Abstract
Nerve growth factor (NGF) regulates B cell activation and differentiation and is an autocrine survival factor for memory B lymphocytes. We have reported recently that the number of memory B cells is reduced during HIV-1 infection. In this study we evaluated whether alteration in the NGF supply was involved in memory B cell loss in HIV-1-infected subjects. High rate of cell death in vitro was observed in memory B cells from HIV-1-infected individuals compared to uninfected donors (26·2 ± 2·5%versus 7·9 ± 1·4%, P < 0·001). The increased expression of Fas on memory B cells from infected subjects did not enhance the susceptibility of the cells to Fas-mediated apoptosis in vitro. The frequency of NGF detection in plasma from HIV-1-infected subjects was significantly lower than in healthy donors (33·6%versus 63·6%, P < 0·001). Also, the median plasma NGF in HIV-1-infected individuals was significantly lower than in uninfected controls (5 versus 14 pg/ml, respectively, P < 0·01). Interestingly, the plasma NGF level was correlated directly 1to the percentage of memory B cells (P < 0·05). HIV-1-infected subjects with a low number of peripheral memory B cells had a reduced incidence of plasmatic NGF (7·4%) compared to patients with a normal level of memory B cells (37%, P < 0·01). Moreover, the addition of recombinant NGF (1 µg/ml) to cultures of purified B cells reduced cell death of memory B cells from HIV-1-infected subjects from 24·04 ± 3·0% to 17·4 ± 1·3% (P < 0·01). HIV-1-infected individuals also carried higher levels of natural anti-NGF autoantibodies compared to uninfected subjects. In conclusion, we found that memory B cells from HIV-1-infected individuals are primed for cell death. Our study suggests an association between low frequency of plasma NGF detection and the increased cell death of memory B lymphocytes observed during HIV-1 infection. Low levels of NGF in plasma may be due to reduced supply or to NGF binding to natural anti-NGF autoantibodies.
Keywords: apoptosis, Fas, HIV-1, memory B lymphocytes, plasma NGF
INTRODUCTION
The nerve growth factor (NGF) is a neurotrophic factor regulating survival and development of cells in the nervous system [1]. Numerous studies have demonstrated that NGF exerts a biological function also on cells of the immune system. Besides its effects on regulation of inflammatory and allergic responses [2], the NGF regulates B cell function. Human B lymphocytes proliferate and differentiate into IgM and IgA secreting cells in the presence of NGF [3]. Furthermore, NGF enhances IgG4 production by tonsillar mononuclear cells and augments proliferation of tonsillar B cells [4,5]. Conversely, NGF was shown to inhibit IL-4-induced IgE secretion by human perpiheral blood mononuclear cells (PBMC) [6]. Such diverse effects of NGF have also been observed in human transformed B-lymphoblastoid and plasma cell lines. NGF was found to stimulate immunoglobulins (Ig) production by certain B-lymphoblastoid cell lines [7], whereas other human B cell lines and plasma cell lines responded to NGF treatment by reducing spontaneous secretion of Ig [8,9].
Among lymphocytes, only B cells express both the low affinity (p75NGFR) and the high affinity (p140trk–A) NGF receptors [5,10]. Both resting and activated B cells have been shown to secrete NGF in culture [10,11]. Torcia and co-workers [10] have reported that NGF is an autocrine survival factor for memory B cells and we have shown that neutralization of NGF reduces IgG production by CD40-activated memory B cells isolated from healthy donors [12]. Also, human CD4+ T cell clones have been shown to produce NGF, further suggesting the immunomodulatory function of NGF [13].
Polyclonal B cell activation is a major immunological dysfunction observed in subjects infected with HIV-1 [14,15]. B cells from HIV-1-infected patients are, however, unresponsive to mitogen and antigen stimulation in vitro[14,15] and poorly responsive to in vivo immunizations [16–18]. B lymphocytes from HIV-1-infected individuals were shown to be primed for apoptosis and to up-regulate Fas ligand (FasL) expression [19]. We have reported recently that peripheral memory B lymphocytes are reduced in HIV-1-infected subjects and suggested that memory B cells could undergo cell death through up-regulation of Fas [20]. Recently, a study by Pica and colleagues have reported high level of NGF in serum from seven patients with AIDS-associated Kaposi's sarcoma (AIDS-KS) [21]. In the present study we investigated whether memory B cells from HIV-1-infected individuals are susceptible to apoptosis. Also, as NGF acts as survival factor for memory B cells, we analysed whether an alteration in plasma NGF levels was related to loss of memory B cells in HIV-1 infection.
