Abstract
Recombination activating gene (RAG) re-expression and secondary Ig gene rearrangement in mature B lymphocytes have been reported. Here, we have studied RAG expression of peripheral blood B lymphocytes in humans. Normal B cells did not express RAG1 and RAG2 spontaneously. More than a half of circulating B cells expressed RAG proteins, when activated with Staphylococcus aureus Cowan I (SAC) + IL-2. DNA binding activity of the RAG complex has been verified by a gel shift assay employing the recombination signal sequence (RSS). Secondary Ig light chain rearrangement in the RAG-expressing B cells was confirmed by linker-mediated (LM)-PCR. Highly purified surface κ+ B cells activated by SAC + IL-2 became RAG+, and thereafter they started to express λ chain mRNA. 2 colour immunofluorescence analysis disclosed that a part of the RAG+ cells derived from the purified κ+ B cells activated by SAC + IL-2 turned to λ+ phenotype in vitro. Similarly, apoptosis induction was observed in a part of the RAG+ B cells. Our study suggests that a majority of peripheral blood B cells re-expresses RAG and the RAG+ B lymphocytes could be eliminated from the B cell repertoire either by changing Ag receptor specificity due to secondary rearrangement or by apoptosis induction. Thus, RAG expression of mature B cells in peripheral blood would contribute to not only receptor revision for further diversification of B cell repertoire but in some cases (or in some B cell subsets) to prevention or induction of autoAb responses at this differentiation stage in humans.
Keywords: RAG, B lymphocytes, autoimmunity, gene rearrangement, recombination
Introduction
Random rearrangement of Ig variable gene segments brings about sufficient diversity of the immune repertoire [1–3]. However, it simultaneously allows development of autoreactive antibodies (Abs). The rearrangement of particular Ig gene segments is tightly regulated with respect to cell lineage and developmental stage. Ig gene rearrangement is dependent on the activation of two key lymphoid-specific proteins, RAG1 and RAG2 [2, 4, 5]. Receptor editing is a mechanism revising their surface Ig receptors by secondary gene rearrangement to create nonautoreactive Ig [6–9]. Receptor editing has been believed to occur in the bone marrow at a transitional stage of B cell differentiation from pre-B to immature B cell [10–15]. Within the receptor editing theory, it has been postulated that emergence of autoreactive receptors in B cells results in expression of high levels of RAG enzymes that will mediate secondary rearrangements on the accessible L chain V genes, revealed by the experiments using murine autoAb gene transgenic models [10–15].
Recently, it has been shown that RAG genes were re-expressed in mature mouse B cells activated in vitro and in germinal centres (GCs) of immunized mouse lymph nodes and spleen [16–18]. Moreover, the RAG proteins induced were reported to be functional as VDJ recombinases, because secondary rearrangements of Ig heavy as well as light chain genes have been observed [19–23]. Thus, it was suggested that the RAG proteins re-expressed in mature mouse B cells mediate secondary rearrangement of Ig genes to further diversify Ig repertoire (receptor revision) at the mature B cell level [24,25]. In addition, re-expression of RAG enzymes in human mature B lymphocytes has been reported [26–29].
A recent study suggested that mouse B1 cells that were predisposed to autoAb production aberrantly expressed RAG proteins, suggesting potential involvement of aberrantly expressed RAGs in the autoAb production [30]. Moreover, it has been shown that receptor revision was actually involved in the pathogenic autoAb production in patients with RA [31–33]. Thus, it is evident that secondary Ig rearrangements of mature B cells contribute to autoAb production in some occasions. However, a majority of RAG+ B cells in the periphery has been suggested to have immature phenotypes by using GFP-reporter mice [34,35]. Thus, it remains uncertain whether RAG proteins re-expressed on mature B lymphocytes contribute to the elimination of autoreactive B cells in humans.
In the present study, we studied RAG protein expression in B lymphocytes purified from normal human peripheral blood, and its role in the light chain replacement and in the induction of apoptosis. We found that RAG proteins were induced upon activation of human circulating B lymphocytes, resulted in either changing their Ag specificity by secondary rearrangement or death via apoptosis.
Materials and Methods
Reagents
Staphylococcus aureus Cowan I (SAC) was purchased from Calbiochem-Behring (La Jolla, CA, USA). IL-2 was kindly provided by Ajinomoto Co. (Kawasaki, Japan). Anti-RAG1 and anti-RAG2 mAbs were purchased from Pharmingen (San Diego, CA, USA). FITC-anti-κ Ab was obtained from Coulter Co. (Hialeah, FL). Anti-λ mAb was purchased from Zymed Laboratories (South San Francisco, CA, USA). FITC-anti-CD19, PE-anti-CD20, FITC-anti-CD40 mAbs were purchased from BeckmanCoulter (Tokyo, Japan) and PE-anti-CD10 mAb was from DAKO (Glostrup, Denmark). As control Abs, FITC and PE labelled isotype matched control mouse Abs were purchased from BeckmanCoulter.
