In the previous issue, Rada et al. [1] demonstrated an aspect of the mechanism by which neutrophils regulate their cytosolic Ca2+. They show a clear link between the activity of the nonmitochondrial (NADPH) oxidase and Ca2+influx in neutrophils. In view of the importance of Ca2+ in regulating various neutrophil activities, this has important implications both for our understanding of chronic granulomatous disease (GCD), where the oxidase is totally inoperative, and, perhaps at first thought, paradoxically, for suppressing inflammatory disease. In this short overview, some of the theory underlying the results of Rada et al. [1] is briefly explained and the potential importance of this type of Ca2+ regulation for restraining neutrophil ‘aggression’ during inflammation is discussed.
A NEW FUNCTION FOR THE NEUTROPHIL OXIDASE?
It can be argued that the purpose of the programme of activity that begins with the neutrophil leaving the circulation and ends in phagocytosis of infecting bacteria is to bring the neutrophil nonmitochondrial oxidase in close proximity to the bacterium. The oxygen metabolites, such as superoxide ions (O2−), which are generated by this oxidase, are highly reactive, and thus have short life-times with consequently small diffusion distances. It is thus reasonable to assume that if the highly reactive oxygen metabolites generated are involved in the killing of the bacterium, they do so within the phagosome. As the product of the dismutation of superoxide ions is peroxide (H2O2), there would also seem to be a role for myeloperoxidase which is secreted into the phagosome after phagosomal closure, especially since its product, hypochlorite (OCl−) is also highly toxic to bacteria. However, there are some arguments against this latter mechanism. For example, while dysfunction of the neutrophil oxidase has serious consequences for the patient (i.e. CGD), myeloperoxidase deficiency is relatively common and has no obvious clinical manifestations. Also, Reeves et al.[2] have argued that the main purpose of the oxidase may be as a proton pump, regulating the intraphagosomal pH or controlling K+ concentration, both releasing the proteolytic activity of neutral proteases in the phagosome. Whatever the details, there is general agreement that the oxidase is involved in bacterial killing. However, could the oxidase have a second function not related to bacterial toxicity?
The key feature of the nonmitochondrial oxidase is its ability to transfer electrons from NADPH (the electron donor) to oxygen (the electron acceptor) across a membrane. While NADPH is within the cytosol, the oxygen acceptor is within the phagosome or external to the cell (Fig. 1a). There is consequently a vectorial movement of electrons (negative charge) across the phagosomal or plasma membrane. Although this movement of charge could be compensated for by an accompanying flow of positive charge (e.g. H+), it has been shown that there is a significant current which results in a change in the potential across the membrane towards the positive [3–5]. Thus the neutrophil oxidase (like the mitochondrial oxidase) is electrogenic, i.e. it creates a change in the potential across the membrane in which it is operating. This raises the question of whether the electrogenic nature of the oxidase is merely an inevitable but unimportant accompaniment to oxidant generation or whether this aspect of its activity also has any biological consequences.
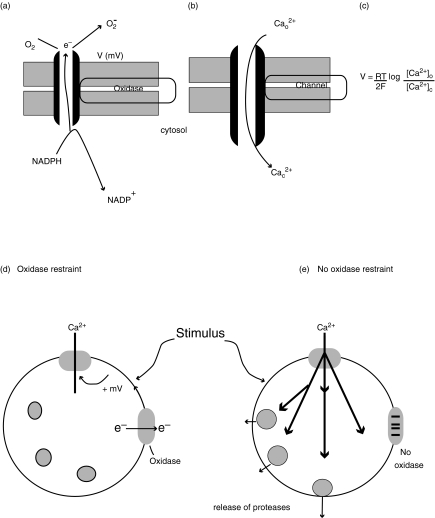
Fig. 1.
