Abstract
Specific serum IgG subclass antibodies against Helicobacter pylori antigens and recombinant CagA were analysed in 75 symptomatic children with histologically confirmed H. pylori infection. H. pylori stimulated an IgG1 predominant response, and IgG3 titres showed a positive association with peptic ulcer disease, chronicity of antral inflammation and density of H. pylori colonization. Two methods used for assessing serum IgG CagA antibody status, i.e. Western blotting and enzyme-linked immunosorbent assay (ELISA), were concordant. CagA stimulated an IgG1 and IgG3 predominant humoral response. Total CagA IgG titres were higher in children with active and more severe chronic antral inflammation. These findings suggest that in children the systemic humoral immune response to H. pylori infection may reflect gastroduodenal pathology.
Keywords: CagA antigens, children, Helicobacter pylori, IgG subclass response
INTRODUCTION
Helicobacter pylori infection, which affects approximately half the world's population, is now accepted as an important pathogenic factor in chronic gastritis, peptic ulcer disease, gastric carcinoma and gastric mucosa-associated lymphoid tissue (MALT) lymphoma [1]. Despite such a high prevalence only a small proportion of infected individuals present clinical manifestations of the disease, and conditions influencing the course of infection have not been determined fully.
Bacterial virulence factors such as the cag pathogenicity island (cag PAI) [2,3], the vacuolating cytotoxin [4] and BabA2 adhesin [5] have been associated in several studies to more severe clinical outcome (reviewed in [6]). Infection with cag positive strains was initially linked with peptic ulcer disease and active gastritis [7]. However, more recently a strong association between infection with cag positive H. pylori strains and increased risk of gastric atrophy [8,9] and gastric cancer [10–13] has become evident. Infection with cag positive strains is associated with increased gastric mucosal C-X-C chemokines [14,15], which are likely to contribute to enhanced neutrophilic responses associated with active gastritis. Previous studies in both children [16] and adults [17,18] have shown that the cag genotype can influence the colonization density.
Accumulating evidence suggests that other factors, such as the host response to the H. pylori infection, are important in the pathogenesis of H. pylori-induced mucosal changes. Genetic polymorphisms in proinflammatory and immunoregulatory cytokines have been linked to an increased risk of developing gastric atrophy and/or gastric cancer [19–21]. These studies provide the most convincing evidence that clinically, host immune responses contribute to clinical outcome. Cytokines such as interleukin (IL)-12 and IL-18, which are increased with H. pylori infection [22,23], are important in polarizing the Th1 mucosal responses [24–26]. The role of the immune response in the outcome of gastric Helicobacter infection has been demonstrated clearly in mice lacking T cells [27].
In many infections the IgG subclass response to infecting pathogens has been associated with severity of clinical symptoms and inflammatory response [28–30]. Although the specific subclass response to H. pylori in adult populations has been analysed in a few studies [31–35], the relationship between H. pylori IgG subclasses and gastric inflammation in children has not been investigated fully. In addition, the IgG subclass antibodies against the immunogenic H. pylori CagA protein have not been studied. The aim of this study was to assess the association between IgG subclass response to H. pylori antigens and recombinant CagA and gastric histology in symptomatic children.
MATERIALS AND METHODS
Patients
Symptomatic children presenting for endoscopy at the Children's Memorial Health Institute, Warsaw, Poland, were eligible for inclusion. From each child, 2 ml of blood was taken for serology and antral biopsies were obtained for histology and culture during routine upper gastrointestinal endoscopy. The study was undertaken with ethics committee approval of the Children's Memorial Health Institute and informed consent was obtained from all patients/parents.
Gastric histology
Antral biopsy specimens were fixed in formalin. Sections stained with haematoxilin–eosin and modified Giemsa stain were assessed histologically according to the updated Sydney System by a single experienced pathologist (M. G.-D), who was unaware of the clinical diagnosis. Sections were graded for the presence and extent of chronic lymphocytic infiltration, active neutrophilic infiltration, atrophy, intestinal metaplasia and the density of H. pylori colonization on a scale of 0–3.
