Abstract
Cutaneous squamous cell carcinoma is typically characterized by the over-expression of the tumour suppressor protein p53. Considerable evidence suggests that immune competence is important in the control of cutaneous SCC. We discuss the immunobiology of p53 and its relevance to cutaneous SCC, including the potential interaction with human papillomavirus.
Keywords: squamous cell carcinoma, p53, cytotoxic T lymphocytes, human papillomavirus
INTRODUCTION
Non-melanoma skin cancer is the most common cancer amongst Caucasians with cutaneous squamous cell carcinoma (SCC) comprising approximately 20% and carrying a significant risk of metastasis [1]. Exposure to ultraviolet (UV) radiation is believed to be a major aetiological cofactor in the development of cutaneous SCC with other risks including ionizing radiation, chemical carcinogens, and viral infection (see below [1]). Immunosuppression also significantly increases the risk of cutaneous SCC development, for example 20 years after renal transplantation the risk of skin cancer development approached 40%[2].
THE IMMUNE RESPONSE TO p53
Up to 90% of cutaneous SCC lesions have UV-induced signature mutations, such as the formation of thymidine dimers, in the p53 gene [3,4]. p53 normally functions in cell cycle arrest, DNA repair and apoptosis, thus mutations in p53 may result in the unrestrained proliferation of keratinocytes (Fig. 1). During the early stages this may manifest as actinic keratoses which can then possibly progress to cutaneous SCC. Indeed some authors suggest that actinic keratoses represent in situ cutaneous SCC [5,6]. It has been observed that mutant p53 accumulates in the cell cytoplasm, probably due to increased half-life of the protein [7,8] and over-expression of p53 in squamous epithelium correlates with sun exposure [9,10]. In nonmalignant tissue, the ubiquitin-proteasome pathway rapidly degrades wild-type p53 [11] and the high levels of p53 expression in cutaneous SCC and other tumours contrasts to the low levels of p53 found in normal tissues.
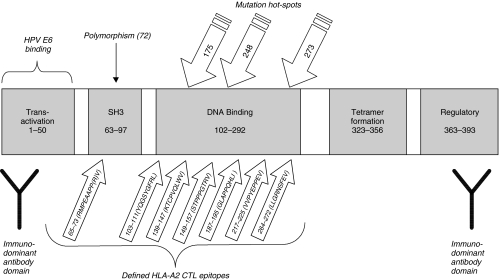
Fig. 1.
p53 CTL epitopes and functional domains. Mutational hotspots are those most commonly found in skin cancer (p53 mutation database;http://perso.curie.fr/tsoussi) Epitopes shown are those defined for CTL recognizing peptides presented on HLA-A2 MHC class I with numbers representing amino acids [18,20,25–31].
Identification of tumour-associated antigens (TAA) remains a major goal of tumour immunology. Once identified, vaccines may be designed to induce immune response against the appropriate TAA. Interest in p53 as a TAA stems from the observation that it is over-expressed in a wide variety of tumour types and therefore a vaccine against p53 could potentially be used in a broad range of malignancies. In cutaneous SCC, as the majority of the mutations are missense point mutations, over-expression of the mutant protein may alter MHC presentation of wild-type epitopes. Induction of an immune response directed against p53 may therefore enhance tumour rejection in cutaneous SCC. However, in order for p53 to be used for therapeutic intervention, a number of issues must be addressed.
Down-regulation and mutation of TAA is one mechanism whereby tumours may be able to evade the immune system. Tumours from patients with head and neck SCC in whom p53-specific cytotoxic T lymphocytes (CTL) were present appeared to have lower expression of p53 compared to those patients in whom no p53-specific CTL were detected. In addition, some tumours were found to have mutations within or adjacent to an immuno-dominant epitope of p53 [12,13]. Processing and presentation of this immuno-dominant epitope can be blocked by mutation of a flanking amino acid situated on a mutation hotspot of p53 [14]. These observations suggest immune evasion by tumours can take place, possibly by outgrowth of tumours expressing low levels of p53 or p53 mutated in or around immunodominant epitopes. An immune response to p53 may enhance this kind of immune evasion.
A further difficulty in the attempt to induce immune responses against p53 arises from the fact that, as a self-antigen, p53 specific CD4 and CD8 T cells may be tolerant, or of low affinity. p53-specific CTL generated from normal (p53+/+) mice have an avidity 10-fold lower than those generated from p53-/– mice probably due to deletion of high avidity p53-specific CTL in the normal mice [15,16]. Significantly, it is possible to induce increased number (but not avidity) of p53-specific CTL by eliciting CD4 T cell help and blocking CTLA-4 whilst immunizing mice with p53 peptide [17]. Immune nonresponsiveness to p53 may also be overcome by use of modified p53 epitopes that are more efficient than wild-type epitopes for inducing CTL responses to p53 [18–21].
