Abstract
Our earlier investigations have demonstrated a critical difference in the efficacy of orally administered porcine compared to human or mouse insulin (no effect) in preventing type I diabetes in two distinct experimental models. Based on these findings one has to assume that certain insulins might not be suitable for the induction of oral ‘tolerance’/bystander suppression, which might be one cause for recent failures in human oral antigen trials. Here we demonstrate that coupling to the non-toxic subunit of cholera toxin (CTB) can abolish these differences in efficacy between human and porcine insulin. As expected, an added benefit was the much smaller oral antigen dose required to induce CD4+ insulin-B specific regulatory cells that bystander-suppress autoaggressive responses. Mechanistically we found that uptake or transport of insulin–CTB conjugates in the gut occurs at least partially via binding to GM-1, which would explain the enhanced clinical efficacy. Both B chains bound well to major histocompatibility complex (MHC) class II, indicating comparable immunological potential once uptake and processing has occurred. Thus, our findings delineate a pathway to overcome issues in oral antigen choice for prevention of type I diabetes.
Keywords: bystander suppression, CTB, oral insulin, porcine and human, RIP–LCMV
INTRODUCTION
Immunization with islet-derived autoantigens is an attractive strategy to prevent type I diabetes and possibly other autoimmune disorders [1–5]. Success has been reported with subcutaneous, intravenous, DNA-mediated [6] and oral [7] antigen administration [8,9] using the well-established non-obese diabetic (NOD) and rat insulin promoter (RIP)–lymphocytic choriomeningitis virus (LCMV) mouse models. However, there are some apparent risks using self-antigens for immunization, as one could expect augmentation of aggressive responses via induction of islet-antigen specific cytotoxic T cells or TH1-like lymphocytes [5,10]. How can these concerns be dealt with– Historically, oral administration of insulin (and other islet antigens) was thought to circumvent induction of autoaggression due to the special microenvironment of the gut. We and others have shown that intermediate, native insulin dosages when administered throughout the prediabetic phase will induce CD4+, insulin-B specific lymphocytes [8,9,11] that act as bystander-suppressors in the pancreatic draining lymph node, where they dampen autoaggressive responses utilizing the IL-4/STAT-6 signalling pathway. However, initial oral antigen trials have failed [12] and it is important at this point to regroup and form a better strategy based on additional experimental information, some of which is provided in this report.
Source of oral insulin
Our earlier studies have shown that porcine but not human or mouse II insulins prevent diabetes in NOD or RIP–LCMV mice at a 1-mg/feeding dose [8]. This observation raises the concern that at a certain dosage, one insulin-type might be less suited for oral antigen therapy than another. Indeed, this could have been one of the reasons why human trials have failed, in particular because induction of bystander suppressor lymphocytes was never demonstrated in oral-insulin-treated individuals. In the present report we provide clear evidence that the B subunit of cholera toxin (CTB) coupling [13–15] will result in an almost complete overlap comparing human and porcine dose–response curves, which would make the correct choice of insulin less important in human trials.
Antigen dose in oral tolerance
It is crucial to give the correct amount of oral antigen. Too-low and too-high amounts per feeding will not be efficacious, because either no regulatory cells are induced or they are deleted (high dose). Comparing human and mouse gut surfaces, one would expect that prevention of human type I diabetes will require a 100–500-fold higher amount of oral insulin compared to mice. This is not possible ethically, if one considers the risk for hormonal side effects. Therefore, much lower amounts of insulin are given in current trials (7 mg in humans compared to 0·5 mg required to protect NOD or RIP–LCMV mice). Because CTB coupling was shown to reduce the oral insulin need by a factor of 500 [9,15,16], this strategy is capable to protect from diabetes at lower dosage and was therefore chosen for our current investigation.
We used the well-established RIP–LCMV mouse model system [17–19] of virally induced autoimmune diabetes, because true ‘bystander suppression’ by regulatory, insulin-specific CD4 lymphocytes had been described by us previously using these transgenic mice [19–24]. RIP–LCMV mice express the nucleoprotein (NP) from LCMV in β-cells under control of the rat insulin promoter. Diabetes develops 2–3 weeks after infection with LCMV due to a strong CD4 and CD8 response directed to the NP expressed in β-cells. Insulitis begins only when the systemic antiviral response reaches its peak and continues well after the LCMV infection has been eliminated [21,22]. Therefore, the localized, islet-specific autoimmune process, although initiated by a response to the viral (self) NP transgene, can be viewed as a true autoimmune process that follows kinetics completely different from systemic antiviral immunity. Indeed, antigenic spreading to insulin and GAD is observed during the prediabetic phase [23]. Both CD4 and CD8 NP-specific T cells are essential for autoimmune diabetes in RIP–LCMV–NP H−2d transgenic mice. Destruction of β-cells requires activation of antigen-presenting cells [24] in the islets and is mediated by both perforin and inflammatory cytokines, predominantly interferon (IFN)-γ[22]. The RIP–LCMV model is, for the above reasons, a good model for autoimmune diabetes, because it comprises many features found in human diabetes as well as other mouse models, for example the NOD mouse with spontaneous type I diabetes. A distinct advantage of RIP–LCMV mice is that the time-point for induction of the autoaggressive, LCMV–NP-specific response can be chosen experimentally (LCMV infection) and that NP-specific, destructive CD4 and CD8 lymphocytes can be tracked reliably [20,25]. This feature was a crucial advantage for our recently published study, where we demonstrated that oral feeding of insulin during the prediabetic period induces insulin B chain-specific CD4 regulatory lymphocytes that act as bystander suppressors and locally down-regulate the autoaggressive (LCMV–NP)-specific response in the pancreatic draining lymph node and islets [20]. Local effects of interleukin (IL)-4 on APCs were required for protection [20]. Thus, RIP–LCMV mice were well suited for our present endeavour.
