory consolidation processes in, or involving, the hippocampus and are consistent with the view that the BLA is not a locus of memory storage but regulates consolidation processes in other brain regions (2).
Extensive evidence indicates that noradrenergic and cholinergic systems in the amygdala participate in modulating memory consolidation (8–13). Although the activation of these two neurotransmitter systems in the BLA may occur under different experimental conditions (14, 15) and induce slightly differential electrophysiological responses in BLA neurons (16–18), they have highly comparable effects on memory consolidation. Posttraining infusion of either a β-adrenoceptor or muscarinic cholinergic agonist into the amygdala enhances inhibitory avoidance retention (11, 12, 19, 20), and inactivation of either receptor type in the BLA blocks the memory-enhancing effects of systemic glucocorticoids (ref. 13; and A.E.P., B.R. and J.L.M, unpublished observation). Moreover, previous findings indicate that the BLA is a critical locus of interaction between glucocorticoids and the noradrenergic system in memory consolidation modulation (13, 21).
The present experiments examined whether β-adrenoceptor or muscarinic cholinergic receptor activation in the BLA is critically involved in enabling facilitation of memory consolidation induced by activation of GRs in the hippocampus. In the first experiment, rats received unilateral microinfusions into the BLA of either the β-adrenoceptor antagonist atenolol or the muscarinic cholinergic antagonist atropine 10 min prior to training in an inhibitory avoidance task. The GR agonist RU 28362 was administered ipsilaterally into the dorsal hippocampus immediately after training, and retention was tested 48 h later. In a second experiment, we examined whether BLA–hippocampus interactions in memory consolidation involve unilateral projections between these brain regions. The experimental procedures were identical to those of the first experiment, except that the BLA and hippocampal infusions were given on contralateral sides. If the influence of the BLA on glucocorticoid-induced effects on hippocampal memory processes is mediated through neural connections between the BLA and hippocampus, only inactivation of the ipsilateral BLA should block the GR effects. On the other hand, if the effects are mediated by peripheral stress responses resulting from BLA activation, then inactivation of either the ipsilateral or contralateral BLA should have similar effects.
MATERIALS AND METHODS
Subjects.
Male Sprague–Dawley rats (n = 342; 270–300 g at time of surgery) from Charles River Breeding Laboratories were used. They were housed individually in a temperature- and humidity-controlled colony room and maintained on a standard 12-h light/12-h dark cycle (0700–1900 h lights on) with ad libitum access to food and water. Training and testing were performed during the light phase of the cycle between 1000 and 1500 h.
Surgery.
The animals were adapted to the vivarium for at least 1 wk before surgery. They were anesthetized with sodium pentobarbital (50 mg/kg of body weight, i.p.) and given atropine sulfate (0.4 mg/kg, i.p.) to maintain respiration. The skull was positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA) and stainless steel guide cannulae (23 gauge) were implanted unilaterally with the cannula tips 1.5 mm above the left dorsal hippocampus [11 mm long; coordinates: anterioposterior (AP), −3.3 mm from bregma; mediolateral (ML), +1.5 mm from midline; dorsoventral (DV), −2.6 mm from skull surface] and 2 mm above either the ipsilateral or contralateral BLA (15 mm long; coordinates: AP, −2.8 mm; ML, ±5.0 mm; DV, −6.5 mm), according to the atlas of Paxinos and Watson (22). The cannulae and two anchoring screws were affixed to the skull with dental cement. Stylets (11- or 15-mm-long 00 insect dissection pins) were inserted into each cannula to maintain patency and were removed only for the infusion of drugs. After surgery, the rats received an s.c. 3.0-ml injection of saline and were placed into an incubator until recovery from anesthesia, after which they were returned into their home cages. The rats were allowed to recover a minimum of 7 days before initiation of training and were handled for 1 min on each of 3 days during this recovery period.
Drugs and Infusion Procedures.