Preparation of purified B cells
We obtained blood from 29 healthy volunteer donors. Their mean (± SD) age was 36·2 ± 6·3 years (range 25–52 years). PBMC was separated into T cells and non T cells by neuraminidase treated SRBC rosetting technique, and plastic dish adherent cells were removed [36]. Non-T, non adherent cells were treated with anti-CD3 (Ortho Diagnostic Systems, Inc., Raritan, NJ, USA), anti-CD14 and CD56 mAbs (Becton Dickinson Monoclonal Centre) and magnetic beads to obtain purified B cells [37]. The resultant B cells were more than 99% positive for CD19, CD20 and CD40. Our B cell preparation did not contain CD10+ immature B cells (less than 0·1%).
In some experiments, human κ+ B cells were negatively purified by using rabbit anti-human λ Ab (Medical & Biological Laboratories, Nagoya, Japan), microbeads conjugated anti-rabbit Ig and MACS apparatus [38,39]. The resultant population contained more than 99%κ+and less than 1%λ+ B cells by flow cytometry (see Fig. 4). In other experiments, human κ+ cells were positively purified by using goat anti-human κ chain Ab (Tago, Burlingame, CA, USA), anti-goat Ig Ab and MACS apparatus.
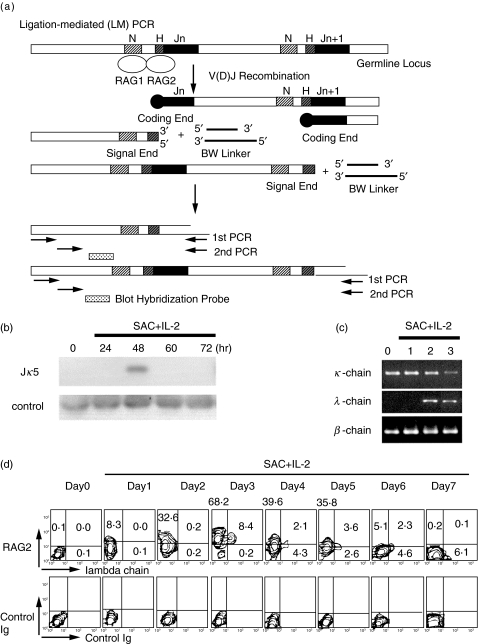
Fig. 4.
Secondary gene rearrangement of normal peripheral blood B cells. a. Schematic representation of LM-PCR assay for broken-ended recombination signal sequence DNA. b. Secondary gene rearrangement of normal peripheral blood B cells stimulated with SAC + IL-2. B cells were stimulated with SAC + IL-2 for indicated periods and LM-PCR was performed to detect Jκ5 gene rearrangement. Before RAG protein expression in nuclei (day 0 and 1), there was no LM-PCR product emerged in normal B cells. At day 2, LM-PCR product was successfully amplified from normal B cells, suggesting secondary rearrangement utilizing Jκ5. Control PCR was conducted using primers specific for human Jκ5 coding sequence. c. Light chain mRNA expression of purified κ+ B cells stimulated with SAC + IL-2. The κ+ B cells were purified from normal peripheral blood and stimulated with SAC + IL-2. Thereafter, κ and λ chain mRNA expressions were studied by RT-PCR. λ chain mRNA was expressed in highly purified κ+ B cells when stimulated with SAC + IL-2 for 2–3 days. d. Intracytoplasmic RAG expression and cell surface expression of Ig λ chain in normal κ+ B cells stimulated with SAC + IL-2. Cytoplasmic RAG2 expression and λ chain expression of the B cells were studied by two colour immunofluorescence analysis. RAG2 protein was expressed in B cells, but λ+ B cells were not detected at day 2. Note that all of the λ+ B cells appeared at day 3 expressed RAG2 simultaneously.
Medium and cell cultures
Medium used in this study was RPMI 1640 containing penicillin (100 µg/ml) and streptomycin (100 U/ml) (Life Technologies, Inc., Gaithersburg, MD, USA) and 10% FCS (Life Technologies).