Electrogenic effect of the oxidase on Ca2+ influx. The figure shows the schematic lay-out of (a) the oxidase, transporting electrons across the phagosomal or plasma membrane and generating a transmembrane potential (V) and (b) the open Ca2+ channel with Ca2+ ions moving against the electron flow. These opposite effects balance when the Nernst equation (c) is true, where R,T and F are the gas constant, the absolute temperature and the Faraday constant, respectively; 2 is the valency of Ca2+ and the square brackets denote the equilibrium concentrations of Ca2+ outside the cell and in the cytosol (denoted by the subscripts o and c, respectively) at the transmembrane potential, V. (d) shows the effect of a stimulus on an oxidase-competent neutrophil, causes Ca2+ influx, which activates outwardly directly electron transport via the oxidase. This results in an increase in membrane potential towards the positive, limiting Ca2+ influx. (e) shows the effect on an oxidase-defective cell, the Ca2+ influx restraint is lost and more Ca2+ enters the cell causing other cellular events including the release of proteases from stored granules to occur.
RESTRAINT OF CA2+ INFLUX BY THE OXIDASE-GENERATED MEMBRANE POTENTIAL
Although neutrophils are not electrically excitable cells, like nerve or muscle, and do not have voltage sensitive ion channels, the electrogenic effect of the oxidase is not trivial. When activated, the oxidase can result in the membrane potential rising to between + 30 and + 50 mV [3–5]. At these positive levels, it would be expected that the movement of charged ions, such as H+, K+ and especially Ca2+ would be affected. Ca2+ influx occurs when Ca2+ channels are open in the phagosomal and plasma membranes [6,7]. With the extracellular (or intraphagosomal) Ca2+ concentration at about 1 mm and the cytosolic Ca2+ at about 100 nm, there is a 10,000-fold difference in concentration. In the absence of any electrical effect, Ca2+ ions would flood in through the open Ca2+ channels (Fig. 1b). In fact, in the resting neutrophil, the membrane potential, which is negative (inside), exerts an even steeper electrochemical gradient. However, when the oxidase is activated and the membrane potential reverses, the inside of the cell becomes positive relative to the outside, so that the influx of positively charged ions, like Ca2+, would be impeded, a phenomenon termed here ‘oxidase restraint’. From the Nernst equation (Fig. 1c), it can be seen that at positive membrane potentials of 30–50 mV (those reported when the oxidase is active), the inward Ca2+ electrochemical gradient would cease when the concentration of Ca2+ on the cytosolic face of the Ca2+ channel was about 24–100 µm. In other words, despite the Ca2+ channels being open, there would be no further net flux of Ca2+ into the cell. The inward concentration gradient would be balanced by the opposing voltage gradient (Fig. 1d). This theoretical possibility has been tested by Ligeti's group [1,8]. They have shown previously with neutrophils from patients with GCD, which are unable to mount an oxidase response, that Ca2+ influx is exaggerated [8]. This result in CGD neutrophils has more recently been confirmed by another group [9]. Now Ligeti's group have also shown, both in a genetically modified cell-line devoid of the oxidase component gp91phox and in neutrophils in which oxidase activity was inhibited pharmacologically, that there is a demonstrable linkage between oxidase activity, its accompanying membrane depolarization and the extent of Ca2+ (or Mn2+) influx [1]. There is thus now good experimental support for the theoretical basis of the ‘oxidase restraint’ model outlined here.