It remains unclear whether there is a protective antip53 response in cancer patients. Antibodies against p53 are rare in normals but may occur in up to 30% of patients with cancer [8]. Mice injected with p53 expressing tumours also develop antibodies to p53, possibly due to necrosis of tumour and release of p53 into the serum [22]. The protective value of these antibodies is not known. Also, the relevance of antip53 antibodies to cutaneous SCC is unclear as one recent study found that, despite the high expression of p53 in tumours, patients with cutaneous SCC had low prevalence of antip53 antibodies [23]. The authors suggested that the antip53 response might be inhibited by UV-induced immuno-suppression.
Finally, it has been shown that tumour recognition and lysis may not be dependent on the level of p53 expression but rather on its rate of turnover. Those tumour cells with a high rate of p53 degradation may generate a high number of epitopes for recognition by T cells [24].
Despite these caveats, research into the immune response to p53 and its potential for therapeutic application has yielded promising results. Many studies have focused on the CD8 cytotoxic T cell (CTL) response to p53 and a number of human CTL epitopes have been described (Fig. 1.) [18,20,25–31]. Human CTL lines and clones specific for epitopes within p53 have been derived from the peripheral blood of normal individuals and cancer patients. Importantly, p53 specific CTL have been shown to recognize and kill a variety of tumour cell lines including SCC lines [32–38].
Little is known about the CD4 response to p53 in cutaneous SCC or other cancers. CD4 T cells from normal individuals have been shown to recognize peptides derived from the core domain of p53 [39] and peptides generated from mutant and cryptic p53 sequences also induce CD4 T cell response [40]. Interestingly, there is a proliferative response to p53 protein in patients with breast cancer who have p53 antibodies but not normals or those without antibodies [41]. In contrast, peripheral blood mononuclear cells from a cohort of colorectal cancer patients without p53 antibodies also displayed proliferative responses in vitro to recombinant p53 and peptides [42]. These observations have implications for cutaneous SCC given that CD4 T cells infiltrate the tumours and have been suggested to play a role in tumour regression [43,44].
p53-specific CTL capable of mediating protective immunity to tumours have been generated in a number of murine models. Adoptive transfer of p53-specific CTL generated in p53-/– mice confers immunity on the recipient to p53 overexpressing murine tumour [45]. p53-specific CTL generated in normal mice by vaccination with peptide also display antitumour immunity when adoptively transferred into recipients with established pulmonary metastasis [46]. The use of HLA-A2 transgenic mice has demonstrated the feasibility of generating protective CTL responses to epitopes within human p53. CTL generated in these mice following peptide vaccination were able to lyse a number of human tumour cell lines [47] and also confer immunity to pancreatic tumour following adoptive transfer to nude mice in vivo[48].
Canarypox virus vectors expressing mutant or wild-type p53 conferred immunity on BALB/c mice challenged with a mouse fibroblast tumour cell line that expressed high levels of p53 [22]. Other vaccines used to induce p53-specific immune responses in mice include peptide pulsed dendritic cells [49], recombinant adenovirus transduced dendritic cells [50,51], recombinant DNA [52] and recombinant vaccinia virus [53].
Concerns about the safety of inducing immune responses to self-antigen have been addressed by immunization of macaques with recombinant canary pox (ALVAC) expressing p53 protein. This regimen does not induce adverse autoimmunity despite the appearance p53 antibodies [54]. More recently, a recombinant ALVAC-p53 vaccine has been used to successfully induce antip53 T cell responses, without obvious autoimmune symptoms, in end-stage colorectal cancer patients [55]. These data contrast to another recent study in which p53-recombinant adenoviral vaccination did not generate p53-specific CTL or antibodies in six cancer patients [56]. These studies may be relevant to cutaneous SCC given the high levels of expression of p53 in the SCC lesions.
INTERACTIONS OF P53 WITH HUMAN PAPILLOMAVIRUSES
Human papillomaviruses (HPV) are believed to be additional cofactors in the development of cutaneous SCC. Interaction with p53 may prove to be a mechanism by which HPV may alter the immunobiology of cutaneous SCC.
HPV are nonenveloped viruses with double stranded DNA approximately 5kb in size. They infect epithelial cells of the skin and mucosa and replicate in differentiating keratinocytes. The viruses consist of seven early proteins (E1-E7) and 2 late structural proteins (L1 and L2). HPV types are defined according to DNA homology and over 80 types have been described but are difficult to culture in vitro– possibly due to their inability to grow differentiated keratinocytes (reviewed in [57]).
A link between HPV infection and cervical cancer has been established for a long time. HPV16 and HPV18 – the ‘high risk’ types – are commonly found in cervical cancer lesions. The transforming properties of HPV 16 are mediated by the E6 protein that disrupts p53 expression [58] and the E7 protein that interferes with the retinoblastoma tumour suppressor protein [59,60]. Human vaccine trials with cervical cancer patients are currently underway using vaccines designed to induce immune response to high risk HPV (reviewed in [61]).