In summary, the findings from the present report should greatly facilitate the use of oral insulin–CTB conjugates in the prevention of type I diabetes. First, the precise source of insulin becomes less important for achieving protection. Secondly, therapeutic success can be achieved at much lower antigenic dosages.
MATERIALS AND METHODS
Mice
Generation of H-2d RIP–LCMV–NP transgenic mice used in this study has been described previously [19]. These mice are screened by Southern blot or polymerase chain reaction (PCR) as described previously [19,26] and develop diabetes only after infection with LCMV. They were crossed to the H-2d (Balb/c) background for 10 generations and express the nucleoprotein (NP) of LCMV in the pancreatic β-cells and thymus but not in any other organs. Female BALB/c mice used for the Ova studies were purchased at M&B (Ry, Denmark) and housed at the central animal facility at Novo Nordisk, Bagsvaerd, Denmark.
Blood glucose
Blood glucose was monitored with AccuCheck III at the time of infection and weekly thereafter. Diabetes was defined by two subsequent blood glucose values over 300 mg/dl.
Virus
Lymphocytic choriomeningitis virus (LCMV) strain Armstrong (Arm), clone 53b [24] was used in all experiments. LCMV was plaque purified three times on Vero cells and stocks were prepared by a single passage on BHK-21 cells. Mice were infected with a single intraperitoneal dose of 105 pfu unless indicated otherwise.
Cytotoxicity assays
LCMV-specific cytotoxic T lymphocyte (CTL) activity in spleens was analysed in a 5-h in vitro51Cr-release assay [19]. All samples were run in triplicate. Primary CTL activity was tested by harvesting spleens at day 7 after intraperitoneal infection with 105 pfu LCMV. Splenocytes were co-incubated with MHC-matched (Balb/CL7 H-2d) target cells that had been loaded with 51Cr for 5 h. Target cells were either LCMV-infected, uninfected but coated with the immune dominant LCMV MHC class I peptype I diabetese Ld-NP (RPQASGVYM), or uninfected and uncoated.
Flow cytometry
Spleen and pancreatic draining lymph nodes (PDLN) were harvested at week 2 or 4 post-LCMV infection. Single cell suspensions were incubated with 50 U/ml IL-2 and 2 µg/ml brefeldin A (Sigma, St Louis, MO, USA) for 5–16 h at 37°C. Cells were stained for cell surface markers using MoAbs against CD8 and CD4, permeabilized and fixed with paraformaldehyde/saponin and stained for intracellular cytokines using PE-conjugated antimouse IFN-γ MoAb (Pharmingen, San Diego, CA, USA). Data were acquired and analysed on a Facsort or Facscalibur flow cytometer (Beckton Dickinson) using Cell Quest software (Beckton Dickinson) as described [20].
Adoptive transfers
Lymphocytes were harvested from the spleen at days 30 or 60 post-LCMV infection (1 × 105 pfu) of oral insulin–CTB-fed (twice per week) RIP–NP transgenics (intermediate 1·0 µg dose or high 50 µg dose), red cells were lysed and 5 × 107 cells were injected i.p. into prediabetic RIP–NP recipients at day 7 post-LCMV infection after a 1-day in vitro stimulation in the presence of ins-B 2 µg/ml, IL-4 8 ng/ml and 10 U/ml recombinant IL-2. Depletion of CD4 lymphocytes was achieved by magnetic beads as described by us previously [20,26,32].
Oral antigens
Porcine insulin was provided by Novo Nordisk, Bagsvaerd, Denmark and obtained as dry crystals from the very last stage of the purification process, immediately before formulation into the injectable product used for patients. Insulin was solubilized in acid buffer, pH adjusted and the solution stored at − 20°C until used. Oral antigen was administered via a blunt-ended curved feeding tube inserted into the oesophagus/stomach. RIP–NP mice were fed biweekly (unless indicated otherwise for experiments shown in Fig. 5) with 0·5 ml of an aqueous solution containing 0·1–10 mg porcine insulin or CTB–ins conjugates. Feeding was started 1 week prior to infection with LCMV and discontinued 6 weeks later. Control groups received 1 mg of bovine serum albumin (BSA) at the same intervals. CTB-conjugates were generated by linking insulin at the B1 residue of the B chain with CTB (SBL Vaccine, Stockholm, Sweden) as described previously [13].
Histological analysis
Tissues taken for histological analysis were placed in Bouin's fixative, then stained with haematoxylin and eosin. Immunochemical studies were carried out on 6–10 µm cryomicrotome sections as described [24].
Competition assays to MHC class II
A modified competition assay was used to determine binding of insulin B chains to MHC class II [27]. 200 ng of purified IAd protein and 1 µm biotinylated ovalbumin323−339 were co-incubated with inhibitory (porcine, human or mouse) B chains diluted in PBS in eight concentrations ranging from 170 µm to 17 pm in U-bottomed polypropylene 96-well plates in binding buffer (6·7 mm citric phosphate pH 7·0, 0·15 m NaCl, 2% NP-40, 2 mm EDTA). Positive control was GAD87–97[28,29], negative control (R01) the IAk-restricted hen egg lysozyme (HEL)50−62. After overnight incubation at 37°C, each incubate was transferred to precoated M5114 (anticlass II antibody; 5 µg/ml, overnight at 4°C) and preblocked (5% BSA, overnight at 37°C) ELISA plates (Nunc Maxisorb, Roskilde, Denmark). After a 3-h incubation and washing (PBS/0·05% Tween), 1 : 100 streptavidin–alkaline phosphatase conjugate was added for 1 h at room temperature. After reaction with paranitrophenolphosphate (overnight at 4°C), bound biotinylated peptype I diabetese-class II complexes were detected at 405 nm on an ELISA plate reader.