SIGNIFICANCE OF CA2+ INFLUX FOR NEUTROPHIL BEHAVIOUR
It is well known that neutrophil responses to a variety of stimuli are triggered by rises in cytosolic free Ca2+[10]. This Ca2+ signal is often initiated by a release of Ca2+ from intracellular stores within the neutrophil, which is coupled, either within a few tens of milliseconds [11] or tens of seconds [12] to the opening of Ca2+ channels on the plasma membrane and a consequent influx of Ca2+. While there is no clear role for the release of stored Ca2, the accompanying Ca2+ influx is crucial for a number of neutrophil responses [10]. Ca2+ influx locally raises the cytosolic free Ca2+ just under the plasma membrane to over 50 µm[13], and is sufficient to activate submembrane µ-calpain to cleave β2 integrin from its cytoskeletal tether [14], with a kD for Ca2+ of about 30 µm. However, at higher submembranous cytosolic free Ca2+ concentrations (100–300 µm), exocytosis of myeloperoxidase-containing and protease-containing granules is also triggered [6,15]. This latter event would be pathogenic if uncontrolled. Fortunately, as Ca2+ influx is also important for activating the oxidase system [10], the accompanying voltage change would have a self-restricting effect on Ca2+ influx. For example, during phagocytosis, Ca2+ channels on the phagosomal membrane (presumably arising from the invaginated plasma membrane) are opened and Ca2+ effluxes from the phagosome into the cytosol [7], causing the oxidase to be activated, and the potential across the phagosomal membrane to increase. As the oxidase-generated membrane potential change approaches the Ca2+ reversal potential, the tendency for Ca2+ to influx into the cytosol would be reduced and so limit the possibility of pathogenic degranulation.
OXIDASE RESTRAINT ON NEUTROPHIL AGGRESSION
From the above discussion, it can be seen that as well as being a mechanism for intraphagosomal killing of bacteria, the oxidase may also be key to limiting the pro-inflammatory effects of cytosolic free Ca2+ within the neutrophil. In this issue, Rada et al. [1] suggest that this effect may underlie some of the pathology of CGD [16]. For example, while changes in cytosolic free Ca2+ are not necessary for chemotaxis by neutrophils [17,18], high cytosolic free Ca2+ causes them to become immobile [17]. In CGD this may result in the accumulation of immobile neutrophils at infection foci producing granulomas. This would be especially the case if high Ca2+ were also antiapoptotic [19]. Perhaps more importantly, if this mechanism exerts a limitation on neutrophil ‘aggression’, there are wider implications for this mechanism. Any reduction in oxidase activity, while perhaps not sufficiently severe to totally inhibit bacterial killing, may have a pathological consequence by failing to limit Ca2+ influx. Such cells would be easily activated, hyper-responsive and prone to degranulate, all conditions that may lead to inappropriate activation and inflammatory disease. It is thus interesting that hyperactivation of neutrophils has been described in CGD, and attributed to an accelerated Ca2+ influx [9]. Perhaps a more striking effect of the lack of ‘oxidase restraint’ is seen in a recent report that inflammatory arthritis in rats was linked to a polymorphism of a gene expressing neutrophil cytosolic factor (Ncf1), the analogue of human p47phox of the neutrophil oxidase, and that decreased neutrophil oxidase activity was associated with increased arthritic severity [20]. Strategies that increased neutrophil oxidase activity were found to be beneficial in reducing inflammatory tissue damage in this animal model [20]. Thus the activity of the neutrophil oxidase may be exerting a crucial role under inflammatory conditions in providing a restraint over Ca2+ influx and consequently acting as a brake on neutrophil aggression. Clearly, there is much to be done to establish this model and to identify ways in which it could be beneficially exploited. However, it is clear that the recent papers connecting the neutrophil oxidase, Ca2+ influx and neutrophil behaviour are already laying the necessary groundwork.