The role of HPV infection in the development of cutaneous SCC is less well established. Evidence comes from the link between HPV infection and cutaneous SCC development in epidermodysplasia verruciformis (EV) patients and immuno-suppressed patients. EV is an autosomal recessive disease in which warts and macular lesions are widespread on the body, particularly on sun-exposed skin. The disease is associated with a high risk of nonmelanoma skin cancer – approximately one third of EV patients develop cutaneous SCC. A susceptibility to infection with specific HPV types (such as HPV 5, 8, 20, 21, 23, 24, 38) is thought to be responsible for the high occurrence of warts and malignancies in EV [62,63].
Immunosuppressed patients, such as transplant recipients, show up to 100 fold increased susceptibility to development of cutaneous SCC on sun-exposed skin [64]. Although the HPV types vary according to the detection method used, it is believed that there is a high prevalence of EV type HPV in the lesions of these patients [65–68].
Some of the inconsistencies associated with detection of HPV DNA are due to use of different primers by different investigators [69]. Such problems have been overcome by the employment of a degenerate PCR technique, making use of nested primers that are specific for a broad range of HPV types [70,71] (reviewed in [72]). These studies have confirmed the presence of multiple HPV types, predominantly EV-types, in lesions from EV and immunosuppressed patients as well as immunocompetent patients with cutaneous SCC. However, no particular HPV type appears to be strongly associated with cutaneous SCC. Detection of HPV DNA in the skin of normal individuals also questions the significance of the presence of HPV in cutaneous SCC [73]. There have been few serological investigations into the role of HPV infection in cutaneous SCC. One study found an increased prevalence of antibodies against the EV-associated HPV 8 in individuals with actinic keratoses [74].
As mentioned above, E6 protein from the cervical associated HPV-16 mediates degradation of p53. A common p53 polymorphism at position 72 replacing proline with arginine renders p53 more susceptible to E6 mediated degradation. The arginine allele was found to be a risk factor in the development of cervical cancers and there was also a significant association with cutaneous SCC development in renal transplant patients [75]. The two allelic variants of p53 also differ in their ability to induce apoptosis following DNA damage [76]. These findings stimulated investigations into the association of the p53 polymorphism and cutaneous SCC. Unfortunately, conflicting data has clouded the results of these studies, possibly due to interlaboratory variation in protocols [77]. Some groups confirmed an association of the polymorphism with development of cutaneous SCC in immunosuppressed patients [78] whilst others have found no association with the development of cutaneous SCC [79–82].
The presence of UV-induced p53 mutations in cutaneous SCC tumours contrasts with tumours induced by ‘high risk’ HPV types, which contain wild-type p53. It remains a tantalizing possibility that the arginine allele of p53, perhaps in combination with UV induced mutation, is more susceptible to interference from particular HPV types and subsequent malignant transformation.
Lastly, sunlight may play a role in the pathogenesis of cutaneous SCC other than the induction of p53 mutation. HPV 77, a type only so far detected in cutaneous lesions of renal transplant patients, contains a p53-DNA binding site. Once activated by UV, p53 may stimulate HPV 77 promoter activity [83]. Also, UV induced pro-inflammatory cytokines released by keratinocytes can regulate promoter activity of HPV 20 and HPV27 [84].
CONCLUSIONS
Overall there is significant evidence supporting a role for CD8+ T cells in the control of cutaneous SCC and induction of a specific CTL response is an important aim of tumour vaccination (reviewed in [85]). As p53 is over-expressed in the majority of cutaneous SCC, vaccination against p53 is an appealing strategy to induce tumour-reactive immunity. This may be particularly relevant for individuals with recurrent or metastatic cutaneous SCC but may also be considered in individuals thought to have a high risk of primary SCCs. Several problems would need to be overcome in order to generate protective immunity using a p53 vaccine. Whilst studies showing loss of p53 CTL epitopes in human cancer patients [12,13] and down-regulation of p53 in ALVAC-p53 immunized mice [22] argue for the significance of p53 specific CTL, they also underline the problem of escape mutations and tumour evasion. However, down-regulation of p53 by a tumour may diminish a dominant negative effect of a p53 missense mutation and thus represent a selective disadvantage for the tumour. Furthermore, vaccination with multiple epitopes or whole protein, concurrent cytokine vaccination, use of p53 variant peptides and/or dendritic cell therapies may overcome these difficulties. The roles of HPV in the aetiology of cutaneous SCC and their interactions with p53 have yet to be fully characterized. The development of techniques to detect HPV-specific T cell responses in an HPV-type independent manner will contribute to our understanding of their role in cutaneous SCC pathogenesis and therapeutics.
Acknowledgments
We are grateful to the Medical Research Council and British Skin Foundation for their support.