The specific β1-adrenoceptor antagonist atenolol (0.5 μg; Sigma) and the muscarinic cholinergic antagonist atropine (0.5 μg; Sigma) were dissolved in 0.9% saline and infused into either the left or right BLA 10 min prior to training. Drug solutions were freshly prepared before each experiment. The doses were selected on the basis of previous experiments in this laboratory (10, 13). These drugs or a saline control solution were infused into the BLA via a 30-gauge injection needle connected to a 10-μl Hamilton microsyringe by polyethylene (PE-20) tubing. The injection needle protruded 2 mm beyond the tip of the cannula to reach the BLA. A 0.2-μl injection volume was infused over a period of 25 s by an automated syringe pump (Sage Instruments, Boston). The infused volume was based on findings that this volume infused into an identical injection site induces selective neurotoxic lesions of the BLA (4) and that drug infusions of this volume into either the BLA or the adjacent central nucleus induce differential effects on memory consolidation (3, 23). The injection needle was retained within the cannula for an additional 20 s after drug infusion to maximize diffusion and to prevent backflow of drug into the cannula. After completion of the infusion, the animal was returned to its home cage until the start of the inhibitory avoidance training 10 min later.
The specific GR agonist RU 28362 (11β,17β-dihydroxy-6,21-dimethyl-17α-pregna-4,6-trien-20-yn-3-one; 3.0, 10.0, or 30.0 ng; Roussel-UCLAF) was infused into the left dorsal hippocampus immediately after training. The drug was first dissolved in 100% ethanol and subsequently diluted with saline to reach its appropriate concentration. The final concentration of ethanol was 2%. The vehicle solution contained 2% ethanol only. The infusion procedure was similar to that described for infusions into the BLA, except that a volume of 0.5 μl was infused over a 36-s period and the injection needle protruded 1.5 mm beyond the cannula tip. The doses were selected on the basis of previous experiments conducted in this laboratory (7).
Inhibitory Avoidance Apparatus and Procedure.
The rats were trained in an inhibitory avoidance apparatus consisting of a trough-shaped alley (91 cm long, 15 cm deep, 20 cm wide at the top, 6.4 cm wide at the floor) divided into two compartments, separated by a sliding door that opened by retracting into the floor (24). The starting compartment (31 cm long) was made of opaque white plastic and was well-lit; the shock compartment (60 cm long) was made of metal plates and was not illuminated. The apparatus was located in a sound- and light-attenuated room.
The rat was placed in the starting compartment of the apparatus, facing away from the door, and was allowed to enter the dark compartment. After the animal stepped completely into the shock compartment, the door was closed and a single footshock (0.35 mA, 1.0 s) was delivered through the metal floor plates. Animals with entrance latencies longer than 30 s were eliminated from the study. The animal was removed from the shock compartment 15 s after termination of the footshock and, after drug injections, returned to its home cage. On the retention test, the latency to reenter the shock compartment with all four paws (maximum latency of 600 s) was recorded and used as the measure of retention. Longer latencies were interpreted as indicating better retention. Shock was not administered during the retention test trial.
Histology.
The rats were anesthetized with an overdose of sodium pentobarbital (≈100 mg/kg, i.p.) and perfused intracardially with 0.9% saline (wt/vol) solution followed by 4% formaldehyde (wt/vol) dissolved in water. After decapitation, the brains were removed and placed in 4% formaldehyde. At least 24 h before sectioning, the brains were submerged in a 20% sucrose (wt/vol) solution for cryoprotection. Sections of 40 μm were made by using a freezing microtome and stained with cresyl violet. The sections were examined under a light microscope and determination of the location of the injection needle tips in the BLA and hippocampus was made according to the standardized atlas plates of Paxinos and Watson (22).
Statistics.
The retention test latencies of the different experiments were analyzed separately by using a two-way ANOVA with animals given saline, the β-adrenoceptor antagonist, or the muscarinic cholinergic antagonist into the BLA (three levels), and intrahippocampal infusions of vehicle or different doses of the GR agonist (four levels) both as between-subject variables. Further analysis used Fisher’s post hoc tests to determine the source of the significance. A probability level of less than 0.05 was accepted as statistically significant. There were 8–12 rats per group, as indicated in the legend of Fig. 2.
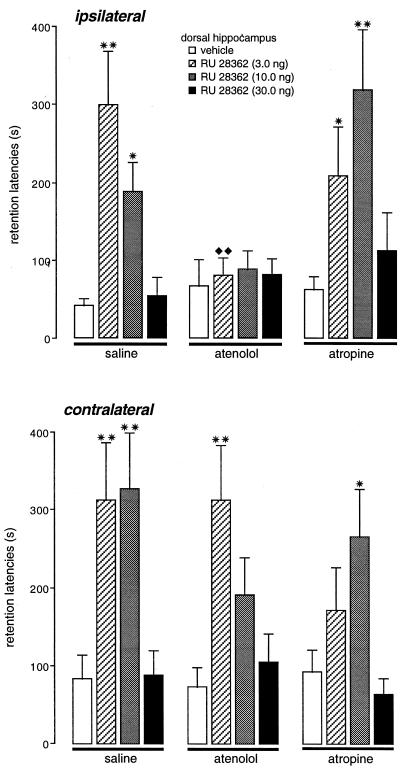
Figure 2.