Serological assays
Serum samples were assayed for the presence of total IgG antibodies to CagA and specific IgG subclasses to H. pylori and CagA. Total CagA IgG antibodies were measured by enzyme-linked immunosorbent assay (ELISA), as described previously [12], and by Western blotting (Helicoblot 2·0; Genelabs Diagnostic, Singapore) according to the manufacturer's instructions. Positivity in the CagA IgG ELISA was determined by reference to a standard curve of positive control serum assayed on each plate as previously described [9,12]. Cut-off values were validated with paediatric sera from H. pylori-negative children with histologically normal mucosa as described previously [36].
For the H. pylori and CagA IgG subclasses ELISAs an ultracentrifuged sonicated whole cell preparation of H. pylori[36,37] and a recombinant fragment of CagA [38] (kindly provided by Dr G. del Guidice, Chiron Vaccines, Siena, Italy) respectively, were used as antigens. Flat-bottomed 96-well microtitre plates were coated with 125 ng/well CagA or 500 ng/well H. pylori antigen in 0·1 m bicarbonate buffer (pH 9·6) for 24 h at 4°C. Plates were washed with phosphate buffered saline (PBS) containing 0·1% Tween 20, and blocked with 1% bovine serum albumin (BSA) in PBS-Tween for 1 h at 26°C. Serum samples diluted 1/200 (H. pylori ELISA) or 1/50 (CagA ELISA) in 1% BSA/PBS-Tween were incubated in duplicate for 90 min at 26°C. Following further washing and incubation with biotin-conjugated monoclonal antihuman IgG1, IgG2, IgG3 and IgG4 antibodies (Sigma Chemicals, Poole, UK) the plates were incubated with avidin alkaline phosphatase (Sigma Chemicals), and bound antibodies were detected with p-nitrophenyl phosphate substrate (Sigma Chemicals) solution at 1 mg/ml in diethanolamine-MgCl2 buffer pH 9·8. On each plate positive control sera diluted 1/200–1/12800 (H. pylori) or 1/50–1/3200 (CagA) were used to generate standard curves of arbitrary units. The cut-off for positivity in the IgG subclass ELISAs was the mean ±2 s.d. of 37 patients who were H. pylori and CagA seronegative by Western blotting and histologically negative for H. pylori.
Culture
Primary isolation was performed on Wilkins Chalgren agar with 7% horse blood and Dent's selective supplement SR 147 (Oxoid, UK). The plates were incubated under microaerophilic conditions (CampyPak Plus, BBL generators) at 37°C for up to 7 days. H. pylori was identified by colony morphology, Gram staining and urease, catalase and oxidase activities.
Statistical analysis
The relation between antibody titres (CagA IgG, H. pylori IgG subclasses, CagA IgG subclasses), histological parameters and the presence of peptic ulcer disease (PUD) were analysed by non-parametric statistics: the Mann–Whitney U-test for comparison of two groups and the Kruskal–Wallis anova for groups greater than two. The Spearman correlation coefficient was used to determine the relation between histological parameters and H. pylori IgG subclass titres and to assess the relation between H. pylori IgG subclass titres and CagA IgG subclass titres. Associations between disease manifestations [PUD versus non-ulcer dyspepsia (NUD)] and specific H. pylori and CagA IgG subclass responses were analysed by Fisher's exact test. The same test was used to assess the relationship between positivity of specific H. pylori IgG subclasses and CagA status. The associations between the ratio of IgG1/IgG2 H. pylori antibodies, age and disease manifestations were analysed by χ2 test.
RESULTS
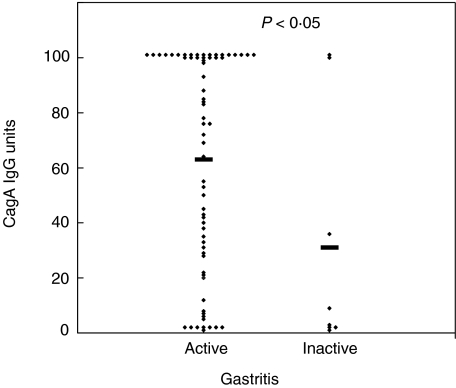
A total of 75 children with histologically confirmed H. pylori infection were included in the study. Histologically, all the children had gastritis. The children were all ethnically Polish; the mean age was 13·2 years (range 7–18 years), 34 were female and 41 male. Twenty-four (32%) children had duodenal ulcers (DU). H. pylori cultures were performed in 68 children, 62 of whom were positive. CagA IgG antibodies were detected by ELISA in 60 of 75 (80%) of the children. To determine the sensitivity of the CagA ELISA in the population, Western blotting for CagA antibodies was undertaken in a subgroup of 57 children. Forty-six of 57 (81%) children were CagA positive by Western blotting and the two methods showed a 100% concordance for detecting CagA IgG antibodies. CagA IgG titres were higher in patients with active (n = 67) compared to inactive gastritis (n = 8) (P < 0·05; Fig. 1) and in children with more severe chronic antral inflammation (P < 0·05). Higher (although not significant) levels of CagA IgG antibodies were observed in children with DU (mean 74 units) compared to a group with non-ulcer dyspepsia (NUD) (mean 53 units) (P = 0·06).