REFERENCES
- 1.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344:975–83. doi: 10.1056/NEJM200103293441306. [DOI] [PubMed] [Google Scholar]
- 2.Hartevelt MM, Bavinck JN, Kootte AM, Vermeer BJ, Vandenbroucke JP. Incidence of skin cancer after renal transplantation in The Netherlands. Transplantation. 1990;49:506–9. doi: 10.1097/00007890-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Ponten J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci USA. 1991;88:10124–8. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziegler A, Jonason AS, Leffell DJ, et al. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–6. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 5.Grossman D, Leffell DJ. The molecular basis of nonmelanoma skin cancer: new understanding. Arch Dermatol. 1997;133:1263–70. [PubMed] [Google Scholar]
- 6.Cockerell CJ. Histopathology of incipient intraepidermal squamous cell carcinoma (‘actinic keratosis’) J Am Acad Dermatol. 2000;42:11–7. doi: 10.1067/mjd.2000.103344. [DOI] [PubMed] [Google Scholar]
- 7.Dowell SP, Wilson PO, Derias NW, Lane DP, Hall PA. Clinical utility of the immunocytochemical detection of p53 protein in cytological specimens. Cancer Res. 1994;54:2914–8. [PubMed] [Google Scholar]
- 8.Soussi T. The p53 tumor suppressor gene: from molecular biology to clinical investigation. Ann NY Acad Sci. 2000;910:121–37. doi: 10.1111/j.1749-6632.2000.tb06705.x. [DOI] [PubMed] [Google Scholar]
- 9.Coulter LK, Wolber R, Tron VA. Site-specific comparison of p53 immunostaining in squamous cell carcinomas. Hum Pathol. 1995;26:531–3. doi: 10.1016/0046-8177(95)90249-x. [DOI] [PubMed] [Google Scholar]
- 10.Liang SB, Ohtsuki Y, Furihata M, Takeuchi T, Iwata J, Chen BK, Sonobe H. Sun-exposure- and aging-dependent p53 protein accumulation results in growth advantage for tumour cells in carcinogenesis of nonmelanocytic skin cancer. Virchows Arch. 1999;434:193–9. doi: 10.1007/s004280050327. [DOI] [PubMed] [Google Scholar]
- 11.Maki CG, Huibregtse JM, Howley PM. In vivo ubiquitination and proteasome-mediated degradation of p53(1) Cancer Res. 1996;56:2649–54. [PubMed] [Google Scholar]
- 12.Hoffmann TK, Nakano K, Elder EM, Dworacki G, Finkelstein SD, Appella E, Whiteside TL, DeLeo AB. Generation of T cells specific for the wild-type sequence p53(264–272): peptide in cancer patients: implications for immunoselection of epitope loss variants. J Immunol. 2000;165:5938–44. doi: 10.4049/jimmunol.165.10.5938. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann TK, Donnenberg AD, Finkelstein SD, et al. Frequencies of tetramer+ T cells specific for the wild-type sequence p53(264–272): peptide in the circulation of patients with head and neck cancer. Cancer Res. 2002;62:3521–9. [PubMed] [Google Scholar]
- 14.Theobald M, Ruppert T, Kuckelkorn U, et al. The sequence alteration associated with a mutational hotspot in p53 protects cells from lysis by cytotoxic T lymphocytes specific for a flanking peptide epitope. J Exp Med. 1998;188:1017–28. doi: 10.1084/jem.188.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theobald M, Biggs J, Hernandez J, Lustgarten J, Labadie C, Sherman LA. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J Exp Med. 1997;185:833–41. doi: 10.1084/jem.185.5.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hernandez J, Lee PP, Davis MM, Sherman LA. The use of HLA A2.1/p53 peptide tetramers to visualize the impact of self tolerance on the TCR repertoire. J Immunol. 2000;164:596–602. doi: 10.4049/jimmunol.164.2.596. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez J, Ko A, Sherman LA. CTLA-4 blockade enhances the CTL responses to the p53 self-tumor antigen. J Immunol. 2001;166:3908–14. doi: 10.4049/jimmunol.166.6.3908. [DOI] [PubMed] [Google Scholar]
- 18.Gnjatic S, Bressac-de Paillerets B, Guillet JG, Choppin J. Mapping and ranking of potential cytotoxic T epitopes in the p53 protein: effect of mutations and polymorphism on peptide binding to purified and refolded HLA molecules. Eur J Immunol. 1995;25:1638–42. doi: 10.1002/eji.1830250625. [DOI] [PubMed] [Google Scholar]
- 19.Bertholet S, Iggo R, Corradin G. Cytotoxic T lymphocyte responses to wild-type and mutant mouse p53 peptides. Eur J Immunol. 1997;27:798–801. doi: 10.1002/eji.1830270332. [DOI] [PubMed] [Google Scholar]