Retention latencies (mean ± SEM) in seconds of rats given immediate posttraining infusions of the GR agonist RU 28362 (3.0, 10.0, or 30.0 ng) into the dorsal hippocampus and pretraining infusions of either the β-adrenoceptor antagonist atenolol (0.5 μg in 0.2 μl) or the muscarinic cholinergic antagonist atropine (0.5 μg in 0.2 μl) into either the ipsilateral or contralateral basolateral amygdala. ∗, P < 0.05; ∗∗, P < 0.01, as compared with the corresponding intrahippocampal vehicle group; ♦♦, P < 0.01, as compared with the corresponding intra-BLA saline group. n = 8–12 animals per group.
RESULTS
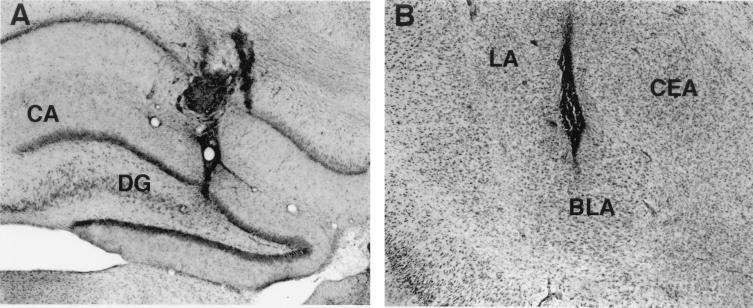
Histological examination revealed that 244 animals had cannula placements in the dorsal hippocampus and the BLA. Animals with improper cannula placement or with extensive damage to the targeted tissue were excluded from the analyses. Fig. 1 A and B shows photomicrographs illustrating representative locations of injection needle tips in the dorsal hippocampus and the BLA, respectively.
Figure 1.
Photomicrographs illustrating the location of microinjection needle tips within the dorsal hippocampus (A) and basolateral amygdala (B). CA, Ammon’s horn; CEA, central amygdala; DG, dentate gyrus; LA, lateral amygdala. (×25)
Inhibitory avoidance retention latencies of rats given pretraining infusions of the β-adrenoceptor antagonist atenolol or the muscarinic cholinergic antagonist atropine into the left BLA and immediate posttraining infusions of vehicle or the GR agonist RU 28362 into the ipsilateral dorsal hippocampus are shown in Upper Fig. 2. A two-way ANOVA revealed significant intrahippocampal glucocorticoid [F (3, 115) = 8.99; P < 0.001] and intra-BLA antagonist effects [F (2, 115) = 5.04; P < 0.01], as well as a significant interaction between these two factors [F (6, 115) = 3.01; P < 0.01]. The retention test latencies of animals given control infusions in both brain structures were relatively short (41.0 ± 9.5 s) as was expected because of the low footshock intensity used. Posttraining infusions of the two lower doses of the GR agonist RU 28362 (3.0 and 10.0 ng) into the hippocampus enhanced retention of rats given saline infusions into the BLA, as compared with the retention of corresponding vehicle-treated animals (3.0 ng, P < 0.01; 10.0 ng, P < 0.05). The highest dose of RU 28362 (30.0 ng) did not enhance retention. Infusions of the β-adrenoceptor antagonist atenolol into the ipsilateral BLA did not impair retention latencies, but blocked the retention-enhancing effects of RU 28362 administered into the hippocampus. Further, retention latencies of RU 28362-treated rats (3.0 ng) given atenolol into the BLA were significantly shorter than retention latencies of RU 28362-treated rats (3.0 ng) given saline into the BLA (P < 0.01). In contrast, infusions of the muscarinic cholinergic antagonist atropine into the ipsilateral BLA did not block the enhancing effects of RU 28362 administered into the hippocampus. Retention latencies of animals given the two lower doses of RU 28362 together with atropine were significantly longer than those of vehicle-treated animals (3.0 ng, P < 0.05; 10.0 ng, P < 0.01). The highest dose of RU 28362 did not significantly affect retention latencies.