Fig. 1.
CagA IgG Units in H. pylori infected children with active (n = 67) versus inactive (n = 8) gastritis (Mann–Whitney U-test, P < 0·05). The bars represent the mean values.
H. pylori IgG subclass response
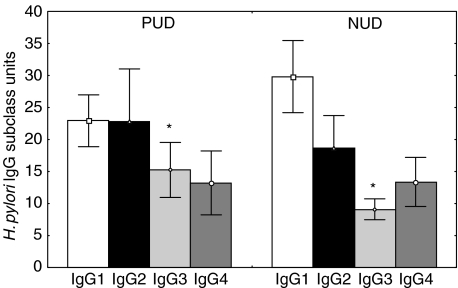
The specific IgG subclass positive responses to H. pylori in the infected children consisted of all four subclasses, with 84% of children having IgG1 subclass antibodies, 68% IgG2 and IgG3 subclasses and 53% IgG4 (Table 1). Levels of all IgG subclasses (expressed as units) to H. pylori were moderately correlated. There was no significant difference in the IgG1, IgG2, IgG3 and IgG4 subclass positivity to H. pylori between DU and NUD groups (Table 1). There was also no difference in the IgG subclass response to H. pylori between CagA seropositive and CagA seronegative groups. The H. pylori IgG1, IgG2 and IgG4 titres did not differ between DU and NUD patients; however, the H. pylori IgG3 titres were significantly higher in children with DU compared to NUD group (P < 0·05; Fig. 2). There was no association between the levels of H. pylori IgG subclasses and activity of antral inflammation. However, titres of specific IgG1 and IgG3 antibodies correlated moderately with chronicity of antral inflammation (Spearman correlation coefficient, r = 0·27, P < 0·05 and r = 0·37, P < 0·01, respectively), and IgG3 titres correlated moderately with the density of H. pylori colonization (r = 0·31, P < 0·01). Comparison of the ratio of IgG1/IgG2 H. pylori antibodies showed no difference between DU and NUD patients and no age-related differences in subclass ratios were observed.
Table 1.
H. pylori IgG subclass positivity in relation to disease status in 75 children
| H. pylori IgG subclass response (no. and % positive) | ||||
|---|---|---|---|---|
| Diagnosis | IgG1 | IgG2 | IgG3 | IgG4 |
| Duodenal ulcer (n = 24) | 23 (96%) | 20 (83%) | 20 (83%) | 14 (58%) |
| Non-ulcer dyspepsia(n = 51) | 40 (78%) | 31 (61%) | 31 (61%) | 26 (51%) |
| Total (75) | 63 (84%) | 51 (68%) | 51 (68%) | 40 (53%) |
Fig. 2.
IgG1, IgG2, IgG3 and IgG4 H. pylori subclass antibodies in H. pylori infected children with duodenal ulcer (DU; n = 24) and non-ulcer dyspepsia (NUD; n = 51). *IgG3 titres were significantly higher in patients with DU compared to NUD (Mann–Whitney U-test, P < 0·05) The squares represent mean ± s.e.m. values.
CagA IgG subclass response
CagA IgG subclasses were analysed in 60 children who were positive for CagA IgG antibodies by ELISA. Preliminary studies showed that in subjects infected with CagA-positive H. pylori strains there is virtually no specific IgG4 response to the CagA protein; therefore, in the present study only CagA IgG1, IgG2 and IgG3 subclass antibodies were assayed. The specific CagA IgG subclass response involved predominantly IgG1 and IgG3 subclasses (Table 2), and titres of the two subclasses showed moderate correlation (r = 0·43; P < 0·001). No significant difference was found in the distribution and levels of CagA IgG subclass response between DU and NUD patients. There was also no association between CagA IgG subclass response and histological features of antral inflammation and density of H. pylori colonization.