- 20.Petersen TR, Buus S, Brunak S, Nissen MH, Sherman LA, Claesson MH. Identification and design of p53-derived HLA-A2-binding peptides with increased CTL immunogenicity. Scand J Immunol. 2001;53:357–64. doi: 10.1046/j.1365-3083.2001.00887.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann TK, Loftus DJ, Nakano K, Maeurer MJ, Chikamatsu K, Appella E, Whiteside TL, DeLeo AB. The ability of variant peptides to reverse the nonresponsiveness of T lymphocytes to the wild-type sequence p53(264–272): epitope. J Immunol. 2002;168:1338–47. doi: 10.4049/jimmunol.168.3.1338. [DOI] [PubMed] [Google Scholar]
- 22.Roth J, Dittmer D, Rea D, Tartaglia J, Paoletti E, Levine AJ. p53 as a target for cancer vaccines: recombinant canarypox virus vectors expressing p53 protect mice against lethal tumor cell challenge. Proc Natl Acad Sci USA. 1996;93:4781–6. doi: 10.1073/pnas.93.10.4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moch C, Moysan A, Lubin R, et al. Divergence between the high rate of p53 mutations in skin carcinomas and the low prevalence of anti-p53 antibodies. Br J Cancer. 2001;85:1883–6. doi: 10.1054/bjoc.2001.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vierboom MP, Zwaveling S, Bos GMJ, Ooms M, Krietemeijer GM, Melief CJ, Offringa R. High steady-state levels of p53 are not a prerequisite for tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. Cancer Res. 2000;60:5508–13. [PubMed] [Google Scholar]
- 25.Houbiers JG, Nijman HW, van der Burg SH, et al. In vitro induction of human cytotoxic T lymphocyte responses against peptides of mutant and wild-type p53. Eur J Immunol. 1993;23:2072–7. doi: 10.1002/eji.1830230905. [DOI] [PubMed] [Google Scholar]
- 26.Nijman HW, Houbiers JG, van der Burg SH, Vierboom MP, Kenemans P, Kast WM, Melief CJ. Characterization of cytotoxic T lymphocyte epitopes of a self-protein, p53, and a non-self-protein, influenza matrix: relationship between major histocompatibility complex peptide binding affinity and immune responsiveness to peptides. J Immunother. 1993;14:121–6. [PubMed] [Google Scholar]
- 27.Zeh HJ, III, Leder GH, Lotze MT, Salter RD, Tector M, Stuber G, Modrow S, Storkus WJ. Flow-cytometric determination of peptide-class I complex formation. Identification of p53 peptides that bind to HLA-A2. Hum Immunol. 1994;39:79–86. doi: 10.1016/0198-8859(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 28.Stuber G, Leder GH, Storkus WT, et al. Identification of wild-type and mutant p53 peptides binding to HLA-A2 assessed by a peptide loading-deficient cell line assay and a novel major histocompatibility complex class I peptide binding assay. Eur J Immunol. 1994;24:765–8. doi: 10.1002/eji.1830240341. [DOI] [PubMed] [Google Scholar]
- 29.Gnjatic S, Cai Z, Viguier M, Chouaib S, Guillet JG, Choppin J. Accumulation of the p53 protein allows recognition by human CTL of a wild-type p53 epitope presented by breast carcinomas and melanomas. J Immunol. 1998;160:328–33. [PubMed] [Google Scholar]
- 30.McArdle SE, Rees RC, Mulcahy KA, Saba J, McIntyre CA, Murray AK. Induction of human cytotoxic T lymphocytes that preferentially recognise tumour cells bearing a conformational p53 mutant. Cancer Immunol Immunother. 2000;49:417–25. doi: 10.1007/s002620000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferries E, Connan F, Pages F, et al. Identification of p53 peptides recognized by CD8 (+) T lymphocytes from patients with bladder cancer. Hum Immunol. 2001;62:791–8. doi: 10.1016/s0198-8859(01)00266-x. [DOI] [PubMed] [Google Scholar]
- 32.Ropke M, Hald J, Guldberg P, et al. Spontaneous human squamous cell carcinomas are killed by a human cytotoxic T lymphocyte clone recognizing a wild-type p53-derived peptide. Proc Natl Acad Sci USA. 1996;93:14704–7. doi: 10.1073/pnas.93.25.14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chikamatsu K, Nakano K, Storkus WJ, Appella E, Lotze MT, Whiteside TL, DeLeo AB. Generation of anti-p53 cytotoxic T lymphocytes from human peripheral blood using autologous dendritic cells. Clin Cancer Res. 1999;5:1281–8. [PubMed] [Google Scholar]
- 34.Eura M, Chikamatsu K, Katsura F, et al. A wild-type sequence p53 peptide presented by HLA-A24 induces cytotoxic T lymphocytes that recognize squamous cell carcinomas of the head and neck. Clin Cancer Res. 2000;6:979–86. [PubMed] [Google Scholar]
- 35.Barfoed AM, Petersen TR, Kirkin AF, Thor Straten P, Claesson MH, Zeuthen J. Cytotoxic T-lymphocyte clones, established by stimulation with the HLA- A2 binding p5365–73 wild type peptide loaded on dendritic cells In vitro, specifically recognize and lyse HLA-A2 tumour cells overexpressing the p53 protein. Scand J Immunol. 2000;51:128–33. doi: 10.1046/j.1365-3083.2000.00668.x. [DOI] [PubMed] [Google Scholar]