The retention latencies of rats given posttraining infusions of the GR agonist into the left hippocampus and pretraining infusions of either atenolol or atropine into the contralateral BLA are shown in Lower Fig. 2. Posttraining infusions of the GR agonist RU 28362 into the hippocampus dose-dependently enhanced retention of rats given saline, as well as those given atenolol or atropine into the BLA. A two-way ANOVA revealed a significant intrahippocampal glucocorticoid effect [F (3, 105) = 12.80; P < 0.001], but no significant intra-BLA antagonist effect [F (2, 105) = 1.16; not significant] or interaction between these two factors [F (6, 105) = 1.28; not significant].
DISCUSSION
The findings of the present experiments provide additional evidence that posttraining activation of hippocampal GRs facilitates long-term storage of recently acquired information in a dose-dependent inverted-U manner (7, 25–27). The findings are consistent with evidence that the dentate gyrus and Ammon’s horn have high densities of GRs (28–30) as well as the evidence that glucocorticoids affect hippocampal excitability and several forms of long-term neuroplasticity, putative mechanisms underlying learning and memory (31–34). Because the glucocorticoid was administered after training to selectively influence consolidation processes, it seems highly likely that the neurobiological mechanisms underlying this effect differ fundamentally from those of studies obtaining cognitive-impairing effects with elevated glucocorticoid levels during training and/or testing (35–38).
The primary finding of the present experiments is that infusions of the β-adrenoceptor antagonist atenolol into the BLA blocked the memory-enhancing effects induced by posttraining infusions of a GR agonist into the hippocampus. Atenolol did not simply shift the dose-response effect of the GR agonist, as higher doses of the GR agonist did not affect retention. Furthermore, the blocking effect was specific to the ipsilateral BLA, as infusions of atenolol into the contralateral BLA did not block the memory enhancement induced by the GR agonist infused into the hippocampus. Thus, these findings provide strong evidence that activation of β-adrenoceptors in the BLA is essential in enabling glucocorticoid memory-modulatory influences in the hippocampus. The finding that pretraining infusions of a β-adrenoceptor antagonist administered alone in the BLA did not impair memory for inhibitory avoidance training is also consistent with the results of previous studies (13, 24, 39), as well as with our finding that excitotoxic lesions of the BLA induced before training do not impair inhibitory avoidance learning (4). However, it should be noted that higher doses of β-adrenoceptor antagonists induce memory impairment (19, 40).
The current experiment brings together several components of earlier studies. In previous studies, we found that infusions of β-adrenoceptor antagonists into the BLA block the effects of posttraining systemic injections of the synthetic glucocorticoid dexamethasone on memory consolidation (13) and that excitotoxically induced lesions of the BLA block the memory-modulating effects induced by a GR agonist infused into the hippocampus (7). The role of the BLA in influencing memory formation involving the hippocampus is not restricted to enhancing effects. BLA lesions also block memory impairment induced by either adrenalectomy or intrahippocampal infusions of a GR antagonist (5–7). In parallel with our behavioral experiments, electrophysiological studies have shown that lesions of the BLA or infusions of a β-adrenoceptor antagonist into the BLA attenuate the induction of long-term potentiation in the dentate gyrus in vivo (41, 42).
We have reported extensive evidence indicating that activation of β-adrenoceptors in the BLA plays a critical role in enabling the memory-influencing effects of several neuromodulatory systems (i.e., γ-aminobutyric acid and opioids) as well as adrenal stress hormones (10–13). The BLA receives noradrenergic input from cell groups in the nucleus of the solitary tract as well as the locus coeruleus (43). Studies using in vivo microdialysis have shown that footshock stimulation of the kind typically used in inhibitory avoidance training (i.e., a single footshock of low intensity and short duration) induces the release of norepinephrine in the amygdala and the magnitude of the release is modulated by drugs and hormones known to affect memory consolidation (14, 44–46).