Table 2.
CagA specific subclass positivity in 60 children positive for CagA IgG antibodies
| H. pylori IgG subclass response (no. and % positive) | |||
|---|---|---|---|
| Diagnosis | IgG1 | IgG2 | IgG3 |
| Duodenal ulcer (n = 21) | 20 (95%) | 4 (19%) | 10 (48%) |
| Non-ulcer dyspepsia (n = 39) | 35 (90%) | 3 (8%) | 14 (36%) |
| Total (60) | 55 (92%) | 7 (12%) | 24 (40%) |
DISCUSSION
There are relatively few data on the IgG subclass response to H. pylori and how it may relate to gastroduodenal disease. Studies to date have largely focused on adult populations [31,32,34,35] and the results have varied depending on the population studied. Mitchell et al. found IgG2 predominant response in Australian and German adults and IgG1 predominant response in Sowetan children and adults, and suggested that the difference could result from simultaneous helminthic infections in Sowetan subjects modulating immune response against H. pylori[35]. In the present study an IgG1 predominant response to H. pylori in Polish children was observed; however, because the prevalence of intestinal parasitic infection in this population has been shown to be relatively low (22%) [39], it seems unlikely that parasitic infection would influence anti-H.pylori response markedly. It is possible, however, that the difference in IgG subclass response to H. pylori could be related to antigenic variation of infecting strains. A similar phenomenon has already been observed in mice vaccinated with different serotypes of Streptococcus pneumoniae capsular polysaccharide [40]. Similarly to the Sowetan population, where over 90% of H. pylori infections are caused by cagA positive strains [41], the vast majority of children (80%) analysed in the present study had cagA+ infections. CagA is a strongly immunogenic protein which, as shown in this study, elicits an IgG1 predominant response. Therefore, it could be speculated that IgG1 predominant response in Sowetan and Polish patients could result potentially from a predominance of CagA IgG1 antibodies in the total pool of anti-H. pylori antibodies. The composition of the antigen preparations used to determine the H. pylori subclass IgG response in earlier studies [31–35] may influence strongly the ratio H. pylori IgG subclass responses. In the current study the antigen preparation used for the H. pylori IgG subclasses ELISA did not contain CagA.
There is some evidence to suggest H. pylori cag positive strains could modulate the immune response to H. pylori, shifting it towards IgG1 predominance. Previous studies have indicated that mucosal IL-12p40 transcript levels are greater in patients with peptic ulcers and those infected with cagA positive strains than cagA negative strains [22]. Additionally, Th1 cell clones generated from the gastric mucosa of H. pylori positive patients frequently recognize CagA [42]. Apart from environmental and bacterial factors, the immune response against H. pylori can be also influenced by host factors, such as age. Andersen et al. reported that H. pylori IgG2 was the IgG subclass which increased the most with age [31]. A similar observation has also been made by Lottenbach et al., who noted that children vaccinated with pneumococcal vaccines responded predominantly with IgG1 antibodies, whereas adults had mainly an IgG2 antibody response [43]. In the present study no age-related differences between IgG1/IgG2 ratios of H. pylori antibodies was observed, but the age range of our patients was relatively narrow (7–18 years). To verify this hypothesis it would be worthwhile to analyse H. pylori subclass response in a large ethnically homogeneous population of adults and children free from diseases known to influence immune response.
The present study has shown significantly higher levels of H. pylori IgG3 antibodies in children with PUD comparing to NUD group. This observation differs from previous studies, which reported either higher IgG2 subclass response in adults with duodenal ulcers compared to NUD patients, or lack of an association between H. pylori subclass response and disease manifestations [31,32,34]. However, Valnes et al. who analysed the distribution of gastric IgG-producing immunocytes in various types of gastritis, found a significantly higher proportion of IgG3 plasma cells in duodenal ulcer patients subjected previously to Billroth II resection than in patients with simple gastritis [44]. Although the latter study did not examine the presence of H. pylori infection, its involvement in chronic gastritis and peptic ulcer disease is now well documented. Valnes et al. [44] suggested that because IgG3 has a strong capacity for complement activation and binding to Fcγ receptors on mononuclear cells, it can be involved in gastroduodenal pathology.