- 36.Nikitina EY, Clark JI, Van Beynen J, Chada S, Virmani AK, Carbone DP, Gabrilovich DI. Dendritic cells transduced with full-length wild-type p53 generate antitumor cytotoxic T lymphocytes from peripheral blood of cancer patients. Clin Cancer Res. 2001;7:127–35. [PubMed] [Google Scholar]
- 37.Wurtzen PA, Pedersen LO, Poulsen HS, Claesson MH. Specific killing of P53 mutated tumor cell lines by a cross-reactive human HLA-A2-restricted P53-specific CTL line. Int J Cancer. 2001;93:855–61. doi: 10.1002/ijc.1417. [DOI] [PubMed] [Google Scholar]
- 38.Wurtzen PA, Claesson MH. A HLA-A2 restricted human CTL line recognizes a novel tumor cell expressed p53 epitope. Int J Cancer. 2002;99:568–72. doi: 10.1002/ijc.10375. [DOI] [PubMed] [Google Scholar]
- 39.Fujita H, Senju S, Yokomizo H, Saya H, Ogawa M, Matsushita S, Nishimura Y. Evidence that HLA class II-restricted human CD4+ T cells specific to p53 self peptides respond to p53 proteins of both wild and mutant forms. Eur J Immunol. 1998;28:305–16. doi: 10.1002/(SICI)1521-4141(199801)28:01<305::AID-IMMU305>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Fedoseyeva EV, Boisgerault F, Anosova NG, Wollish WS, Arlotta P, Jensen PE, Ono SJ, Benichou G. CD4+ T cell responses to self- and mutated p53 determinants during tumorigenesis in mice. J Immunol. 2000;164:5641–51. doi: 10.4049/jimmunol.164.11.5641. [DOI] [PubMed] [Google Scholar]
- 41.Tilkin AF, Lubin R, Soussi T, et al. Primary proliferative T cell response to wild-type p53 protein in patients with breast cancer. Eur J Immunol. 1995;25:1765–9. doi: 10.1002/eji.1830250642. [DOI] [PubMed] [Google Scholar]
- 42.van der Burg SH, de Cock K, Menon AG, et al. Long lasting p53-specific T cell memory responses in the absence of anti-p53 antibodies in patients with resected primary colorectal cancer. Eur J Immunol. 2001;31:146–55. doi: 10.1002/1521-4141(200101)31:1<146::aid-immu146>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 43.Halliday GM, Patel A, Hunt MJ, Tefany FJ, Barnetson RS. Spontaneous regression of human melanoma/nonmelanoma skin cancer: association with infiltrating CD4+ T cells. World J Surg. 1995;19:352–8. doi: 10.1007/BF00299157. [DOI] [PubMed] [Google Scholar]
- 44.Patel A, Halliday GM, Barnetson RS. CD4+ T lymphocyte infiltration correlates with regression of a UV- induced squamous cell carcinoma. J Dermatol Sci. 1995;9:12–9. doi: 10.1016/0923-1811(94)00344-e. [DOI] [PubMed] [Google Scholar]
- 45.Vierboom MP, Nijman HW, Offringa R, et al. Tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. J Exp Med. 1997;186:695–704. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hilburger Ryan M, Abrams SI. Characterization of CD8+ cytotoxic T lymphocyte/tumor cell interactions reflecting recognition of an endogenously expressed murine wild-type p53 determinant. Cancer Immunol Immunother. 2001;49:603–12. doi: 10.1007/s002620000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Theobald M, Biggs J, Dittmer D, Levine AJ, Sherman LA. Targeting p53 as a general tumor antigen. Proc Natl Acad Sci USA. 1995;92:11993–7. doi: 10.1073/pnas.92.26.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCarty TM, Liu X, Sun JY, Peralta EA, Diamond DJ, Ellenhorn JD. Targeting p53 for adoptive T-cell immunotherapy. Cancer Res. 1998;58:2601–5. [PubMed] [Google Scholar]
- 49.Mayordomo JI, Loftus DJ, Sakamoto H, et al. Therapy of murine tumors with p53 wild-type and mutant sequence peptide- based vaccines. J Exp Med. 1996;183:1357–65. doi: 10.1084/jem.183.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ishida T, Chada S, Stipanov M, Nadaf S, Ciernik FI, Gabrilovich DI, Carbone DP. Dendritic cells transduced with wild-type p53 gene elicit potent anti- tumour immune responses. Clin Exp Immunol. 1999;117:244–51. doi: 10.1046/j.1365-2249.1999.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nikitina EY, Chada S, Muro-Cacho C, Fang B, Zhang R, Roth JA, Gabrilovich DI. An effective immunization and cancer treatment with activated dendritic cells transduced with full-length wild-type p53. Gene Ther. 2002;9:345–52. doi: 10.1038/sj.gt.3301670. [DOI] [PubMed] [Google Scholar]