Ova immunization model
Female BALB/c mice were treated orally with vehicle, human insulin or CTB-insulin daily five times per week for a total of 4 weeks. After two2 weeks of treatment mice were immunized in the footpad with 50-µg human insulin, 100-µg ovalbumin (Sigma) in Freund's complete adjuvant (Sigma). After an additional 2 weeks of treatment, mice were sacrificed and pancreatic draining lymph nodes harvested. Quadruplicate cultures of 2 × 105 pancreatic lymph node cells were established in round-bottomed 96-well culture plates (Nunc, Roskilde, Denmark) in RPMI-1640 supplemented with 5% fetal bovine serum and antibiotics in a final volume of 200 µl with graded concentrations of Ova and cultured for 3 days in a humid 37°C CO2 atmosphere. After 48 h, 1 µCi of [3H] thymidine was added to each well and the cells were harvested 20 h later. The uptake of [3H] thymidine by the lymphocytes is used to assess lymphocyte proliferation. Results are expressed as stimulation indices (SI); the ratio of radioactivity incorporated in antigen-stimulated cells compared to unstimulated cells.
RESULTS
The previously observed difference in therapeutic efficacy between porcine and human insulin can be overcome by coupling to CTB
Our earlier studies showed that 0·5–1 mg of porcine insulin prevented diabetes in both, NOD and RIP–LCMV mice, whereas the same amount of human insulin (only one amino acid difference at position 30 of the insulin B chain) had no effect on diabetes development. We then performed a detailed dose–response analysis for both, human and porcine non-CTB-coupled insulin. Porcine insulin was effective in protecting from diabetes at much lower dosages than human insulin (Fig. 1). Only very high amounts (>5 mg per feeding) of human insulin partially protected RIP–LCMV mice but lower dosages did not provide any beneficial effects, as reported previously by us [8]. This difference in efficacy was overcome by coupling to CTB. As shown in Fig. 1, both CTB-conjugates exhibited dose–response curves that overlapped in a very large dose range: porcine as well as human conjugates protected, when amounts ranging from 0·2 µg to 10 µg were given. In addition, optimal protection was achieved with similar amounts, 1–2 µg for porcine insulin-CTB conjugate and 1–5 µg for the human insulin–CTB conjugate. In contrast, native insulin dose–response curves showed no overlap using clinically practicable amounts of less than 1 mg/feeding dosages, where only porcine insulin was protective. Only with much higher oral antigen amounts (>5 mg) was protection by observed both insulins. Furthermore, the optimal ranges showed significantly less overlap when non-conjugated insulin was used (0·5–2 mg for porcine insulin, >5 mg for human insulin that exhibited no efficacy at any dose below 1 mg/feeding). Histologically, islet infiltration was reduced significantly in insulin–CTB-protected mice, as has been observed previously for mice treated orally with whole insulin. Thus, subtle differences in antigenic sequences that can influence the ability to protect from diabetes do not play a significant role when CTB conjugation of insulin is used. This strategy should therefore greatly facilitate the oral use of different insulins.
Fig. 1.
Conjugation of orally administered insulin to CTB overcomes differences between porcine and human insulin and reduces antigen required for protection from diabetes by a factor of 500. Groups of 8–20 RIP–LCMV–NP (H-2d) mice were infected with 1 × 105 pfu LCMV i.p. and blood glucose levels were determined once per week by Accucheck (see Methods). Insulin and insulin–CTB conjugates were fed per oral gavage twice per week at various dosages as indicated in 0·25–0·5 ml of buffer (for details see Methods section). Mice with two consecutive blood glucose values exceeding 300 mg/dl were considered diabetic and euthanized. Non-diabetic mice were observed for a maximum of 2·5–3 months. Our previous studies have shown that diabetes never occurs in such protected RIP–LCMV mice over a time-frame of 1 years. Insulins and conjugates were prepared as described in the Methods section. All experiments using different dosages were performed simultaneously with smaller group sizes; the final number of mice per dosage/treatment group was obtained by repeating the study once. The difference in protection between those two studies was <10% and results on display show overall mice used. Statistical differences between dose–response curves were significant between human and porcine insulins and between human and porcine insulins and the two CTB–conjugates, respectively. In contrast, differences between the two CTB–conjugates (human and porcine) were not statistically significant (log-rank analysis).