Posttraining activation of amygdala adrenoceptors (both α1 and β) enhances memory for several kinds of training, including (the hippocampus-dependent) Morris water-maze spatial task (12, 19, 20, 47–49). Furthermore, the memory enhancement induced by pharmacological stimulation of the amygdala is blocked by inactivation of the ipsilateral hippocampus with lidocaine immediately after training or shortly before the retention test (refs. 50 and 51; and B.R., B.T.N. and J.L.M., unpublished observation). Our present findings provide additional evidence that the influence of the BLA on hippocampal memory processing involves ipsilateral neural connections. These findings are of critical importance in understanding the basis of the enabling influence of the BLA on hippocampal functioning. They clearly indicate that the amygdala influence is not mediated by nonspecific effects on the animal’s arousal state or activation of peripheral stress hormone systems that might alter hippocampal functioning. This is important because the BLA projects to the central nucleus of the amygdala (CEA), and it is known that the CEA projects to brainstem and hypothalamic regulatory centers (52, 53). Furthermore, CEA lesions attenuate several central and peripheral stress responses (54–56). The present findings are also consistent with previous evidence indicating that selective lesions of the CEA do not block the effects of systemic or intrahippocampal administration of glucocorticoids (4, 7) and do not attenuate the induction of long-term potentiation in the dentate gyrus (40). Although the mechanisms enabling noradrenergic activation of the BLA to influence memory formation involving the hippocampus are not known, evidence suggests the possible involvement of N-methyl-d-aspartate (NMDA)-dependent processes as activation of β-adrenoceptors induces NMDA-dependent enhancement of excitatory synaptic transmission in pyramidal neurons of the BLA in vitro (16, 57).
The BLA projects directly to the hippocampus, as well as indirectly via the entorhinal cortex (58–60). It has been reported that BLA stimulation affects electroencephalogram activity and long-term potentiation in the hippocampus (61, 62), and that NMDA infused into the amygdala induces c-fos expression in the ipsilateral dorsal hippocampus (63). In addition to the activation of such direct connections between the BLA and the hippocampus, other indirect pathways may also be involved. The BLA may influence glucocorticoid effects on hippocampal memory processes by means of its stria terminalis projections to the nucleus accumbens (64–66). In support of this view, there is evidence that activation of the BLA increases the likelihood of fimbria–fornix stimulation, inducing spike activity in the nucleus accumbens (67). The BLA may also directly influence memory formation in the cortex (68, 69). More generally, recent studies have suggested that memory processing requires the participation of several anatomically and sequentially linked, reverberating subcortical and cortical networks (70–72). Understanding whether the various pathways play differential roles in the transmission of influences from the BLA will require further inquiry.
Unlike the effects of atenolol, blockade of muscarinic cholinergic receptors in the BLA with atropine did not prevent the facilitating effects of GR activation in the hippocampus. This finding is consistent with evidence that muscarinic cholinergic blockade of the BLA does not attenuate dentate gyrus long-term potentiation (39). However, it remains possible that atropine may have induced a slight, but nonsignificant, shift in the dose-response effects of RU 28362. It seems unlikely that the dose of atropine was insufficient to block the receptors because the same dose has been proven effective in preventing the memory-enhancing effects of systemically administered drugs (A.E.P., B.R. and J.L.M., unpublished observation). Rather, these findings suggest that release of acetylcholine in the amygdala depends less on the level of emotional arousal. This view is supported by the finding that inescapable footshock does not induce the release of acetylcholine in the amygdala (73) as well as the evidence that acetylcholine release in the hippocampus, unlike that of norepinephrine, does not reflect emotional valence but, rather, is related to the animal’s task performance (74). However, infusion of a muscarinic cholinergic agonist into the amygdala induces enhancement of memory for inhibitory avoidance and contextual fear conditioning (10, 75). Moreover, a muscarinic cholinergic antagonist administered into the amygdala attenuates the memory-enhancing effects of concurrent administration of a β-adrenoceptor agonist and potentiates the memory-impairing effects of a β-adrenoceptor antagonist (8, 10, 76). Thus, although muscarinic cholinergic receptors interact with noradrenergic mechanisms in the BLA in modulating memory consolidation, muscarinic cholinergic activity in the BLA appears not to be critical for enabling glucocorticoids to facilitate memory consolidation involving the hippocampus.
In conclusion, the present findings provide further evidence for a critical role of β-adrenoceptor mechanisms in the BLA in mediating stress hormone effects on memory consolidation. Our findings are consistent with the view that the BLA regulates the strength of memories in other brain structures, reflecting their emotional significance (1, 2, 50, 51, 77). Our findings are also consistent with those of recent brain imaging studies in human subjects, supporting the hypothesis that the amygdala has a time-limited role in consolidation processes underlying long-term explicit/declarative memory for emotionally arousing events (78–80).
Acknowledgments
The authors thank Dr. Lucille A. Lumley for her comments on an earlier version of the paper; and Dao Tran, Jason Buranday, and Jamin Pablo for excellent technical assistance. RU 28362 was generously provided by Roussel-UCLAF. Research was supported by an R. W. and L. Gerard Family Trust Fellowship (to B.R.), the University of California Irvine Undergraduate Research Opportunities Program (to B.T.N.), a University of California Cota-Robles Fellowship (to A.E.P.), and National Institute of Mental Health Grant MH12526 (to J.L.M.).