- 52.Petersen TR, Bregenholta S, Pedersen LO, Nissen MH, Claesson MH. Human p53(264–272): HLA-A2 binding peptide is an immunodominant epitope in DNA-immunized HLA-A2 transgenic mice. Cancer Lett. 1999;137:183–91. doi: 10.1016/s0304-3835(98)00353-x. [DOI] [PubMed] [Google Scholar]
- 53.Chen B, Timiryasova TM, Andres ML, Kajioka EH, Dutta-Roy R, Gridley DS, Fodor I. Evaluation of combined vaccinia virus-mediated antitumor gene therapy with p53, IL-2, and IL-12 in a glioma model. Cancer Gene Ther. 2000;7:1437–47. doi: 10.1038/sj.cgt.7700252. [DOI] [PubMed] [Google Scholar]
- 54.Rosenwirth B, Kuhn EM, Heeney JL, Hurpin C, Tartaglia J, Bonnet MC, Moingeon P, Erdile L. Safety and immunogenicity of ALVAC wild-type human p53 (vCP207) by the intravenous route in rhesus macaques. Vaccine. 2001;19:1661–70. doi: 10.1016/s0264-410x(00)00416-3. [DOI] [PubMed] [Google Scholar]
- 55.van der Burg SH, Menon AG, Redeker A, et al. Induction of p53-specific immune responses in colorectal cancer patients receiving a recombinant ALVAC-p53 candidate vaccine. Clin Cancer Res. 2002;8:1019–27. [PubMed] [Google Scholar]
- 56.Kuball J, Schuler M, Antunes Ferreira E, et al. Generating p53-specific cytotoxic T lymphocytes by recombinant adenoviral vector-based vaccination in mice, but not man. Gene Ther. 2002;9:833–43. doi: 10.1038/sj.gt.3301709. [DOI] [PubMed] [Google Scholar]
- 57.McMurray HR, Nguyen D, Westbrook TF, McAnce DJ. Biology of human papillomaviruses. Int J Exp Pathol. 2001;82:15–33. doi: 10.1046/j.1365-2613.2001.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kessis TD, Slebos RJ, Nelson WG, et al. Human papillomavirus 16, E6 expression disrupts the p53–mediated cellular response to DNA damage. Proc Natl Acad Sci USA. 1993;90:3988–92. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chellappan S, Kraus VB, Kroger B, Munger K, Howley PM, Phelps WC, Nevins JR. Adenovirus E1A, simian virus 40 tumor antigen, and human papillomavirus E7 protein share the capacity to disrupt the interaction between transcription factor E2F and the retinoblastoma gene product. Proc Natl Acad Sci USA. 1992;89:4549–53. doi: 10.1073/pnas.89.10.4549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berezutskaya EYuB, Morozov A, Raychaudhuri P, Bagchi S. Differential regulation of the pocket domains of the retinoblastoma family proteins by the HPV16 E7 oncoprotein. Cell Growth Differ. 1997;8:1277–86. [PubMed] [Google Scholar]
- 61.Luxton J, Shepherd P. Human papillomavirus antigens and T-cell recognition. Curr Opin Infect Dis. 2001;14:139–43. doi: 10.1097/00001432-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 62.Orth G, Favre M, Majewski S, Jablonska S. Epidermodysplasia verruciformis defines a subset of cutaneous human papillomaviruses. J Virol. 2001;75:4952–3. doi: 10.1128/JVI.75.10.4952-4953.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Majewski S, Jablonska S. Epidermodysplasia verruciformis as a model of human papillomavirus- induced genetic cancer of the skin. Arch Dermatol. 1995;131:1312–8. [PubMed] [Google Scholar]
- 64.Cohen EB, Komorowski RA, Clowry LJ. Cutaneous complications in renal transplant recipients. Am J Clin Pathol. 1987;88:32–7. doi: 10.1093/ajcp/88.1.32. [DOI] [PubMed] [Google Scholar]
- 65.Shamanin V, Glover M, Rausch C, Proby C, Leigh IM, zur Hausen H, de Villiers EM. Specific types of human papillomavirus found in benign proliferations and carcinomas of the skin in immunosuppressed patients. Cancer Res. 1994;54:4610–3. [PubMed] [Google Scholar]
- 66.de Jong-Tieben LM, Berkhout RJ, Smits HL, Bouwes Bavinck JN, Vermeer BJ, van der Woude FJ, ter Schegget J. High frequency of detection of epidermodysplasia verruciformis- associated human papillomavirus DNA in biopsies from malignant and premalignant skin lesions from renal transplant recipients. J Invest Dermatol. 1995;105:367–71. doi: 10.1111/1523-1747.ep12320803. [DOI] [PubMed] [Google Scholar]
- 67.Shamanin V, zur Hausen H, Lavergne D, et al. Human papillomavirus infections in nonmelanoma skin cancers from renal transplant recipients and nonimmunosuppressed patients. J Natl Cancer Inst. 1996;88:802–11. doi: 10.1093/jnci/88.12.802. [DOI] [PubMed] [Google Scholar]
- 68.Berkhout RJ, Bouwes Bavinck JN, ter Schegget J. Persistence of human papillomavirus DNA in benign and (pre) malignant skin lesions from renal transplant recipients. J Clin Microbiol. 2000;38:2087–96. doi: 10.1128/jcm.38.6.2087-2096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Meyer T, Arndt R, Christophers E, Stockfleth E. Frequency and spectrum of HPV types detected in cutaneous squamous-cell carcinomas depend on the HPV detection system: a comparison of four PCR assays. Dermatology. 2000;201:204–11. doi: 10.1159/000018489. [DOI] [PubMed] [Google Scholar]