Regulatory CD4 lymphocytes are induced only by intermediate but not high insulin–CTB dosages
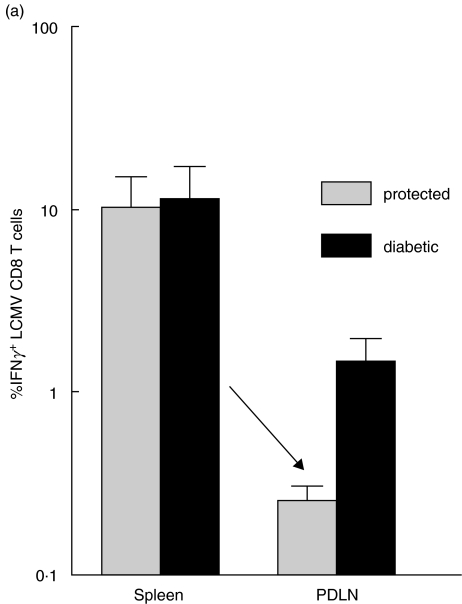
Previous studies by Weiner and others had suggested that the precise amount of fed autoantigen is crucial for obtaining suppression of autoimmune disease. In their experiments, ‘intermediate’ (0·5–1·0 mg) but not high antigen dosages (>10 mg) resulted in induction of regulatory lymphocytes [30]. These findings had formed the rationale for choosing the oral porcine dosages used for our earlier studies [20,31]. Too-high amounts of oral antigens are thought to lead to deletion of regulatory bystander-suppressor lymphocytes, an outcome that is not desirable when the goal is to activate them continuously. However, direct proof for deletion (high antigen dose) versus regulation (intermediate dose) had never been established using the same experimental diabetes model system. Our findings, presented in Fig. 1, show that only an ‘intermediate’ amount of porcine insulin or insulin–CTB conjugate was protective, suggesting that induction of regulatory cells would require an optimal dose of antigen per feeding. Indeed, good protection was achieved using splenocytes from donors protected with intermediate oral CTB–ins doses. In contrast, splenocytes from donors that had been treated with high dosages of porcine insulin (>10 mg per feeding) or porcine/human insulin–CTB conjugates (>50 µg) were never capable of protecting prediabetic RIP–LCMV recipients in adoptive transfers (Fig. 2), not even after in vitro stimulation in the presence of APCs, IL-4 and insB peptype I diabetese (not shown, stimulation performed as described in 20). Depletion of CD4 lymphocytes by magnetic bead sorting in the transferred splenocyte population abrogated this protective effect and a high incidence of diabetes was observed in recipients of CD4-depleted splenocytes from oral-insulin protected donors. Similar to our earlier observations with RIP–LCMV mice using 0·5–1·0 mg of porcine insulin [20], we find that 0·5–1 µg of CTB–insulin led to suppression of autoaggressive (LCMV-specific) CD4 (not shown) and CD8 (Fig. 3a) responses in pancreatic draining lymph nodes. Thus, oral administration of CTB–insulin conjugates is acting through induction of regulatory CD4 lymphocytes, as evidenced by our adoptive transfers in non-immunocompromised hosts. This finding is not unexpected and fits well with earlier observations from Thivolet's group in NOD mice using CTB–insulin conjugates and our group using native oral porcine insulin.
Fig. 2.
CD4 lymphocytes from donor mice treated with ‘intermediate’ oral CTB–insulin dosages can transfer protection into prediabetic recipients − more frequent feeding temporarily delays development of type I diabetes. Groups of 8–15 RIP–LCMV–NP mice were infected with 1 × 105 pfu LCMV i.p. and blood glucose levels were determined once per week by Accucheck (see Methods). Insulin and insulin–CTB conjugates were fed per oral gavage twice or seven times per week as indicated in 0·25–0·5 ml of buffer (for details see Methods section). Mice with two consecutive blood glucose values exceeding 300 mg/dl were considered diabetic and euthanized. Non-diabetic mice were observed for a maximum of 2·5–3 months. Adoptive transfers with splenocytes were performed as indicated in the Methods section using insulin–CTB (intermediate dose 0·5–1·0 µg) protected or non-protected (high dose > 50 µg) RIP–NP mice as donors. Whereas good protection of recipients was achieved when using donor spleens from insulin–CTB-treated non-diabetic mice, splenocytes from diabetic mice could never prevent diabetes. Depletion of CD4 lymphocytes by magnetic beads (see Methods) in the transferred cell population abrogated the protective effect. The differences between transfers using splenocytes from protected donors and non-protected donors are statistically significant by Fisher's exact test.More frequent feeding (5 times/week) of insulin–CTB conjugates delayed development of diabetes. This delay is significant by log-rank analysis at 3 weeks post-LCMV infection to trigger the diabetogenic process (P < 0·05), but not other time-points, and reduction of diabetes is not significant.
Fig. 3.
Bystander suppressive activity of ins-B-specific CD4 lymphocytes in RIP–LCMV as well as Ova models. (a) Lymphocytes were harvested from pancreatic draining lymph nodes and spleens of at least three RIP–NP transgenic mice that had received a therapeutically efficacious 1·0 µg dose of insulin–CTB twice per week and were protected from diabetes or from RIP–NP mice that were diabetic after being fed oral saline (buffer) solution. All mice had been infected with 105 pfu of LCMV on day 0 and analysis was done on day 30 post-infection. LCMV specific interferon-γ-producing CD8 lymphocytes were quantified by FACS analysis as described in the Methods section and previously. The mean and s.e. of all mice from the two independent studies are on display. The reduction of LCMV-specific CD8 cells in the PDLN of protected mice is statistically significant by Student's t-test in comparison to CTL found in non-protected PDLNs (P < 0·005). (b) Groups of six female BALB/c mice were treated with vehicle or the indicated doses of insulin–CTB five times per week for a total of 4 weeks and immunized with Ova and insulin as described in Materials and methods. The Ova-specific T cell proliferation was assessed in vitro 2 weeks after immunization and is shown in reference to positive control proliferation following oral administration of vehicle (= 100%). Data represent the mean from groups of six mice, s.d. values are indicated. Insulin B chain-specific proliferation (not shown) was reduced significantly (factor 3) after administration of 100 µg insulin–CTB but not lower amounts, indicating loss of insulin B chain-specific lymphocytes possibly by activation induced cell death occurring only after high oral antigen dosages.
We also observed induction of regulatory CD4 lymphocytes after administration of insulin–CTB using an additional experimental model. Oral feeding of insulin–CTB was able to suppress responses to another ‘bystander’ antigen, ovalbumin (Ova), when intermediate dosages were fed (Fig. 3b). In contrast, feeding of high insulin–CTB amounts did not lead to suppression of immune responses to the ‘bystander’ antigen Ova (Fig. 3b). Thus, optimal (intermediate) amounts of oral insulin induced regulatory/suppressor CD4 lymphocytes that down-regulated immune responses to other, unrelated antigens (Ova or LCMV–NP) and therefore acted as true bystander suppressors.
Human and porcine insulin both bind to MHC class II (I-Ad or I-Ag7)
As shown in Fig. 4, the one amino acid difference at position 30 setting human and porcine B chains apart does not significantly affect binding to the I-Ad MHC class II molecule. Thus, the observed difference in preventing type I diabetes between uncoupled human and porcine insulin is not due to different binding of insulin B chains to MHC class II but rather processing or uptake in the gut by APCs. We hypothesized that this was overcome by CTB-coupling through increasing uptake efficiency by binding to the CTB GM-1 receptor in the gut and performed additional studies.