ABBREVIATIONS
- BLA
basolateral nucleus of the amygdala
- GR
glucocorticoid receptor
- RU 28362
11β,17β-dihydroxy-6,21-dimethyl-17α-pregna-4,6-trien-20-yn-3-one
References
- 1.Cahill L, McGaugh J L. Trends Neurosci. 1998;17:208–214. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 2.McGaugh J L, Cahill L, Roozendaal B. Proc Natl Acad Sci USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1997;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- 4.Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- 5.Roozendaal B, Portillo-Marquez G, McGaugh J L. Behav Neurosci. 1996;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- 6.Roozendaal B, Sapolsky R M, McGaugh J L. Neuroscience. 1998;84:453–465. doi: 10.1016/s0306-4522(97)00538-1. [DOI] [PubMed] [Google Scholar]
- 7.Roozendaal B, McGaugh J L. Eur J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 8.Dalmaz C, Introini-Collison I B, McGaugh J L. Behav Brain Res. 1993;58:167–174. doi: 10.1016/0166-4328(93)90101-u. [DOI] [PubMed] [Google Scholar]
- 9.Decker M W, McGaugh J L. Brain Res. 1989;477:29–37. doi: 10.1016/0006-8993(89)91391-7. [DOI] [PubMed] [Google Scholar]
- 10.Introini-Collison I B, Dalmaz C, McGaugh J L. Neurobiol Learn Mem. 1996;65:57–64. doi: 10.1006/nlme.1996.0006. [DOI] [PubMed] [Google Scholar]
- 11.Introini-Collison I B, Miyazaki B, McGaugh J L. Psychopharmacology. 1991;104:541–544. doi: 10.1007/BF02245663. [DOI] [PubMed] [Google Scholar]
- 12.Liang K C, McGaugh J L, Yao H. Brain Res. 1990;508:225–233. doi: 10.1016/0006-8993(90)90400-6. [DOI] [PubMed] [Google Scholar]
- 13.Quirarte G L, Roozendaal B, McGaugh J L. Proc Natl Acad Sci USA. 1997;94:14048–14053. doi: 10.1073/pnas.94.25.14048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quirarte G L, Galvez R, Roozendaal B, McGaugh J L. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- 15.McIntyre C K, Ragozzino M E, Gold P E. Behav Brain Res. 1998;95:219–226. doi: 10.1016/s0166-4328(97)00161-7. [DOI] [PubMed] [Google Scholar]
- 16.Huang C C, Tsai J J, Gean P O. Chin J Physiol. 1994;37:73–78. [PubMed] [Google Scholar]
- 17.Womble M D, Moises H C. J Neurophysiol. 1993;70:2056–2065. doi: 10.1152/jn.1993.70.5.2056. [DOI] [PubMed] [Google Scholar]
- 18.Yajeya J, De La Fuente Juan A, Merchan M A, Riolobos A S, Heredia M, Criado J M. Neuroscience. 1997;78:731–743. doi: 10.1016/s0306-4522(96)00614-8. [DOI] [PubMed] [Google Scholar]
- 19.Liang K C, Chen L L, Huang T-E. Chin J Physiol. 1995;38:81–91. [PubMed] [Google Scholar]
- 20.Ferry B, Roozendaal B, McGaugh J L. J Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roozendaal B, Williams C L, McGaugh J L. Eur J Neurosci. 1999;11:13177–1323. doi: 10.1046/j.1460-9568.1999.00537.x. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 23.Parent M B, McGaugh J L. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- 24.McGaugh J L, Introini-Collison I B, Nagahara A H. Brain Res. 1988;446:37–49. doi: 10.1016/0006-8993(88)91294-2. [DOI] [PubMed] [Google Scholar]
- 25.Cottrell G A, Nakajima S. Pharmacol Biochem Behav. 1977;7:277–343. doi: 10.1016/0091-3057(77)90146-0. [DOI] [PubMed] [Google Scholar]
- 26.de Kloet E R, de Kock S, Schild V, Veldhuis H D. Neuroendocrinology. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- 27.Micheau J, Destrade C, Soumireu-Mourat B. Eur J Pharmacol. 1985;106:39–46. doi: 10.1016/0014-2999(84)90675-7. [DOI] [PubMed] [Google Scholar]
- 28.McEwen B S, Weiss J M, Schwartz L S. Nature (London) 1968;220:911–912. doi: 10.1038/220911a0. [DOI] [PubMed] [Google Scholar]