2 × 105 purified B cells were stimulated in vitro with SAC (0·005% v/v) and/or IL-2 (5 ng/ml) for various periods at 37°C in 5% CO2/95% air. Multiple replicate cultures were set up in a final volume of 1 ml in 48-well flat-bottomed plates (Costar, Cambridge, MA, USA).
RNA extraction, reverse transcription and PCR reaction
RAG1 and RAG2 mRNA expression was studied by PCR based technique [37]. In brief, poly A tailed RNA was purified by mRNA extraction kit (Invitrogen, Carlsbad, CA, USA). A half of the precipitated RNA was reverse transcribed (the cDNA) as described [40], and the remaining half was kept as the mRNA. PCR amplification of both the cDNA and the mRNA was performed in parallel to detect any contamination of genomic DNA. When PCR products were amplified from the mRNA preparations, we discarded the corresponding cDNA samples, because of genomic DNA contamination. All the PCR results shown were thus free from genomic DNA contamination. β-actin primers were used to compare and monitor efficient cDNA synthesis between different samples. For β-actin and RAG1 reactions, temperature cycling was as follows: step 1, 94°C 1 min; step 2, 57°C 1 min; and step 3, 72°C 2 min. For RAG2 reaction, temperature cycling was as follows: step 1, 94°C 1 min; step 2, 61°C 1 min; and step 3, 72°C 2 min. Step 1 through 3 was repeated 40 times followed by 72°C for 10 min. The DNA fragments were separated on 1·5% agarose gels and visualized by staining with ethidium bromide. We have confirmed the internal DNA sequences of the amplified products by the TA cloning method and subsequent DNA sequencing.
Nuclear extracts and DNA-protein binding assay
Nuclear extracts were prepared as previously described [41,42]. A gel shift assay was performed using digoxigenin (DIG) gel shift kit (Boehringer Mannheim Biochemica, Mannheim, Germany) [41]. In brief, DIG-labelled DNA fragments were incubated at room temperature for 15 min with 5 µg of nuclear proteins. Protein-DNA complexes were separated from free probe on a polyacrylamide gel. Thereafter, the protein-DNA complexes in the gels were electrically transferred to nylon membrane, and detected by chemiluminescence. Cold inhibition study and immunodepletion study were conducted to confirm the specificity of the binding [41].
Immunoblotting analysis
The proteins were electrophoresed, and were transferred onto polyvinylidene difluoride membranes. Immunoblotting was performed using the Abs described above. Blots were probed with appropriate biotin-conjugated secondary Ab, followed by streptavidin-peroxidase and detection by chemiluminescence [41].
Annexin V staining and measurement of caspase-3 activity
Annexin V was used to determine the percentage of cells undergoing apoptosis [43]. Annexin V-FITC was obtained from Pharmingen (San Diego, CA, USA) and the staining was performed as follows; the B cells were resuspended in annexin V binding buffer (10 mm HEPES/NaOH, pH 7·4, 140 mm NaCl, 2·5 mm CaCl2) containing annexin V-FITC for 15 min in the dark. Thereafter, the cells were analysed by flow cytometry. In two colour staining experiments, annexin V-FITC was applied first (see below), and then intracytoplasmic protein was stained.
The measurement of caspase-3-activated cells was performed with a PhiPhiLux G1D2 and G2D2 kits (MBL, Nagoya, Japan) according to the manufacturer's protocol. Briefly, cells were cultured and then, substrate was added and cells were incubated at 37°C for 1 h. The percentage of caspase-3-active cells was then measured by a flow cytometer.
Immunofluorescence staining of intracytoplasmic proteins
Immunofluorescence staining of intracytoplasmic proteins was carried out by the method of Sander with minor modification [44]. In brief, the cells were fixed by using 4% paraformaldehyde and permeabilized by 0·1% saponin (Sigma Chemical, St. Louis, MO, USA) in PBS with 0·01 m HEPES buffer solution. Thereafter, the cells were stained with FITC-labelled goat anti-human λ Ab and mouse anti-human RAG2 mAb, followed by biotin conjugated anti-mouse IgG and streptavidin-PE. The stained cells were analysed by a flow cytometer. Appropriate control Abs were included to define background immunofluorescence of the cells in the present study.