Fig. 4.
Human, porcine and mouse II insulin B chains bind to I-Ad. Competition assays of various insulin B chains to the mouse I-Ad (BALB/c) class II molecule. Positive control peptide was GAD78−97, negative controls were the I-Ak restricted HEL50−62 (R01) or no peptide. Under the assay conditions employed, the previously as a non-binder identified HEL50−62 peptide [29] bound very weakly to I-Ad (left panel). Competition between biotinylated ovalbumin323−339 and non-labelled porcine, human or mouse B chains for binding to either I-Ag7 or I-Ad was measured by ELISA (right panel). Human, porcine and mouse BII B chains bound to I-Ad (RIP–NP), and only rather small, non-significant differences in binding efficacy were observed. The Y-scale shows OD units at 405 nm. The average of two tests is shown; difference between the two experimental values was smaller than 10%.
GM-1 plays an important role for the CTB effect
We evaluated whether CTB would act by binding to GM-1 that is expressed on M-cells and intestinal epithelium lining the gut. Soluble GM-1 was added to orally administered insulin–CTB conjugates and incidence of diabetes was compared in groups of mice that received porcine insulin–CTB with and without GM-1. As shown in Fig. 5, addition of soluble GM-1 reduced the protective effect observed usually (66–70%, Figs 1 and 5) with insulin–CTB conjugates to 37% and the majority (63%) of RIP–LCMV mice receiving porcine insulin–CTB plus GM-1 developed autoimmune diabetes. Thus, blocking the GM-1 binding sites on CTB reduced its ‘helper effect’ for orally administered insulin significantly. As expected, insulin–CTB was still able to protect RIP–LCMV mice in the presence of soluble GM-1, but the therapeutic dose was approximately 500-fold higher (>500 µg per feeding) reflecting the loss of CTB's carrier/adjuvant capacity if GM-1 is blocked (data not shown). Increasing the amount of GM-1 further did not enhance diabetes development and reduce protection more. Thus, one has to postulate additional effects of CTB as a direct adjuvant [for example, by activating antigen-presenting cells (APCs) directly] in addition to its binding to GM-1.
Fig. 5.
GM-1-binding plays a role for efficient diabetes prevention by CTB–insulin conjugates. Groups of 3–10 RIP–LCMV–NP mice were treated with porcine insulin–CTB or porcine insulin as described in the Methods section twice per week. Soluble GM-1 was added as indicated at 1 mg/ml. Blood glucose values were assessed once per week and diabetic mice (two consecutive glucose values >300 mg/dl) were euthanized. Significant differences by log-rank survival between the three groups if combined with the porcine insulin–CTB-treated group from Fig. 1 that exhibited similar protection.
Increasing feeding frequency of insulin–CTB but not the dose delays type I diabetes
We were searching for novel strategies to increase protection after oral administration of insulin-CTB conjugates. Our studies, as shown in Fig. 1, demonstrated that increasing the dose per feeding is not beneficial, because high amounts of oral insulin result in deletion of insulin-specific regulatory CD4 lymphocytes. Interestingly, increasing the feeding frequency from twice per week to daily administration of insulin–CTB significantly delayed diabetes development early after treatment, but not the overall degree of protection (10% versus 50% diabetes 2 weeks after LCMV triggering, Fig. 2). Thus, more frequent feeding of intermediate but not high doses of oral insulin is beneficial for activating insulin-specific CD4 regulatory lymphocytes and could be of clinical benefit in trials evaluating oral antigens in type I diabetes.
DISCUSSION
Oral administration of autoantigens has been used with success in experimental animal models to treat various autoimmune disorders [1,12,31–33]. However, recent trials in humans have produced rather disappointing results and the cause(s) for these failures are mainly unclear [5,10,34–36]. One probable reason is that the amounts of oral insulin or myelin basic protein fed to humans in diabetes or multiple sclerosis prevention trials, respectively, have probably been too low. For example, based on the relative difference between mouse and human gut sizes and body weights, the effective insulin dose should have been approximately 1 g/feeding. In agreement with this notion are the results of a human exploratory trial using oral administration of keyhole limpet haemocyanin (KLH) to suppress subsequent T cell responses following KLH immunization, where doses of 50–100 mg were effective [37]. Thus, the amounts of less than 10 mg insulin used in current human clinical trials for type I diabetes [35,36] were probably too low. When we used KLH to induce similar ‘oral tolerance’ in mice we found that 50–100-fold less antigen is needed to obtain a comparable reduction of the KLH specific response (1·5 mg KLH required in mice versus 50–100 mg in humans). Cost plus safety considerations were the reasons for using lesser amounts of insulin in human trials, because direct absorption of hormonally active insulin through the upper gastrointestinal tract could not be excluded and severe health risks due to potential insulin-induced hypoglycaemia cannot be tolerated. This situation formed our rationale for using insulin–CTB conjugates, because this strategy had been shown previously to reduce the quantity of oral antigen required for protection in NOD mice drastically [9,13], and therefore appears to be an excellent candidate for future oral antigen trials.