In the current study the titres of H. pylori IgG1 and IgG3 antibodies correlated moderately with chronicity of antral inflammation, and IgG3 titres correlated moderately with the density of antral H. pylori colonization. In adults an association between H. pylori IgG3 levels and gastric inflammation was also observed by Mitchell et al., who noted a positive relationship between IgG3 levels and grade of chronic inflammation in the fundus and active inflammation in the transitional zone [34]. A similar phenomenon has also been found in other bacterial infections. In patients with cystic fibrosis increased levels of IgG3 to Pseudomonas aeruginosa were associated with enhanced inflammation and poorer pulmonary status [45]. Additionally, in patients with leprosy progression of the disease correlated significantly with a selective increase of IgG1 and IgG3 antibodies, and levels of these antibodies correlated with bacterial load within lesions [28].
This study has also demonstrated that in children infected with H. pylori there is a positive association between CagA antibody titres and active and chronic inflammatory response in the antral mucosa. Two methods were used to determine CagA IgG seropositivity, Western blotting and ELISA. The methods showed 100% correlation demonstrating that both are equally reliable for assessing serum IgG CagA antibody status in this paediatric population. The specific IgG subclass response to CagA has not been examined previously. In this study it was found that CagA elicits predominantly an IgG1 and IgG3 antibody response and virtually no IgG4 antibodies. This is in agreement with previous observations indicating that protein antigens stimulate mainly IgG1 and IgG3 synthesis [46]. No association was observed between the distribution and levels of CagA IgG subclass response and disease manifestation or antral inflammation. Because there are geographical allelic variations in cagA[47], it would be interesting to investigate the relation between CagA IgG subclass response and gastric pathology in different populations.
In summary, this study has demonstrated a positive association between H. pylori IgG3 titres and peptic ulcer disease, chronicity of antral inflammation and density of H. pylori colonization in children. CagA elicits predominantly an IgG1 and IgG3 antibody response and there is no relationship between CagA subclass response and gastroduodenal pathology in paediatric patients.
Acknowledgments
Financial support was received from the British Council and State Committee for Scientific Research, Polish–British Research Partnership Programme, Yorkshire Cancer Research and the European Commission (contract ICA4-CT-1999-10010).
REFERENCES
- 1.Walker MM, Crabtree JE. Helicobacter pylori infection and the pathogenesis of duodenal ulceration. Ann NY Acad Sci. 1998;859:96–111. doi: 10.1111/j.1749-6632.1998.tb11114.x. [DOI] [PubMed] [Google Scholar]
- 2.Censini S, Lange N, Xiang Z, et al. Cag, a pathogenicity island of Helicobacter pylori encodes Type I-specific and disease associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–53. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akopyants NS, Clifton SW, Kersulyte D, et al. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–54. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 4.Atherton JC, Cao P, Peek RMJ, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–7. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 5.Gerhard M, Lehn N, Neumayer N, et al. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778–83. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peek RM, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 7.Crabtree JE, Taylor JD, Wyatt JI, Heatley RV, Shallcross TM, Tompkins DS, Rathbone BJ. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration and gastric pathology. Lancet. 1991;338:332–5. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- 8.Beales ILP, Crabtree JE, Scunes D, Covacci A, Calam J. Antibodies to CagA are associated with gastric atrophy in Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 1996;8:645–9. [PubMed] [Google Scholar]
- 9.Webb PM, Crabtree JE, Forman D. Gastric cancer, cytotoxin associated gene A positive Helicobacter pylori and serum pepsinogens: an international study. Gastroenterology. 1999;116:269–76. doi: 10.1016/s0016-5085(99)70122-8. [DOI] [PubMed] [Google Scholar]