METHODS
Patients
A total of 131 HIV-1-infected and 108 uninfected subjects of similar age were included in the study. All patients gave informed consent and the study was approved by the ethical committee of Huddinge University Hospital (DNR 370/95). According to availability of different biological samples, the analysis of NGF plasma levels, B cell apoptosis and phenotyping of peripheral B lymphocytes was performed on smaller groups of patients, as indicated below. Patients’ subgroups did not differ in terms of treatment status, CD4 counts or CDC stage. The median CD4+ T cell count among the patient population was 320 cells/µl (range 16–869). One hundred subjects were undergoing antiretroviral treatment while 31 patients were drug-naive. According to the CDC classification 40 patients had clinical manifestations of AIDS (CDC A3, B3, C) while 50 patients were in CDC stage A and 41 patients in stage B.
Cell culture
PBMC and plasma samples were obtained as reported previously [20]. Total B lymphocytes were obtained by positive selection using CD19 magnetic microbeads (Miltenyi Biotec, Germany). Spontaneous cell death of naive and memory B cells was measured in purified B cells cultured overnight in RPMI medium. For susceptibility of purified B cells to Fas-induced apoptosis the agonistic anti-Fas MoAb clone CH11 (MBL, Nagoya, Japan) and an isotype mouse IgM were used at a concentration of 1 µg/ml. Mouse recombinant NGF was purchased from Promega (Madison, WI, USA).
Flow cytometry
Phenotyping of B cells was performed on PBMC from 66 HIV-1-infected and 51 uninfected subjects, as already reported [20]. Two- or three-colour flow cytometry was used on freshly isolated PBMC with the following MoAbs conjugated with flourescein isothiocyanate (FITC), phycoerythrin (PE) or RPE-Cy5: CD19-RPECy5, Fas-FITC (Dako, Denmark), and CD27-PE (Pharmingen, San Diego, CA, USA), and Fas ligand (FasL)-FITC (Alexis Corporation, San Diego, CA, USA). Isotype matched FITC, PE and RPECy5 conjugated mouse antibodies (Dako) were used as negative controls for unspecific staining. The percentage of memory B lymphocytes was calculated as percentage of CD27+ cells on the gate of CD19+ B cells [19]. Quantification of cell death in cultures of purified B lymphocytes was performed after overnight culturing by staining with anti-CD27-PE MoAb and Annexin-V-FITC (Pharmingen, San Diego, CA, USA). Annexin-V binds selectively to phosphatidylserine residues exposed on the outer membrane of apoptotic cells and represents a valuable tool for quantification of apoptosis in cultured lymphocytes [22].
Quantification of plasma NGF
The amount of plasma NGF was measured in 107 HIV-1-infected and 77 uninfected subjects by sandwich ELISA following the manufacture recommendations (Boehringer Mannheim, Germany). Briefly, 96-well microplates were coated with 50 µl of 0·4 µg/ml anti-NGF MoAb 27/21 in coating buffer (Sodium Carbonate 0·05 m, pH 9·0) overnight at room temperature (RT). The plates were blocked with 0·5% bovine serum albumin (BSA) in coating buffer for 2 h at RT. Recombinant NGF and plasma samples diluted 1 : 2 in sample solution (50 mm Tris-HCl, 200 mm NaCl, 10 mm CaCl2, 1% BSA, 0·1%Triton X-100, 0·05% NaN3, pH 7·0) were added in triplicate wells and incubated overnight at RT. Anti-NGF MoAb 21/27 conjugated with β-galactosidase in sample solution was added at a concentration of 80 mU/ml for 2 h at 37°C. One hundred µl of chlorophenol red-β-d-galactopyranoside (2 mg/ml) diluted in substrate solution (100 mm Hepes, 150 mm NaCl, 2 mm MgCl2, 1% BSA, 0·1% NaN3, pH 7·0) were added and incubated at 37°C for 2 h. The absorbance was measured at 540 nm. Reconstitution experiments were performed on 10 samples by adding known amounts of recombinant NGF to plasma samples in order to check for the sensitivity and reproducibility of the assay. The detection limit of the ELISA was 10 pg/ml and samples with NGF below the threshold were considered negative (arbitrary value of 5 pg/ml). Moreover, analysis of plasma and serum samples taken from the same subject was performed on 10 healthy individuals. NGF levels in plasma and serum samples from the same individuals were similar, with a variability coefficient of 20%.
Detection of anti-NGF antibodies
Recombinant NGF was diluted to 2·5 µg/ml in 0·05 m sodium carbonate, pH 9·0. Microtitre Maxisorp plates (Nunc, Denmark) were coated with 100 µl/well at RT for 2 h. Blocking was performed with 100 µl phosphate-buffered saline (PBS) containing 1% BSA for 1 h at RT. Plates were washed three times with PBS, 0·1% Tween-20. Plasma samples were diluted 1 : 100 in PBS containing 0·5% gelatin, 0·1% Tween-20 and 100 µl were applied in duplicate wells and incubated for 2 h at RT. After three washes, rabbit antihuman total Igs HRP-conjugated (Dako) was diluted 1 : 6000 and incubated for 1 h at RT. Plates were washed five times and 100 µl of tetramethylbenzidine solution were added to each well and incubated for 30 min at RT. The reaction was stopped with 1·8 m H2SO4 and the optical densities were recorded at 450 nm. OD values for samples were obtained after subtracting the background absorbance.