- 29.Reul J M H M, de Kloet E R. Endocrinology. 1985;117:2505–2512. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 30.Reul J M H M, de Kloet E R, van Sluys F J, Rijnberk A, Rothuizen J. Endocrinology. 1990;127:907–915. doi: 10.1210/endo-127-2-907. [DOI] [PubMed] [Google Scholar]
- 31.Diamond D M, Bennett M C, Flesher M, Rose G M. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- 32.Joëls M, de Kloet E R. Prog Neurobiol. 1994;43:1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 33.Pavlides C, Watanabe Y, McEwen B S. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- 34.Xu L, Holscher C, Anwyl R, Rowan M J. Proc Natl Acad Sci USA. 1998;95:3204–3208. doi: 10.1073/pnas.95.6.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Quervain D J-F, Roozendaal B, McGaugh J L. Nature (London) 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 36.Kirschbaum C, Wolf O T, May M, Wippich W, Hellhammer D H. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- 37.Lupien S J, McEwen B S. Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 38.Newcomer J W, Craft S, Hershey T, Askins K, Bardgett M E. J Neurosci. 1994;14:2047–2053. doi: 10.1523/JNEUROSCI.14-04-02047.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liang K C, Juler R G, McGaugh J L. Brain Res. 1986;368:125–133. doi: 10.1016/0006-8993(86)91049-8. [DOI] [PubMed] [Google Scholar]
- 40.Gallagher M, Kapp B S, Musty R E, Driscoll P A. Science. 1977;198:423–425. doi: 10.1126/science.20664. [DOI] [PubMed] [Google Scholar]
- 41.Ikegaya Y, Nakanishi K, Saito H, Abe K. Neuro Report. 1997;8:3143–3146. doi: 10.1097/00001756-199709290-00027. [DOI] [PubMed] [Google Scholar]
- 42.Ikegaya Y, Saito H, Abe K. Brain Res. 1994;656:157–174. doi: 10.1016/0006-8993(94)91377-3. [DOI] [PubMed] [Google Scholar]
- 43.Fallon J H, Ciofi P. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction. Aggleton J P, editor. New York: Wiley–Liss; 1992. pp. 431–451. [Google Scholar]
- 44.Galvez R, Mesches M H, McGaugh J L. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- 45.Hatfield T, Spanis C, McGaugh J L. Brain Res. 1999;835:340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- 46.Williams C L, Men D, Clayton E C, Gold P E. Behav Neurosci. 1998;112:1414–1422. doi: 10.1037//0735-7044.112.6.1414. [DOI] [PubMed] [Google Scholar]
- 47.Ferry B, McGaugh J L M. Neurobiol Learn Mem. 1999;72:8–12. doi: 10.1006/nlme.1998.3904. [DOI] [PubMed] [Google Scholar]
- 48.Hatfield T, McGaugh J L. Neurobiol Learn Mem. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- 49.Salinas J A, Introini-Collison I B, Dalmaz C, McGaugh J L. Neurobiol Learn Mem. 1997;68:51–59. doi: 10.1006/nlme.1997.3776. [DOI] [PubMed] [Google Scholar]
- 50.Packard M G, Cahill L, McGaugh J L. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Packard M G, Teather L. Neurobiol Learn Mem. 1998;69:163–203. doi: 10.1006/nlme.1997.3815. [DOI] [PubMed] [Google Scholar]
- 52.Danielsen E H, Magnuson D J, Gray T S. Brain Res Bull. 1989;22:705–715. doi: 10.1016/0361-9230(89)90090-7. [DOI] [PubMed] [Google Scholar]
- 53.Krettek J E, Price J L. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 54.Beaulieu S, Di Paolo T, Côte J, Barden N. Neuroendocrinology. 1987;45:37–46. doi: 10.1159/000124701. [DOI] [PubMed] [Google Scholar]
- 55.Roozendaal B, Koolhaas J M, Bohus B. Physiol Behav. 1991;50:771–775. doi: 10.1016/0031-9384(91)90016-h. [DOI] [PubMed] [Google Scholar]