One major obstacle for using antigen-specific therapies in humans is shown in previous evidence by others and us showing that the precise sequence of the protein or peptype I diabetese is crucial for its therapeutic efficacy [8,38]. For example, the one amino acid difference in position 30 of the insulin B chain, distinguishing human from porcine insulin, is of importance [8]. This is probably another reason why oral antigen trials have failed, because giving the ‘wrong’ antigen (human versus porcine insulin or human versus bovine myelin) will have no therapeutic effect at certain doses (see Fig. 1). Unfortunately, there is no firm immunological basis at this point on which one could define the optimally suited sequence for human trials. As a solution to this problem, we demonstrate here that CTB coupling can overcome the therapeutic difficulties caused by differences in efficacy between human and porcine native insulin (Fig. 1). Consequently, human trials using CTB conjugates would probably not depend on minor differences in antigenic sequence and have a higher chance of success. Which are the possible reasons that link antigenic sequence with protection or lack of protection? The first possibility is that presentation of the essential peptype I diabetese(s) by MHC class II to regulatory CD4 lymphocytes is impaired. In our case this is unlikely, because porcine and human insulin B chains both bind well to I-Ad (Fig. 4). However, as the only difference between the two insulins is one amino acid at the carboxy-terminus of the B chain, the probable cause for the lack of efficacy of human insulin at lower doses is processing by APCs or uptake in the gut, where carboxypeptype I diabetesases are probably involved. We found that coupling to CTB can overcome this difference, which is due at least partly to CTB interaction with the GM-1 receptor, because blockade of GM-1 binding sites can reduce the clinical efficacy of ins-B conjugates in the present study (Fig. 5). It is conceivable that this apparently more efficient delivery system is insensitive to minor changes in the insulin B chain sequence. Differences in the A-chain are unlikely to play a role, as our previous observations have demonstrated that the B chain restricts regulatory lymphocytes.
How does ‘oral tolerance’ work– The term tolerance has been used very loosely in the literature in this context and encompasses a multitude of scenarios, ranging from induction of deletion and/or anergy to the activation of regulatory circuits. We provide here a detailed dose–response curve using orally administered porcine and human insulin (native or coupled to CTB) and show in two experimental mouse models that induction of regulatory lymphocytes occurs only at ‘intermediate’ dosages (Figs 2 and 3). Activation of such cells is therapeutically the desired outcome, because they can act as ‘bystander suppressors’ capable of down-regulating detrimental autoaggressive responses targeting other autoantigens. In our model systems we demonstrated that responses to LCMV–NP or Ova could be decreased after feeding mice with the appropriate amount of insulin twice per week. Similar observations were made with intermediate dosages in other models [35] and a detailed mechanistic evaluation of insulin B chain-specific bystander suppressor lymphocytes has been achieved in our earlier experiments [8]. The unique value of these cells lies in their ability to suppress autoaggressive responses locally in the pancreatic draining lymph node without causing systemic immune-suppressive side effects. They rely on an intact IL-4 signalling pathway and can modulate APCs in vitro, which is the most likely mechanism for how ‘bystander suppression’ works in vivo[8,39]. Indeed, splenocyte cultures derived from mice protected with insulin–CTB conjugates exhibited increased production of IL-4 and IL-10 in strong similarity to our earlier report [20] showing cytokine production by insulin-induced regulatory CD4 lymphocytes. In contrast to intermediate doses, high amounts of oral antigen lead to deletion of lymphocytes, reflected by a decrease of insulin B chain-specific responses, and no protection from diabetes is observed. It is important to consider that many earlier reports talk about this type of deletion of autoreactive lymphocytes in conjunction with oral tolerance [40,41]. Therapeutically, deletion may be desired, if the response that is to be suppressed is directed solely towards one (auto)antigen. For many autoimmune disorders this is not the case during advanced pathogenesis due to antigenic spreading. Consequently, elimination of all autoreactive T cells is difficult to achieve, because the initiating autoantigens are not known and the autoimmune process will most probably have spread to target more than one self-antigen at the time of treatment. For example, prediabetic individuals at risk to develop type I diabetes frequently have autoantibodies to insulin, GAD as well as IA-2 [42]. Secondly, deletion of autoaggressive lymphocytes is preceded frequently by their initial activation [43], which should be avoided at any cause to prevent acceleration of disease. Supporting these concerns, several reports described aggravation of autoimmunity after feeding high dosages of oral antigen [44]. However, it is very encouraging that insulin–CTB at high doses never resulted in acceleration of diabetes in the RIP–LCMV model.
A last point that deserves attention is our observation that more frequent feeding (daily) but not increasing the dose per feeding delays diabetes, although overall protection is not significantly enhanced. It thus appears beneficial for the induction and activation of insulin B chain-specific regulatory lymphocytes to receive a more frequent rather than a too-strong stimulus. This might augment their numbers without resulting in deletion or activation-induced cell death. At the present stage it is difficult to track these cells in vivo precisely, probably because their numbers are too low and/or the affinity is weak. Further systemic tracking studies and affinity measurements using MHC class II tetramers will possibly offer better insight into this issue in the future [45].
In conclusion, coupling of oral insulin to CTB increases efficacy and safety through several routes. First, up to 500-fold less antigen is required to achieve protection from diabetes, which will make it easier to administer high enough amounts to humans without risking direct hormonal side-effects. Secondly, differences in antigenic sequence are much less likely to affect therapeutic success. Thirdly, more frequent feeding delays disease development, suggesting daily antigen administration for future trials. These findings should provide rationale guidelines to bring the CTB-based strategy to the clinic.
Acknowledgments
This is publication no. 527 from the Division of Immune regulation, La Jolla Institute for Allergy and Immunology, San Diego, CA. This work was supported by NIH grants R01AI44451 and R29 DK51091 to M.G.V.H. D.H. is supported by a fellowship from the Juvenile Diabetes Foundation JDFI 3-1999-629. We thank Diana Frye for assistance with the manuscript and Rachel Enselman for technical assistance.