Statistical analysis
Statistical analysis was performed with the softwares Prophet 6·0 (AbTech Corporation, VA, USA) and SigmaStat (SPSS Inc., Germany). Normal distribution of the data was tested by the Shapiro–Wilk test. Differences between HIV-1-infected and uninfected subjects were analysed by Mann–Whitney test (memory B cells), paired Student's t-test (B cells apoptosis), Wilcoxon signed-rank paired test (Fas-induced apoptosis) or Pearson χ2 test (frequency of plasma NGF detection). Data in the text are shown as mean ± s.e.m. or median (5–95 percentiles). Correlation analysis was performed by Spearman's rank test. When comparing differences in plasma NGF between populations, the variable plasma NGF has been analysed in two different ways. When absolute values are analysed, plasma NGF is described as median (5–95 percentiles) and Kruskall–Wallis anova was used. Due to the high number of samples with undetectable NGF, differences between populations were also analysed by comparing the frequency of positive samples (% of samples with detectable NGF/total samples).
RESULTS
Cell death of memory B cells in HIV-1 infection
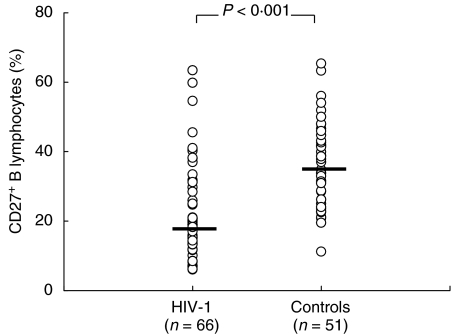
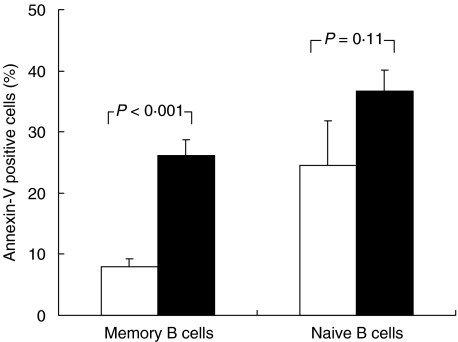
Peripheral lymphocytes from 66 HIV-1-infected and 51 uninfected subjects were double-stained for CD19 and CD27 expression. As indicated in Fig. 1, the median frequency of memory (CD27+) B lymphocytes was reduced significantly in HIV-1-infected subjects as compared to uninfected donors [19·25% (6·8–59·8) and 34·3% (19·5–57·5)], respectively. We therefore investigated whether loss of memory B cells was due to priming for cell death in vitro. As shown in Fig. 2, a fourfold increase in cell death was observed in the CD27+ B cells from 26 HIV-1-infected compared to eight healthy subjects (26·2 ± 2·5%versus 7·9 ± 1·4%, P < 0·001). Conversely, the rate of cell death detected in the CD27- (naive) B cells from HIV-1-infected subjects was only 1·5-fold higher than the controls (36·7 ± 3·4 versus 24·5 ± 7·3, P = 0·11). The increase in cell death of memory B cells from HIV-1-infected subjects was accompanied by the up-regulation of Fas expression on freshly isolated memory B cells compared to healthy controls (P < 0·05, Table 1), while no significant difference was observed in FasL expression (P = 0·08). In order to investigate whether increased Fas expression would render memory B cells from patients more susceptible to Fas-mediated apoptosis, we cultured purified B cells with agonistic anti-Fas MoAb (CH-11). Our results showed that up-regulation of Fas did not increase the susceptibility of memory B cells to apoptosis induced by agonistic anti-Fas MoAb in vitro (Table 1). The latter finding suggests that other pathways of survival/apoptosis rather than Fas/FasL could be responsible for memory B cells death in HIV-1 infection.
Fig. 1.
Memory B lymphocytes in HIV-1 infection. Distribution of the percentage of memory B lymphocytes in HIV-1-infected and uninfected subjects. Memory B cells are defined as the CD27-positive fraction of B cells. Differences were analysed by Mann–Whitney U-test.
Fig. 2.
Cell death in memory and naïve B lymphocytes. B cells were purified from HIV-1-infected and uninfected subjects and cultured overnight. Cell death was measured by flow cytometry as the percentage of Annexin-V positive memory (CD27+) or naïve (CD27-) B cells. □, HIV- (n = 8); ▪, HIV+ (n = 26).
Table 1.