- 10.Blaser MJ, Perez-Perez GI, Kleanthous H, et al. Infection with Helicobacter pylori strains possessing cagA is associated with increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–5. [PubMed] [Google Scholar]
- 11.Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimoyama T, Fukada S, Tanaka M, Mikami T, Munakata A, Crabtree JE. CagA seropositivity associated with development of gastric cancer in a Japanese population. J Clin Pathol. 1998;51:225–8. doi: 10.1136/jcp.51.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Figueiredo C, van Doorn LJ, Nogueira C, et al. Helicobacter pylori genotypes are associated with clinical outcome in Portuguese patients and show a high prevalence of infections with multiple strains. Scand J Gastroenterol. 2001;36:128–35. doi: 10.1080/003655201750065861. [DOI] [PubMed] [Google Scholar]
- 14.Shimoyama T, Everett SM, Dixon MF, Axon ATR, Crabtree JE. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J Clin Pathol. 1998;51:765–70. doi: 10.1136/jcp.51.10.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamaoka Y, Kita M, Kodama T, et al. Chemokines in the gastric mucosa in Helicobacter pylori infection. Gut. 1998;42:609–17. doi: 10.1136/gut.42.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dzierzanowska-Fangrat K, Crabtree JE, Rozynek E, et al. Helicobacter pylori genotype and density of colonization in relation to gastric inflammation in children. Eur J Gastroenterol Hepatol. 2002;14:1303–7. doi: 10.1097/00042737-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Alam K, Schubert TT, Bologna SD, Ma CK. Increased density of Helicobacter pylori on antral biopsy is associated with severity of acute and chronic inflammation and likelihood of duodenal ulceration. Am J Gastroenterol. 1992;87:424–8. [PubMed] [Google Scholar]
- 18.Atherton JC, Than KT, Peek RM, Cover TL, Blaser MJ. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J Infect Dis. 1996;174:552–6. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- 19.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 20.Figueiredo C, Machado JC, Pharoah P, et al. Heliocbacter pylori and interleukin-1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst. 2002;94:1680–7. doi: 10.1093/jnci/94.22.1680. [DOI] [PubMed] [Google Scholar]
- 21.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymophisms. Gastroenterol. 2003;124:1193–201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 22.Hida N, Shimoyama T, Neville P, et al. Increased expression of interleukin 10 and IL-12 (p40) mRNA in Helicobacter pylori infected gastric mucosa: relationship to bacterial cag status and peptic ulceration. J Clin Pathol. 1999;52:658–64. doi: 10.1136/jcp.52.9.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tomita T, Jackson A, Hida N, et al. Expression of interleukin-18, a TH1 cytokine, in human gastric mucosa is increased in Helicobacter pylori infection. J Infect Dis. 2001;183:620–7. doi: 10.1086/318541. [DOI] [PubMed] [Google Scholar]
- 24.Crabtree JE. Cytokine responses in Helicobacter pylori infection. In: Achtman M, Suerbaum S, editors. Helicobacter pylori: molecular and cellular biology. Wymondham, UK: Horizon Press; 2002. pp. 63–83. [Google Scholar]
- 25.Crabtree JE. The role of cytokines in Helicobacter pylori induced mucosal damage. Dig Dis Sci. 1998;43:46S–55S. [PubMed] [Google Scholar]
- 26.D’Elios MM, Amedei A, Del Prete G. Helicobacter pylori antigen-specific T cell responses at gastric level in chronic gastritis, peptic ulcer, gastric cancer and low-grade mucosa-associated lymphoid tissue (MALT) lymphoma. Microbes Infect. 2003;5:723–30. doi: 10.1016/s1286-4579(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 27.Roth KA, Kapadia SB, Martin SM, Lorenz RG. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–7. [PubMed] [Google Scholar]
- 28.Hussain R, Kifayet A, Chiang TJ. Immunoglobulin G1 (IgG1) and IgG3 antibodies are markers of progressive disease in leprosy. Infect Immun. 1995;63:410–5. doi: 10.1128/iai.63.2.410-415.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashbee HR, Muir SR, Cunliffe WJ, Ingham E. IgG subclasses specific to Staphylococcus epidermidis and Propionibacterium acnes in patients with acne vulgaris. Br J Dermatol. 1997;136:730–3. [PubMed] [Google Scholar]
- 30.Lagace J, Peloquin L, Kermani P, Montie T. IgG subclass responses to Pseudomonas aeruginosa a- and b-type flagellins in patients with cystic fibrosis. J Med Microbiol. 1995;43:270–6. doi: 10.1099/00222615-43-4-270. [DOI] [PubMed] [Google Scholar]