REFERENCES
- 1.Martinez NR, Harrison LC. Disabling a constitutive CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptype I diabetese. J Clin Invest. 2003;111:1365–7. doi: 10.1172/JCI17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach J-F. Predictive medicine in autoimmune diseases: from the identification of genetic predisposition and environmental influence to precocious immunotherapy. Clin Immunol Immunopathol. 1994;72:156–61. doi: 10.1006/clin.1994.1122. [DOI] [PubMed] [Google Scholar]
- 3.Tisch R, Wang B, Weaver DJ, et al. Antigen-specific mediated suppression of beta cell autoimmunity by plasmid DNA vaccination. J Immunol. 2001;166:2122–32. doi: 10.4049/jimmunol.166.3.2122. [DOI] [PubMed] [Google Scholar]
- 4.Tisch R, McDevitt HO. Antigen-specific immunotherapy: is it a real possibility to combat T-cell-mediated autoimmunity–. Proc Natl Acad Sci USA. 1994;91:437–8. doi: 10.1073/pnas.91.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McFarland HF. Complexities in the treatment of autoimmune disease. Science. 1996;274:2037–8. doi: 10.1126/science.274.5295.2037. [DOI] [PubMed] [Google Scholar]
- 6.Coon B, An LL, Whitton JL, et al. DNA immunization to prevent autoimmune diabetes. J Clin Invest. 1999;104:189–94. doi: 10.1172/JCI7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zang JA, Davidson L, Eisenbarth G, et al. Suppression of diabetes in NOD mice by oral administration of porcine insulin. Proc Natl Acad Sci USA. 1991;88:10252–6. doi: 10.1073/pnas.88.22.10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homann D, Dyrberg T, Petersen J, et al. Insulin in oral immune ‘tolerance’: a one-amino acid change in the B chain makes the difference. J Immunol. 1999;163:1833–8. [PubMed] [Google Scholar]
- 9.Bergerot I, Arreaza GA, Cameron MJ, et al. Insulin B-chain reactive CD4+ regulatory T-cells induced by oral insulin treatment protect from type 1 diabetes by blocking the cytokine secretion and pancreatic infiltration of diabetogenic effector T-cells. Diabetes. 1999;48:1720–9. doi: 10.2337/diabetes.48.9.1720. [DOI] [PubMed] [Google Scholar]
- 10.Bielekova B, Goodwin B, Richert N, et al. Encephalitogenic potential of the myelin basic protein peptype I diabetese (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptype I diabetese ligand. Nat Med. 2000;6:1167–75. doi: 10.1038/80516. [DOI] [PubMed] [Google Scholar]
- 11.Maron R, Blogg NS, Polanski M, et al. Oral tolerance to insulin and the insulin B-chain: cell lines and cytokine patterns. Ann NY Acad Sci. 1996;778:357. doi: 10.1111/j.1749-6632.1996.tb21142.x. [DOI] [PubMed] [Google Scholar]
- 12.Harrison LC, Hafler DA. Antigen-specific therapy for autoimmune disease. Curr Opin Immunol. 2000;12:704–11. doi: 10.1016/s0952-7915(00)00166-7. [DOI] [PubMed] [Google Scholar]
- 13.Bergerot I, Ploix C, Petersen J, et al. A cholera toxoid–insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc Natl Acad Sci USA. 1997;94:4610–4. doi: 10.1073/pnas.94.9.4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arakawa T, Yu J, Chong DK, et al. A plant-based cholera toxin B subunit–insulin fusion protein protects against the development of autoimmune diabetes. Nat Biotechnol. 1998;16:934–8. doi: 10.1038/nbt1098-934. [DOI] [PubMed] [Google Scholar]
- 15.Czerkinsky C, Sun JB, Lebens M, et al. Cholera toxin B subunit as transmucosal carrier-delivery and immunomodulating system for induction of antiinfectious and antipathological immunity. Ann NY Acad Sci. 1996;778:185–93. doi: 10.1111/j.1749-6632.1996.tb21127.x. [DOI] [PubMed] [Google Scholar]
- 16.Ploix C, Bergerot I, Durand A, et al. Oral administration of cholera toxin B-insulin conjugates protects NOD mice from autoimmune diabetes by inducing CD4+ regulatory T-cells. Diabetes. 1999;48:2150–6. doi: 10.2337/diabetes.48.11.2150. [DOI] [PubMed] [Google Scholar]
- 17.Ohashi PS, Oehen S, Buerki K, et al. Ablation of ‘tolerance’ and induction of diabetes by virus infection in viral antigen transgenic mice. Cell. 1991;65:305–17. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 18.Oldstone MB, Nerenberg M, Southern P, et al. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65:319–31. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 19.von Herrath MG, Dockter J, Oldstone MB. How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity. 1994;1:231–42. doi: 10.1016/1074-7613(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 20.Homann D, Holz A, Bot A, et al. Autoreactive CD4+ T cells protect from autoimmune diabetes via bystander suppression using the IL-4/Stat6 pathway. Immunity. 1999;11:463–72. doi: 10.1016/s1074-7613(00)80121-1. [DOI] [PubMed] [Google Scholar]
- 21.von Herrath M, Holz A. Pathological changes in the islet milieu precede infiltration of islets and destruction of beta-cells by autoreactive lymphocytes in a transgenic model of virus-induced IDDM. J Autoimmun. 1997;10:231–8. doi: 10.1006/jaut.1997.0131. [DOI] [PubMed] [Google Scholar]
- 22.Seewaldt S, Thomas HE, Ejrnaes M, et al. Virus-induced autoimmune diabetes: most beta-cells die through inflammatory cytokines and not perforin from autoreactive (anti-viral) cytotoxic T-lymphocytes. Diabetes. 2000;49:1801–9. doi: 10.2337/diabetes.49.11.1801. [DOI] [PubMed] [Google Scholar]