Fas/FasL expression on memory B cells ex vivo and sensitivity to Fas-induced apoptosis in cultured memory B lymphocytes1
| Mean fluorescence intensity | Cell death(%) | |||
|---|---|---|---|---|
| Fas | Fas ligand | neg IgM | anti-Fas | |
| HIV-1-infected (n = 26) | 18 (7–124) | 7 (6–57) | 25·7 ± 3·7 | 26·4 ± 3·9 |
| Controls (n = 11) | ″7 (4–48) | 6 (4–10) | 10·3 ± 1·1 | 10·1 ± 0·8 |
The expression of Fas and FasL on memory B cells was analysed ex vivo on the gate of CD19+ CD27+ cells and expressed as mean fluorescence intensity. Susceptibility to CH-11 mediated apoptosis was performed on 11 HIV-1-infected and four uninfected individuals. Apoptosis of memory B cells was analysed on cultures of purified total B cells by two-colour FACS by using anti-CD27-PE MoAb and AnnexinV-FITC.
Plasma NGF
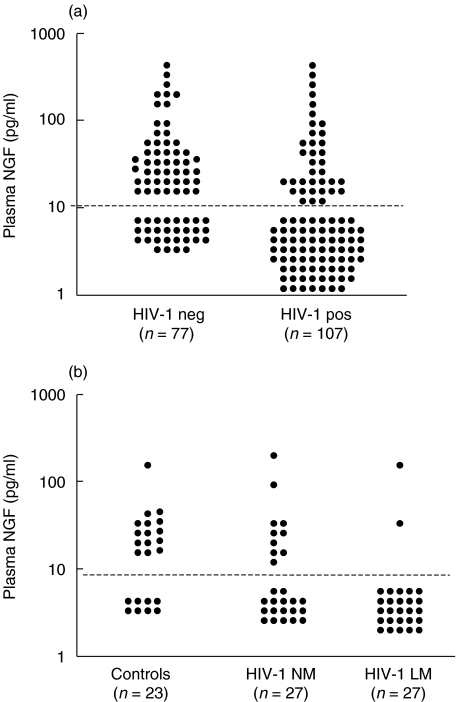
Because NGF is a survival factor for memory B lymphocytes, we measured the amount of circulating NGF in patients with HIV-1 infection. The variable plasma NGF does not follow a normal distribution due to the wide range and also to the high frequency of samples with undetectable NGF. Indeed, as shown in Fig. 3a, 28 plasma samples from 77 healthy subjects had undetectable NGF levels, with a frequency of detection equal to 63·6%. The frequency of NGF detection was significantly lower in the 107 HIV-1-infected patients, with only 36 samples with detectable plasma NGF (33·6%, P < 0·001). Moreover, the difference in plasma NGF levels between the two populations was also confirmed when Mann–Whitney rank test was applied. Indeed, the median plasma NGF in HIV-1-infected subjects was 5 pg/ml (5–112), whereas in the healthy controls it was 14 pg/ml (5–175) (P = 0·003). Spearman's rank correlation analysis on HIV-1-infected samples showed that plasma NGF was not correlated to CD4+ T cell count (ρ = − 0·04, P = 0·65). According to samples availability, simultaneous analysis of plasma NGF and memory B cells was performed on 54 patients and 23 donors. In the total population of patients and controls, plasma NGF and percentage of memory B cells were correlated (ρ = 0·246, P = 0·037). Patients were divided into two groups (normal and low memory, respectively, NM and LM) according to the frequency of memory B cells above or below the median value (19%) in this group. As shown in Fig. 3b, NGF was detected in only two of 27 patients with low memory B cells (7·4%) whereas 10 of 27 subjects with normal memory B cells had detectable NGF in plasma (37%). In healthy subjects, NGF was detected in 15 of 23 patients (65·2%). The frequency of plasma NGF detection in the HIV-1 LM patients was significantly lower compared to HIV-1 NM (P < 0·01) and to the controls group (P < 0·001). Conversely, the frequency of NGF detection between controls and HIV-1 NM subjects was not statistically significant (P = 0·22).
Fig. 3.
Detection of plasma NGF in HIV-1 infection. (a) Levels of plasma NGF in HIV-1-infected and uninfected subjects. (b) Levels of plasma NGF in healthy subjects and in HIV-1-infected subjects with memory B cells below or above the median value of 19%. These subjects were, respectively, defined as patients with low (HIV-1 LM) and normal (HIV-1 NM) memory B lymphocytes. NGF was measured by sandwich ELISA. The dotted line indicates the detection limit of plasma NGF (10 pg/ml). P-values were calculated by Pearson χ2 test.
Anti-NGF natural autoantibodies in HIV-1 infection
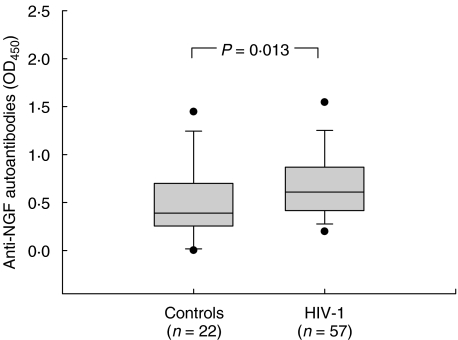
Because we observed a reduction in plasma NGF levels in patients with HIV-1 infection, we considered the possibility that plasma NGF might be complexed to anti-NGF autoantibodies. The levels of anti-NGF antibodies were determined in 57 HIV-1-infected and 22 uninfected individuals. HIV-1-infected subjects were shown to carry higher levels of anti-NGF autoantibodies compared to controls (Fig. 4). The median optical density of anti-NGF autoantibodies in HIV-1-infected subjects was 0·606 (0·421–0·863), while it was 0·392 (0·258–0·698) in control subjects (P = 0·01).