LM-PCR assay for broken-ended DNA
LM-PCR assay was performed as previously reported [45,46]. In brief, DNA was extracted from the B cells, and ligated to the BW linker at 20 pm using T4 DNA ligase. Ligated DNA (100 ng) was used in the first round of PCR containing linker primer BW-1H and Jκ5-1. Samples were amplified for 15 cycles of 40 s at 94°C, 40 s at 68°C, and 1 min at 72°C, followed by a final 10-min extension step at 72°C. The reaction product (1 µl) was then used for an additional 40 cycles of PCR in an identical buffer containing a second, nested locus-specific primer Jκ5-2 and BW-1H. Control PCR assays used the Jκ5-R primer specific for the human Jκ5 coding exon combined with Jκ5-2 to amplify a germline 260-bp internal Igκ locus fragment from the same linker-ligated DNA samples. PCR products were analysed on 1·5% agarose gels and hybridized with Jκ5 internal probes [Table 1].
Table 1.
Primers and probes used in this study
| β-actin (548 bp) | sense | GTGGGGCGCCCCAGGCAC | antisense | CTCCTTAATGTCACGCACGAT |
| RAG1 (316 bp) | sense | ATGCACTCCATGTGACA | antisense | GCATGATGATCGCCATA |
| RAG2 (417 bp) | sense | TCTTGGCATACCAGGAGACA | antisense | ATGAGTGTGCGTTCTGCCAG |
| κ constant primer | sense | TGATGAGCAGTTGAAATCTG | antisense | AGGCCCTGATGGGTGACTTC |
| λ constant primer | sense | CTCCTCTGAGGAGCTTCAAG | antisense | CCACTGTCTTCTCCACGGTG |
| Jκ5-1 primer | CAAACGTAAGTGCACTTTCCTAATGC | |||
| Jκ5-2 primer | GTTTGAGATATTAGCTCAGGTCAATTC | |||
| Jκ5-R primer | GTTTAATCTCCAGTCGTGTCCCTTG | |||
| Jκ5-internal probe | GAACAGCCAAGCGCTAGCCAGTTAAGTGAGGCATCTCAATTGCAAG | |||
| BW-1 primer | GCGGTGACCCGGGAGATCTGAATTC | |||
| BW-2 primer | CCGGGAGATCTGAATTCCAC | |||
| BW-1H primer | GAATTCAGATC | |||
| RSS probe | TCACAGTGCTCCAGGGCTGAACAAAAACCGTCGA | |||
Results
Induction of RAG mRNA and protein expression in normal B lymphocytes
Recent studies suggested the expression of RAGs and secondary Ig gene rearrangement in mature B cells in mice and in humans [19–25]. They used mouse splenocytes and human tonsillar B cells for the studies. These provocative reports prompted us to study RAG expression of human peripheral blood B cells.
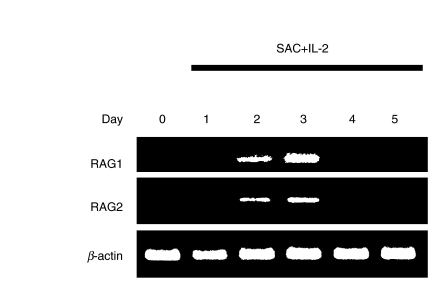
The B cell preparation was more than 99% positive for CD19, CD20 and CD40, and did not include CD10+ B cells (less than 0·1%). Thus, our B cell preparation did not contain immature B cells. Highly purified circulating B cells from normal donors were stimulated with SAC + IL-2. RT-PCR analysis demonstrated that normal B cells did not express RAG1 and RAG2 mRNA spontaneously, and that both RAG1 and RAG2 mRNA expressions were induced in normal B cells after stimulation with SAC + IL-2 [Fig. 1]. A time course analysis of RAG mRNA expression of normal B cells showed that the observed up-regulation in RAG expression returned to be undetectable levels after stimulation for several days [Fig. 1].
Fig. 1.
RAG1 and RAG2 mRNA expression in normal B cells. Purified B cells from normal donors were stimulated with SAC + IL-2 for indicated periods and expression of RAG1 and RAG2 mRNA was analysed by RT-PCR. The result shown is a representative of 10 independent experiments.
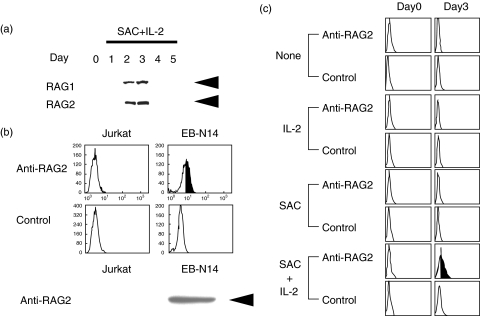
Immunoblotting analysis demonstrated that normal circulating B cells did not express RAG2 proteins spontaneously, and that RAG2 protein expression was induced in normal B cells after stimulation with SAC + IL-2 (Fig. 2a) [47]. We confirmed that RAG1 protein expression was paralleled with RAG2 expression (Fig. 2a). It was evident that unstimulated B cells did not express RAG proteins but they have appeared in the B cells after activation.