- 23.Holz A, Dyrberg T, Hagopian W, et al. Neither B lymphocytes nor antibodies directed against self antigens of the islets of Langerhans are required for development of virus-induced autoimmune diabetes. J Immunol. 2000;165:5945–53. doi: 10.4049/jimmunol.165.10.5945. [DOI] [PubMed] [Google Scholar]
- 24.von Herrath MG, Guerder S, Lewicki H, et al. Coexpression of B7-1 and viral (‘self’) transgenes in pancreatic beta cells can break peripheral ignorance and lead to spontaneous autoimmune diabetes. Immunity. 1995;3:727–38. doi: 10.1016/1074-7613(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 25.Homann D, Tishon A, Berger DP, et al. Evidence for an underlying CD4 helper and CD8 T-cell defect in B-cell-deficient mice: failure to clear persistent virus infection after adoptive immunotherapy with virus-specific memory cells from muMT/muMT mice. J Virol. 1998;72:9208–16. doi: 10.1128/jvi.72.11.9208-9216.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kagi D, Ledermann B, Burki K, et al. Cytotoxicity mediated by T cells and natural killer cells greatly impaired in perforin-deficient mice. Nature. 1994;369:1–7. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 27.Harrison LC, Honeyman MC, Trembleau S, et al. A peptype I diabetese-binding motif for I-A (g7), the class II major histocompatibility complex (MHC) molecule of NOD and Biozzi AB/H mice. J Exp Med. 1997;185:1013–21. doi: 10.1084/jem.185.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sette A, Sidney J, Albertson M, et al. A novel approach to the generation of high affinity class II-binding peptype I diabeteses. J Immunol. 1990;145:1809–13. [PubMed] [Google Scholar]
- 29.Sette A, Buus S, Colon S, et al. Structural analysis of peptype I diabeteses capable of binding to more than one Ia antigen. J Immunol. 1989;142:35–40. [PubMed] [Google Scholar]
- 30.Weiner HL. Oral tolerance, an active immunologic process mediated by multiple mechanisms. J Clin Invest. 2000;106:935–7. doi: 10.1172/JCI11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.von Herrath MG, Dyrberg T, Oldstone MB. Oral insulin treatment suppresses virus-induced antigen-specific destruction of beta cells and prevents autoimmune diabetes in transgenic mice. J Clin Invest. 1996;98:1324–31. doi: 10.1172/JCI118919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsui M. Application of oral tolerance to the treatment of autoimmune diseases − active suppression and bystander suppression. Nippon Rinsho. 1997;55:1537–42. [PubMed] [Google Scholar]
- 33.Weiner HL. Oral tolerance for the treatment of autoimmune diseases. Annu Rev Med. 1997;48:341–51. doi: 10.1146/annurev.med.48.1.341. [DOI] [PubMed] [Google Scholar]
- 34.Weiner HL, Mackin GA, Matsui M, et al. Double-blind pilot trial of oral tolerization with myelin antigens in multiple sclerosis. Science. 1993;259:1321–4. doi: 10.1126/science.7680493. [DOI] [PubMed] [Google Scholar]
- 35.Chaillous L, Lefevre H, Thivolet C, et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Lancet. 2000;356:545–9. doi: 10.1016/s0140-6736(00)02579-4. Diabete Insuline Orale Group. [DOI] [PubMed] [Google Scholar]
- 36.Pozzilli P, Pitocco D, Visalli N, et al. No effect of oral insulin on risidual beta-cell function in recent-onset type I diabetes (the IMDIAB VII) Diabetologia. 2000;43:1000. doi: 10.1007/s001250051482. IMDIAB Group. [DOI] [PubMed] [Google Scholar]
- 37.Husby S, Mestecky J, Moldoveanu Z, et al. Oral tolerance in humans. T cell but not B cell tolerance after antigen feeding. J Immunol. 1994;152:4663–70. [PubMed] [Google Scholar]
- 38.Weissert R, Lobell A, de Graaf KL, et al. Protective DNA vaccination against organ-specific autoimmunity is highly specific and discriminates between single amino acid substitutions in the peptype I diabetese autoantigen. Proc Natl Acad Sci USA. 2000;97:1689–94. doi: 10.1073/pnas.030390097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King C, Mueller Hoenger R, Malo Cleary M, et al. Interleukin-4 acts at the locus of the antigen-presenting dendritic cell to counter-regulate cytotoxic CD8+ T-cell responses. Nat Med. 2001;7:206–14. doi: 10.1038/84659. [DOI] [PubMed] [Google Scholar]
- 40.Wardrop RM, III, Whitacre CC. Oral tolerance in the treatment of inflammatory autoimmune diseases. Inflamm Res. 1999;48:106–19. doi: 10.1007/s000110050433. [DOI] [PubMed] [Google Scholar]
- 41.Benson JM, Whitacre CC. The role of clonal deletion and anergy in oral tolerance. Res Immunol. 1997;148:533–41. doi: 10.1016/s0923-2494(98)80147-8. [DOI] [PubMed] [Google Scholar]
- 42.Jackson RA, Soeldner JS, Eisenbarth GS. Predicting insulin-dependent diabetes. Lancet. 1988;10:627–8. doi: 10.1016/s0140-6736(88)90663-0. [DOI] [PubMed] [Google Scholar]
- 43.von Herrath M, Coon B, Wolfe T. Tolerance induction with agonist peptype I diabeteses recognized by autoaggressive lymphocytes is transient. Therapeutic potential for type 1 diabetes is limited and depends on time-point of administration, choice of epitope and adjuvant. J Autoimmun. 2001;16:193–9. doi: 10.1006/jaut.2000.0497. [DOI] [PubMed] [Google Scholar]
- 44.Blanas E, Carbone FR, Allison J, et al. Induction of autoimmune diabetes by oral administration of autoantigen. Science. 1996;274:1707–9. doi: 10.1126/science.274.5293.1707. [DOI] [PubMed] [Google Scholar]
- 45.Martin R, Ruddle NH, Reingold S, et al. T helper cell differentiation in multiple sclerosis and autoimmunity. Immunol Today. 1998;19:495–8. doi: 10.1016/s0167-5699(98)01345-0. [DOI] [PubMed] [Google Scholar]