- 56.Van de Kar L D, Piechowski R A, Rittenhouse P A, Gray T S. Neuroendocrinology. 1991;54:89–95. doi: 10.1159/000125856. [DOI] [PubMed] [Google Scholar]
- 57.Wang S J, Huang C C, Hsu K S, Tsai J J, Huang C C, Gean P W. Neurosci Lett. 1996;214:87–90. doi: 10.1016/0304-3940(96)12892-5. [DOI] [PubMed] [Google Scholar]
- 58.Pikkarainen M, Ronkko S, Savander V, Insausti R, Pitkanen A. J Comp Neurol. 1999;403:229–260. [PubMed] [Google Scholar]
- 59.Racine R J, Mingram N W, Hafner S. Brain Res. 1983;260:915–922. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- 60.Thomas S R, Assaf S Y, Iversen S D. Brain Res. 1984;307:363–365. doi: 10.1016/0006-8993(84)90496-7. [DOI] [PubMed] [Google Scholar]
- 61.Dringenberg H C, VanderWolf C H. Exp Brain Res. 1996;108:285–296. doi: 10.1007/BF00228101. [DOI] [PubMed] [Google Scholar]
- 62.Ikegaya Y, Saito H, Abe K. Neurosci Res. 1995;22:203–207. doi: 10.1016/0168-0102(95)00894-7. [DOI] [PubMed] [Google Scholar]
- 63.Packard M G, Williams C L, Cahill L, McGaugh J L. In: Neurobehavioral Plasticity: Learning, Development, and Response to Brain Insults. Spear N E, Spear L P, Woodruff M L, editors. Hillsdale, NJ: Erlbaum; 1995. pp. 149–184. [Google Scholar]
- 64.de Quervain D J-F, Roozendaal B, Ferry B, Wannier T, McGaugh J L. Soc Neurosci Abstr. 1998;24:1901. [Google Scholar]
- 65.Roozendaal B, McGaugh J L. Brain Res. 1996;709:243–250. doi: 10.1016/0006-8993(95)01305-9. [DOI] [PubMed] [Google Scholar]
- 66.Setlow B, Roozendaal B, McGaugh J L. Soc Neurosci Abstr. 1998;24:174. [Google Scholar]
- 67.Mulder A B, Hodenpijl M G, Lopes da Silva F H. J Neurosci. 1998;18:5095–5102. doi: 10.1523/JNEUROSCI.18-13-05095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Armony J L, Quirk G J, LeDoux J E. J Neurosci. 1998;18:2592–2601. doi: 10.1523/JNEUROSCI.18-07-02592.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang K C, Hu S J, Chang S C. Chin J Physiol. 1996;39:155–166. [PubMed] [Google Scholar]
- 70.Iijima T, Witter M P, Ichikawa M, Tominaga T, Kajiwara R, Matsumoto G. Science. 1996;272:1176–1179. doi: 10.1126/science.272.5265.1176. [DOI] [PubMed] [Google Scholar]
- 71.Izquierdo I, Quillfeldt J A, Zanatta M S, Quevedo J, Schaeffer E, Schmitz P K, Medina J H. Eur J Neurosci. 1997;9:786–793. doi: 10.1111/j.1460-9568.1997.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 72.McIntosh A R, Gonzalez-Lima F. J Neurophysiol. 1994;72:1717–1733. doi: 10.1152/jn.1994.72.4.1717. [DOI] [PubMed] [Google Scholar]
- 73.Mark G P, Rada P V, Shors T J. Neuroscience. 1996;74:767–774. doi: 10.1016/0306-4522(96)00211-4. [DOI] [PubMed] [Google Scholar]
- 74.Men, D., McIntyre., C. K., McCarty, R. & Gold, P. E. (1999) Neurobiol. Aging, in press. [DOI] [PubMed]
- 75.Vazdarjanova A, McGaugh J L. J Neurosci. 1999;19:6615–6622. doi: 10.1523/JNEUROSCI.19-15-06615.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daws L C, Lopez R, Frazer A. Neuropsychopharmacology. 1998;19:300–313. doi: 10.1016/S0893-133X(98)00016-5. [DOI] [PubMed] [Google Scholar]
- 77.Shors T J, Mathew P R. Learn Mem. 1998;5:220–230. [PMC free article] [PubMed] [Google Scholar]
- 78.Cahill L, Haier R J F, Alkire M, Tang C, Keator D, Wu J, McGaugh J L. Proc Natl Acad Sci USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hamann S B, Ely T D, Grafton S T, Kilts C D. Nat Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- 80.LaBar K S, Gatenby J C, Gore J C, LeDoux J E, Phelps E A. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]