- 70.Harwood CA, Spink PJ, Surentheran T, Leigh IM, de Villiers EM, McGregor JM, Proby CM, Breuer J. Degenerate and nested PCR. a highly sensitive and specific method for detection of human papillomavirus infection in cutaneous warts. J Clin Microbiol. 1999;37:3545–55. doi: 10.1128/jcm.37.11.3545-3555.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harwood CA, Surentheran T, McGregor JM, Spink PJ, Leigh IM, Breuer J, Proby CM. Human papillomavirus infection and non-melanoma skin cancer in immunosuppressed and immunocompetent individuals. J Med Virol. 2000;61:289–97. doi: 10.1002/1096-9071(200007)61:3<289::aid-jmv2>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 72.Harwood CA, Proby CM. Human papillomaviruses and non-melanoma skin cancer. Curr Opin Infect Dis. 2002;15:101–14. doi: 10.1097/00001432-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 73.Antonsson A, Forslund O, Ekberg H, Sterner G, Hansson BG. The ubiquity and impressive genomic diversity of human skin papillomaviruses suggest a commensalic nature of these viruses. J Virol. 2000;74:11636–41. doi: 10.1128/jvi.74.24.11636-11641.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bouwes Bavinck JN, Stark S, Petridis AK, et al. The presence of antibodies against virus-like particles of epidermodysplasia verruciformis-associated humanpapillomavirus type 8 in patients with actinic keratoses. Br J Dermatol. 2000;142:103–9. doi: 10.1046/j.1365-2133.2000.03248.x. [DOI] [PubMed] [Google Scholar]
- 75.Storey A, Thomas M, Kalita A, et al. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998;393:229–34. doi: 10.1038/30400. [DOI] [PubMed] [Google Scholar]
- 76.Thomas M, Kalita A, Labrecque S, Pim D, Banks L, Matlashewski G. Two polymorphic variants of wild-type p53 differ biochemically and biologically. Mol Cell Biol. 1999;19:1092–100. doi: 10.1128/mcb.19.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Makni H, Franco EL, Kaiano J, Villa LL, Labrecque S, Dudley R, Storey A, Matlashewski G. P53 polymorphism in codon 72 and risk of human papillomavirus-induced cervical cancer: effect of inter-laboratory variation. Int J Cancer. 2000;87:528–33. [PubMed] [Google Scholar]
- 78.McGregor JM, Harwood CA, Brooks L, et al. Relationship between p53 codon 72 polymorphism and susceptibility to sunburn and skin cancer. J Invest Dermatol. 2002;119:84–90. doi: 10.1046/j.1523-1747.2002.01655.x. [DOI] [PubMed] [Google Scholar]
- 79.Marshall SE, Bordea C, Wojnarowska F, Morris PJ, Welsh KI. p53 codon 72 polymorphism and susceptibility to skin cancer after renal transplantation. Transplantation. 2000;69:994–6. doi: 10.1097/00007890-200003150-00056. [DOI] [PubMed] [Google Scholar]
- 80.Bastiaens MT, Struyk L, Tjong AHSP, et al. Cutaneous squamous cell carcinoma and p53 codon 72 polymorphism: a need for screening? Mol Carcinog. 2001;30:56–61. doi: 10.1002/1098-2744(200101)30:1<56::aid-mc1013>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 81.O'Connor DP, Kay EW, Leader M, Atkins GJ, Murphy GM, Mabruk MJ. p53 codon 72 polymorphism and human papillomavirus associated skin cancer. J Clin Pathol. 2001;54:539–42. doi: 10.1136/jcp.54.7.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gustafsson AC, Guo Z, Hu X, et al. HPV-related cancer susceptibility and p53 codon 72 polymorphism. Acta Derm Venereol. 2001;81:125–9. doi: 10.1080/00015550152384272. [DOI] [PubMed] [Google Scholar]
- 83.Purdie KJ, Pennington J, Proby CM, Khalaf S, de Villiers EM, Leigh IM, Storey A. The promoter of a novel human papillomavirus (HPV77) associated with skin cancer displays UV responsiveness, which is mediated through a consensus p53 binding sequence. EMBO J. 1999;18:5359–69. doi: 10.1093/emboj/18.19.5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ruhland A, de Villiers EM. Opposite regulation of the HPV 20-URR and HPV 27-URR promoters by ultraviolet irradiation and cytokines. Int J Cancer. 2001;91:828–34. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1129>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 85.Yu Z, Restifo NP. Cancer vaccines: progress reveals new complexities. J Clin Invest. 2002;110:289–94. doi: 10.1172/JCI16216. [DOI] [PMC free article] [PubMed] [Google Scholar]