Fig. 4.
Levels of natural anti-NGF autoantibodies in HIV-1 infection. The presence of autoantibodies against NGF was investigated in plasma samples by ELISA. The differences between levels of anti-NGF antibodies between HIV-1-infected and uninfected subjects was analysed by Mann–Whitney sum rank test.
NGF and cell death of memory B cells
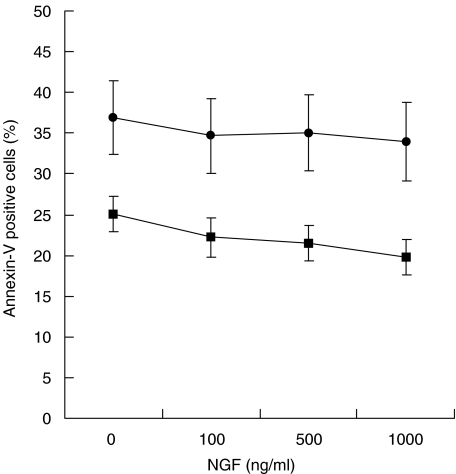
To test whether lack of NGF was involved in the death of memory B lymphocytes, we isolated B cells from 16 HIV-1-infected patients and cultured them overnight in the presence of increasing amount of recombinant NGF (0, 100, 500, 1000 ng/ml). The addition of NGF induced a low but statistically significant reduction of apoptosis of memory B cells at any concentration (Fig. 5). The highest concentration of NGF (1000 ng/ml) induced about 20% reduction of memory B cell death compared to absence of NGF (from 24·04 ± 3·0 to 17·4 ± 1·3%, P = 0·001). However, a significant decrease in memory B cell death was also observed at 100 and 500 ng/ml concentrations (P = 0·03 and P = 0·002). On the contrary, the addition of NGF did not have any significant effect on cell death of naïve cells (Fig. 5).
Fig. 5.
NGF and cell death of memory B lymphocytes in HIV-1 infection. Purified total B cells from 16 HIV-1-infected subjects were cultured overnight in presence of increasing amount of recombinant NGF and cell death was detected, as described in Fig. 2. ▪, Memory B cells; •, naive B cells.
DISCUSSION
We have reported recently that memory B lymphocytes are reduced during HIV-1 infection [20] and as NGF is an important survival factor for memory B cells [10] we investigated whether alterations in plasma NGF was associated with memory B cell loss in HIV-1-infected individuals. We here report that memory B cells from HIV-1-infected subjects are primed for apoptosis and that the plasma NGF detection is lower in HIV-1 infection compared to healthy subjects.
In the only study published on this subject to date, Pica and colleagues reported that serum levels of NGF were high in HIV-1-infected patients with AIDS-KS [21]. In this study, HIV-1-infected patients without KS showed serum NGF levels similar to healthy subjects. The discrepancy between this study and ours may not be due to different sampling of biological material (serum versus plasma) as NGF levels in plasma or serum from the same individuals were totally comparable in our hands. In addition, the median serum NGF in control subjects reported by Pica and co-authors (20 pg/ml) was similar to the median plasma NGF we report in the present study (14 pg/ml). In the study mentioned there was no indication of the frequency of NGF detection (number positive samples/total samples), which has been reported to represent an alternative way of analysis as compared to the absolute values as NGF is also undetectable in a large proportion of healthy subjects [23,24].
High levels of plasma NGF have been reported in subjects with autoimmune diseases characterized by B cell hyperactivity and an excessive antibody production [23–25]. Although these dysfunctions also occur in HIV-1 infection, we observed a decreased detection of plasma NGF in HIV-1-infected individuals. One possible explanation for this apparently controversial finding is that upon HIV-1 infection the potential sources of NGF, such as CD4+ T cells, macrophages and mast cells, are infected, deleted and functionally impaired. Also, HIV-1-infected macrophages have been shown to up-regulate NGF receptors and to use NGF as a survival factor [26]. This latter mechanism may thus contribute to the deprivation of NGF from the peripheral circulation. The impaired functionality and loss of all these cell types in infected subjects may thus account for a reduced supply and/or availability of NGF. Moreover, natural anti-NGF autoantibodies have been described in autoimmune diseases and herpes simplex virus infection [24,27,28] and because HIV-1 infection is characterized by increased levels of autoantibodies [29] the possibility exists that the plasma NGF is masked and/or neutralized by anti-NGF antibodies. We observed for the first time that HIV-1-infected patients carried higher levels of natural anti-NGF antibodies as compared to normal subjects. Although the biological function of natural anti-NGF autoantibodies is not known, an increased amount of these antibodies may modulate NGF function and contribute to neutralize the biological activity of circulating NGF.