References
- 1.Rada BK, Geiszt M, van Bruggen R, Német K, Roos D, Ligeti E. Calcium signaling is altered in myeloid cells with a deficiency in NADPH oxidase activity. Clin Exp Immunol. 2003;132:53–60. doi: 10.1046/j.1365-2249.2003.02138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeves EP, Lu H, Jacobs HL, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–7. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 3.Henderson LM, Chappell JB, Jones OT. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with a H+ channel. Biochem J. 1987;246:325–9. doi: 10.1042/bj2460325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrenzel J, Serrander L, Banfi B, Nusse O, Fouyouzi R, Lew DP, Demaurex N, Krause KH. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 1998;392:734–7. doi: 10.1038/33725. [DOI] [PubMed] [Google Scholar]
- 5.Jankowski A, Grinstein S. A noninvasive fluorimetric procedure for measurement of membrane potential – quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J Biol Chem. 1999;274:26098–104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- 6.Nüsse O, Serrander L, Lew DP, Krause KH. C2-induced exocytosis in individual human neutrophils: high- and low-affinity granule populations and submaximal responses. EMBO J. 1998;17:1279–88. doi: 10.1093/emboj/17.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lundqvist-Gustafsson H, Gustafsson M, Dahlgren C. Dynamic Ca2+ changes in neutrophil phagosomes – A source for intracellular Ca2+ during phagolysosome formation? Cell Calcium. 2000;27:353–62. [Google Scholar]
- 8.Geiszt M, Kapus A, Nemet K, Farkas L, Ligeti E. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes – alterations in chronic granulomatous disease. J Biol Chem. 1997;272:26471–8. doi: 10.1074/jbc.272.42.26471. [DOI] [PubMed] [Google Scholar]
- 9.Tintinger GR, Theron AJ, Steel HC, Anderson R. Accelerated calcium influx and hyperactivation of neutrophils in chronic granulomatous disease. Clin Exp Immunol. 2001;123:254–63. doi: 10.1046/j.1365-2249.2001.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hallett MB, Lloyds D. Springer-Verlag, Heidelberg &, Chapman & Hall. New York, Austin: Landes Bioscience Publications; 1997. Molecular and Ionic Signalling of Neutrophils. [Google Scholar]
- 11.Pettit EJ, Hallett MB. Early Ca2+ Biochem J. 1995;310:445–8. doi: 10.1042/bj3100445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettit EJ, Hallett MB. Localised and global cytosolic Ca2+ J Cell Sci. 1996;109:1689–94. doi: 10.1242/jcs.109.7.1689. [DOI] [PubMed] [Google Scholar]
- 13.Davies EV, Hallett MB. High micromolar Ca2+ beneath the plasma membrane in stimulated neutrophils. Biochem Biophys Res Commun. 1998;248:679–83. doi: 10.1006/bbrc.1998.9031. [DOI] [PubMed] [Google Scholar]
- 14.Dewitt S, Hallett MB. Cytosolic free Ca2+ changes and calpain activation are required for β2 integrin-accelerated phagocytosis by human neutrophils. J Cell Biol. 2002;159:181–9. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theander S, Lew DP, Nusse O. Granule-specific ATP requirements for Ca2+ induced exocytosis in human neutrophils. Evidence for substantial ATP-independent release. J Cell Sci. 2002;115:2975–83. [Google Scholar]
- 16.Geiszt M, Kapus A, Ligeti E. Chronic granulomatous disease: more than the lack of superoxide? J Leukoc Biol. 2001;69:191–6. [PubMed] [Google Scholar]
- 17.Laffafian I, Hallett MB. Does cytosolic free Ca2+ signal neutrophil chemotaxis? J Cell Sci. 1995;108:3199–205. doi: 10.1242/jcs.108.10.3199. [DOI] [PubMed] [Google Scholar]
- 18.Li Z, Jiang HP, Xie W, Zhang ZC, Smrcka AV, Wu DQ. Roles of PLC-β2. Science. 2000;287:1046–9. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 19.Whyte MK, Hardwick SJ, Meagher LC, Savill JS, Haslett C. Transient elevations of cytosolic free calcium retard subsequent apoptosis in neutrophils in vitro. J Clin Invest. 1993;92:446–55. doi: 10.1172/JCI116587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olofsson P, Holmberg J, Tordsson J, Lu S, Åkerström B, Holmdahl R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nature Genetics. 2003;33:25–32. doi: 10.1038/ng1058. [DOI] [PubMed] [Google Scholar]