- 31.Andersen LP, Gaarslev K. IgG subclass antibodies against Helicobacter pylori heat-stabile antigens in normal persons and dyspeptic patients. APMIS. 1992;100:747–51. [PubMed] [Google Scholar]
- 32.Bontkes HJ, Veenendaal RA, Pena AS, et al. IgG subclass responses to Helicobacter pylori in patients with chronic active gastritis and duodenal ulcer. Scand J Gastroenterol. 1992;27:129–33. doi: 10.3109/00365529209165432. [DOI] [PubMed] [Google Scholar]
- 33.Itoh T, Wakatsuki Y, Yoshida M, et al. The vast majority of gastric T cells are polarized to produce T helper 1 type cytokines upon antigenic stimulation despite the absence of Helicobacter pylori infection. J Gastroenterol. 1999;34:560–70. doi: 10.1007/s005350050373. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell HM, Mascord K, Hazell SL, Daskalopoulos G. Association between the IgG subclass response, inflammation and disease status in Helicobacter pylori infection. Scand J Gastroenterol. 2001;36:149–55. doi: 10.1080/003655201750065898. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell HM, Ally R, Wadee A, Wiseman M, Segal I. Major differences in the IgG subclass response to Helicobacter pylori in the first and third worlds. Scand J Gastroenterol. 2002;37:517–22. doi: 10.1080/00365520252903044. [DOI] [PubMed] [Google Scholar]
- 36.Crabtree JE, Mahony MJ, Taylor JD, Heatley RV, Littlewood JM, Tompkins DS. Immune responses to Helicobacter pylori in children with recurrent abdominal pain. J Clin Pathol. 1991;44:768–71. doi: 10.1136/jcp.44.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crabtree JE, Shallcross TM, Wyatt JI, et al. Mucosal immune responses to Helicobacter pylori in patients with duodenitis. Dig Dis Sci. 1991;36:1266–73. doi: 10.1007/BF01307520. [DOI] [PubMed] [Google Scholar]
- 38.Xiang Z, Bugnoli M, Ponzetto A, et al. Detection in an enzyme immunoassay of an immune response to a recombinant fragment of the 128 kilodalton protein (CagA) of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 1993;12:739–45. doi: 10.1007/BF02098460. [DOI] [PubMed] [Google Scholar]
- 39.Plonka W, Dzbenski TH. The occurrence of intestinal parasites among children attending first classes of the elementary schools in Poland in the school year 1997/1998. Przeg Epid. 1999;53:331–8. [PubMed] [Google Scholar]
- 40.Mawas F, Feavers IM, Corbel MJ. Serotype of Streptococcus pneumoniae capsular polysaccharide can modify the Th1/Th2 cytokine profile and IgG subclass response to pneumococcal-CRM (197) conjugate vaccines in a murine model. Vaccine. 2000;19:1159–66. doi: 10.1016/s0264-410x(00)00314-5. [DOI] [PubMed] [Google Scholar]
- 41.Ally R, Mitchell H, Segal I. CagA+ve H. pylori aplenty in South Africa: the first systematic study of H. pylori infection in asymptomatic children in Soweto. Gut. 1999;45:A97. [Google Scholar]
- 42.D’Elios MM, Manghetti M, Almerigogna F, et al. Different cytokine profile and antigen-specificity repertoire in Helicobacter pylori-specific T cell clones from the antrum of chronic gastritis patients with or without peptic ulcer disease. Eur J Immunol. 1997;27:1751–5. doi: 10.1002/eji.1830270723. [DOI] [PubMed] [Google Scholar]
- 43.Lottenbach KR, Mink CM, Barenkamp SJ, Anderson EL, Homan SM, Powers DC. Age-associated differences in immunoglobulin G1 (IgG1) and IgG2 subclass antibodies to pneumococcal polysaccharides following vaccination. Infect Immun. 1999;67:4935–8. doi: 10.1128/iai.67.9.4935-4938.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valnes K, Brandzaeg P. Subclass distribution of mucosal IgG-producing cells in gastritis. Gut. 1989;30:322–6. doi: 10.1136/gut.30.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Likavcanova E, Lagace J. Quantitative analysis of immunoglobulin G subclass response to Pseudomonas aeruginosa antigens in cystic fibrosis. Microbiology. 1992;36:437–44. doi: 10.1099/00222615-36-6-437. [DOI] [PubMed] [Google Scholar]
- 46.Yount WJ, Dorner NN, Kunkel HG, Kabat EA. Studies on human antibodies. VI. Selective variations in subgroup composition and genetic markers. J Exp Med. 1968;27:633–46. doi: 10.1084/jem.127.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miehlke S, Kibler K, Kim JG, et al. Allelic variation in the cagA gene of Helicobacter pylori obtained from Korea compared to the United States. Am J Gastroenterol. 1996;91:1332–5. [PubMed] [Google Scholar]