We have previously shown loss of the memory B cell population during HIV-1 infection [20] and here we report a high rate of in vitro spontaneous cell death in memory B cells from HIV-1-infected subjects. In the present study cell death was accompanied by up-regulation of Fas on memory B cells. The treatment of purified B cells with an apoptosis-inducing anti-Fas agonistic MoAb however, did not enhance cell death in the memory cell pool from HIV-1-infected subjects. This observation is in agreement with previous reports showing that activated memory B cells may not be sensitive to anti-Fas induced apoptosis [30,31]. Although this observation needs to be confirmed in a larger group of patients, our finding suggests that factors other than Fas/FasL may be involved in memory B cell death in HIV-1 infection.
Expression of bcl-2, an anti-apoptotic molecule important for memory B cell longevity [32], is down-regulated by HIV-1 tat protein in B and T lymphocytes [33]. Alteration in bcl-2 expression was reported recently to increase tumour necrosis factor receptor (TNFR)-mediated apoptosis of T lymphocytes from HIV-1-infected subjects [34]. These findings raise the possibility that TNFR-mediated apoptosis is deregulated in B cells from HIV-1-infected patients. Moreover, it was reported recently that the survival factor function of NGF relies critically upon the continuous inactivation of the p38 mitogen-activated protein kinase (MAPK) [35], a bcl-2 modifying enzyme. Indeed, withdrawal of NGF leads to activation of MAPK, release of cytocrome c from mitochondria and apoptotic cell death of memory B lymphocytes [34]. In lymphocytes, HIV gp120 was shown to induce MAPK activation [36,37] and selective deletion of VH3-expressing memory B lymphocytes [38]. This scenario might suggest a direct effect of the virus in mediating memory B cell death through the continuous activation of MAPK which, in turn, leads to inactivation of bcl-2 and circumvents the survival effect of NGF. A detailed analysis of the apoptosis pathways possibly involved in memory B cell death in HIV-1 infection is currently ongoing in our laboratory.
We found that in HIV-1-infected subjects with very low memory B lymphocytes the frequency of NGF detection was significantly reduced. In parallel with this in vivo finding, we observed that the addition of exogenous NGF in cultures of purified B cells from HIV-1 patients could partially reduce cell death of memory B cells. NGF has been described previously to act as a survival factor for human memory B lymphocytes [10]. In addition, this growth factor may have diverse effects depending on the activation status of the target cells [3–9]. Our data suggest that deprivation (or neutralization) of circulating NGF may contribute to the loss of peripheral memory B lymphocytes in HIV-1 infection. Memory B cells in HIV-1 infection are in a continuous status of activation as suggested by the up-regulated expression of Fas and by the high spontaneous secretion of IgG in vitro. In a recent report we suggested that cell death could be a mechanism contributing to loss of memory B cells [20]. The data we report here provide evidence that memory B cells from HIV-1-infected subjects are primed for apoptosis and that low supply or deprivation of growth factors such as NGF may contribute to cell death.
Acknowledgments
The authors would like to thank Dr Francesca Chiodi for helpful discussion and critical reading of the manuscript. This work was supported by the Swedish Medical Research Council, the Swedish Physicians Against AIDS Research Foundation, the Swedish International Development Agency (SIDA-SAREC), the Swedish Association for Medical Research (SSMF) and Consiglio Nazionale delle Ricerche (Italy).
References
- 1.Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–62. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- 2.Aloe L, Bracci-Laudiero L, Bonini S, Manni L. The expanding role of nerve growth factor: from neurotrophic activity to immunologic diseases. Allergy. 1997;52:883–94. doi: 10.1111/j.1398-9995.1997.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 3.Otten U, Ehrhard P, Peck R. Nerve growth factor induces growth and differentiation of human B lymphocytes. Proc Natl Acad Sci USA. 1989;86:10059–63. doi: 10.1073/pnas.86.24.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimata H, Yoshida A, Ishioka C, Kusunoki T, Hosoi S, Mikawa H. Nerve growth factor specifically induces human IgG4 production. Eur J Immunol. 1991;21:137–41. doi: 10.1002/eji.1830210121. [DOI] [PubMed] [Google Scholar]
- 5.Brodie C, Gelfand EW. Functional nerve growth factor receptors on human B lymphocytes. Interaction with IL-2. J Immunol. 1992;148:3492–7. [PubMed] [Google Scholar]
- 6.Brodie C, Oshiba A, Renz H, Bradley K, Gelfand EW. Nerve growth-factor and anti-CD40 provide opposite signals for the production of IgE in interleukin-4-treated lymphocytes. Eur J Immunol. 1996;26:171–8. doi: 10.1002/eji.1830260127. [DOI] [PubMed] [Google Scholar]
- 7.Kimata H, Yoshida A, Ishioka C, Mikawa H. Stimulation of Ig production and growth of human lymphoblastoid B-cell lines by nerve growth factor. Immunology. 1991;72:451–2. [PMC free article] [PubMed] [Google Scholar]
- 8.Brodie C, Gelfand EW. Regulation of immunoglobulin production by nerve growth factor: comparison with anti-CD40. J Neuroimmunol. 1994;52:87–96. doi: 10.1016/0165-5728(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 9.Kimata H, Yoshida A, Ishioka C, Mikawa H. Nerve growth factor inhibits immunoglobulin production by but not proliferation of human plasma cell lines. Clin Immunol Immunopathol. 1991;60:145–51. doi: 10.1016/0090-1229(91)90120-y. [DOI] [PubMed] [Google Scholar]
- 10.Torcia M, Bracci-Laudiero L, Lucibello M, et al. Nerve growth factor is an autocrine survival factor for memory B lymphocytes. Cell. 1996;85:345–56. doi: 10.1016/s0092-8674(00)81113-7. [DOI] [PubMed] [Google Scholar]
- 11.Santambrogio L, Benedetti M, Chao MV, et al. Nerve growth factor production by lymphocytes. J Immunol. 1994;153:4488–95. [PubMed] [Google Scholar]
- 12.De Milito A, Nagy N, Samuelsson A, Chiodi F, Rajnavölgyi E. NGF released by CD40 ligand-transfected L cells: implications for functional and phenotypic studies on CD40 positive cells. Blood. 1998;11:4482–4. [PubMed] [Google Scholar]
- 13.Lambiase A, Bracci-Laudiero L, Bonini S, et al. Human CD4+ T cell clones produce and release nerve growth factor and express high-affinity nerve growth factor receptors. J Allergy Clin Immunol. 1997;100:408–14. doi: 10.1016/s0091-6749(97)70256-2. [DOI] [PubMed] [Google Scholar]
- 14.Terpstra FG, Al BJM, Roos M, et al. Longitudinal study of leukocyte functions in homosexual men seroconverted for HIV: rapid and persistent loss of B cell function after HIV infection. Eur J Immunol. 1989;19:667–73. doi: 10.1002/eji.1830190415. [DOI] [PubMed] [Google Scholar]
- 15.Lane HC, Masur H, Edgar LC, Whalen G, Rook AH, Fauci AS. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1983;309:453–8. doi: 10.1056/NEJM198308253090803. [DOI] [PubMed] [Google Scholar]
- 16.Ballet JJ, Couderc LJ, Rabian-Herzog C, et al. Impaired T-lymphocyte-dependent immune responses to microbial antigens in patients with HIV-1-associated persistent generalized lymphadenopathy. AIDS. 1988;68:479–87. doi: 10.1097/00002030-198808000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Miotti PG, Nelson KE, Dallabetta GA, Farzadegan H, Margolick J, Clements ML. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA. 1989;262:779–83. [PubMed] [Google Scholar]
- 18.Opravil M, Fierz W, Matter L, Blaser J, Luthy R. Poor antibody response after tetanus and pneumococcal vaccination in immunocompromised, HIV-infected patients. Clin Exp Immunol. 1991;84:185–9. doi: 10.1111/j.1365-2249.1991.tb08146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Samuelsson A, Sönnerborg A, Heuts N, Coster J, Chiodi F. Progressive B cell apoptosis and expression of Fas ligand during human immunodeficiency virus type 1 infection. AIDS Res Hum Retroviruses. 1997;13:1031–8. doi: 10.1089/aid.1997.13.1031. [DOI] [PubMed] [Google Scholar]
- 20.De Milito A, Mörch C, Sönnerborg A, Chiodi F. Loss of memory (CD27+) B lymphocytes during HIV-1 infection. AIDS. 2001;15:957–64. doi: 10.1097/00002030-200105250-00003. [DOI] [PubMed] [Google Scholar]
- 21.Pica F, Volpi A, Barillari G, et al. Detection of high nerve growth factor serum levels in AIDS-related and -unrelated Kaposi's sarcoma patients. AIDS. 1998;12:2025–9. doi: 10.1097/00002030-199815000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Lecoeur H, Ledru E, Prevost MC, Gougeon ML. Strategies for phenotyping apoptotic lymphocytes comparing ISNT, annexin-V and 7-AAD cytofluorometric staining methods. J Immunol Meth. 1997;209:111–23. doi: 10.1016/s0022-1759(97)00138-5. [DOI] [PubMed] [Google Scholar]
- 23.Dicou E, Masson C, Jabbour W, Nerriere V. Increased frequency of NGF in sera of rheumatoid arthritis and systemic lupus erythematosus patients. Neuroreport. 1993;5:321–4. doi: 10.1097/00001756-199312000-00036. [DOI] [PubMed] [Google Scholar]
- 24.Dicou E, Perrot S, Menkes CJ, Masson C, Nerriere V. Nerve growth factor (NGF) autoantibodies and NGF in in the synovial fluid: implications in spondylarthropathies. Autoimmunity. 1996;24:1–9. doi: 10.3109/08916939608995352. [DOI] [PubMed] [Google Scholar]
- 25.Bracci-Laudiero L, Aloe L, Levi-Montalcini R, et al. Increased levels of NGF in sera of systemic lupus erythematosus patients. Neuroreport. 1993;4:563–5. doi: 10.1097/00001756-199305000-00025. [DOI] [PubMed] [Google Scholar]
- 26.Garaci E, Caroleo MC, Aloe L, et al. Nerve growth factor is an autocrine factor essential for the survival of macrophages infected with HIV. Proc Natl Acad Sci USA. 1999;96:14013–8. doi: 10.1073/pnas.96.24.14013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dicou E, Nerriere R, Labropoulou V. Naturally occurring antibodies against nerve growth factor in human and rabbit sera: comparison between control and herpes simplex virus-infected patients. J Neuroimmunol. 1991;34:153–8. doi: 10.1016/0165-5728(91)90124-p. [DOI] [PubMed] [Google Scholar]
- 28.Dicou E, Hurez D, Nerriere V. Natural autoantibodies against the nerve growth factor in autoimmune diseases. J Neuroimmunol. 1993;47:159–67. doi: 10.1016/0165-5728(93)90026-u. [DOI] [PubMed] [Google Scholar]
- 29.Fust G, Dierich MP, Hidvegi T. Role of humoral factors in the progression of HIV disease. Immunol Today. 1995;16:167–9. doi: 10.1016/0167-5699(95)80114-6. [DOI] [PubMed] [Google Scholar]
- 30.Lagresle C, Bella C, Daniel PT, Krammer PH, DeFrance T. Regulation of germinal center B cell differentiation. Role of the human APO-1/Fas (CD95) molecule. J Immunol. 1995;154:5746–56. [PubMed] [Google Scholar]
- 31.Koopman G, Keehnen RMJ, Lindhout E, Zhou DF, de Groot C, Pals ST. Germinal center B cells rescued from apoptosis by CD40 ligation or attachment to follicular dendritic cells, but not by engagement of surface immunoglobulin or adhesion receptors, become resistant to CD95-induced apoptosis. Eur J Immunol. 1997;27:1–7. doi: 10.1002/eji.1830270102. [DOI] [PubMed] [Google Scholar]
- 32.Bovia F, Nabili-Tehrani AC, Werner-Favre C, Barnet M, Kindler V, Zubler RH. Quiescent memory B cells in human peripheral blood co-express bcl-2 and bcl-x (L) anti-apoptotic proteins at high levels. Eur J Immunol. 1998;28:4418–23. doi: 10.1002/(SICI)1521-4141(199812)28:12<4418::AID-IMMU4418>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Sastry KJ, Marin MC, Nehete PN, McConnell K, el-Naggar AK, McDonnell TJ. Expression of human immunodeficiency virus type I tat results in down-regulation of bcl-2 and induction of apoptosis in hematopoietic cells. Oncogene. 1996;13:487–93. [PubMed] [Google Scholar]
- 34.de Oliveira Pinto LM, Garcia S, Lecoeur H, Rapp C, Gougeon ML. Increased sensitivity of T lymphocytes to tumor necrosis factor receptor 1 (TNFR1) and TNFR2-mediated apoptosis in HIV infection: relation to expression of Bcl-2 and active caspase-8 and caspase-3. Blood. 2002;99:1666–75. doi: 10.1182/blood.v99.5.1666. [DOI] [PubMed] [Google Scholar]
- 35.Torcia M, De Chiara G, Nencioni L, et al. Nerve growth factor inhibits apoptosis in memory B lymphocytes via inactivation of p38 MAPK, prevention of Bcl-2 phosphorilation, and cytochrome c release. J Biol Chem. 2001;276:39027–36. doi: 10.1074/jbc.M102970200. [DOI] [PubMed] [Google Scholar]
- 36.Popik W, Pitha PM. Early activation of mitogen-activated protein kinase kinase, extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and c-Jun N-terminal kinase in response to binding of simian immunodeficiency virus to Jurkat T cells expressing CCR5 receptor. Virology. 1998;252:210–7. doi: 10.1006/viro.1998.9466. [DOI] [PubMed] [Google Scholar]
- 37.Popik W, Hesselgesser JE, Pitha PM. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J Virol. 1998;72:6406–13. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scamurra RW, Miller DJ, Dahl L, et al. Impact of HIV-1 infection on VH3 gene repertoire of naive human B cells. J Immunol. 2000;164:5482–91. doi: 10.4049/jimmunol.164.10.5482. [DOI] [PubMed] [Google Scholar]