Fig. 2.
RAG protein expression in normal B cells. a. Normal circulating B cells were stimulated with SAC + IL-2, and RAG protein expression was analysed by immunoblotting. The B cells expressed RAG1 and RAG2 when stimulated with SAC + IL-2 for 2–3 days. Equal amounts of the cellular proteins were analysed. Arrows indicate RAG1 (105 kD) and RAG2 (56 kD). The result shown is a representative of 8 independent experiments. b. Intracytoplasmic RAG2 expression of authentic RAG+ (EB-N14; an EB virus transformed cell line) and RAG- (Jurkat T cell line) cells was analysed with flow cytometry and immunoblotting analysis. An arrowhead indicates RAG2 (56 kD). c. Normal B cells were stimulated with either IL-2, SAC or a combination of SAC + IL-2 and intracytoplasmic RAG2 expression was studied by a flow cytometer. The B cells expressed RAG2 protein only when stimulated with SAC + IL-2. The result shown is a representative of 4 independent experiments.
We next analysed RAG2 expression with flow cytometry. Jurkat T cells did not express RAG2 protein, whereas EB-N14, an EB virus transformed B cell line, expressed it (Fig. 2b, upper panels). The results obtained by flow cytometry were confirmed by immunoblotting analysis (Fig. 2b, lower panel). Thus, we have been able to detect intracellular RAG2 expression by flow cytometry. With this method, we found that normal circulating B cells have to be stimulated with the combination of SAC + IL-2 to induce RAG protein expression (Fig. 2c). Neither SAC stimulation nor IL-2 stimulation induced RAG proteins in the B cells (Fig. 2c).
DNA binding activity of RAG proteins expressed in normal circulating B lymphocytes
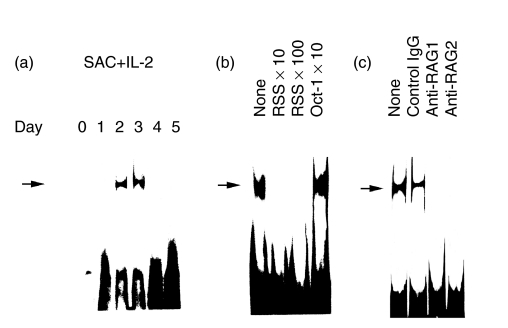
We next studied whether RAG proteins expressed in normal circulating B cells really had DNA binding activity to the relevant gene sequence. As shown in Fig. 3a, nuclear proteins purified from B cells stimulated with SAC + IL-2 for 2–3 days had RSS binding activity. We confirmed the specificity of the binding by the cold inhibition study (Fig. 3b) and immunodepletion study employing anti-RAG1 and anti-RAG2 mAbs (Fig. 3c). These results presented evidence that normal circulating B cells expressed RAG protein complex, which had a RSS binding activity.
Fig. 3.
Recombination signal sequences (RSS) binding activity of RAG proteins in normal human peripheral blood B cells. a. Induction of RSS binding activity in the nuclear extracts from normal B cells stimulated with SAC + IL-2 was analysed by a gel shift assay. b. Specificity of the binding was confirmed by the cold inhibition study employing unlabelled double stranded RSS oligonucleotides (10 fold and 100 fold) and irrelevant Oct-1 oligonucleotides (10 fold). c. Binding activity to the RSS of the RAG complex was confirmed by immunodepletion study. Anti-RAG1 (1 µg/reaction) and anti-RAG2 (1 µg/reaction) mAbs were employed. As a control, mouse IgG (1 µg/reaction) was used.The results shown were representatives of at least 4 independent experiments.
Secondary gene rearrangement of normal peripheral blood B lymphocytes by RAGs
We studied whether the RAGs expressed in the normal peripheral blood B cells actually induced secondary gene rearrangement. We thus conducted LM-PCR to detect Jκ5 gene rearrangement, as reported by Meffre et al. ([45,46]; see Fig. 4a). We found that before RAG protein expression in nuclei of normal B cells (days 0, and 1; Fig. 2a), there was no LM-PCR product emerged, suggesting that Ig light chain gene rearrangement utilizing Jκ5 gene did not occur (Fig. 4b). At day 2, LM-PCR product was successfully amplified, suggesting that secondary gene rearrangement utilizing downstream Jκ5 gene has actually occurred in the activated B cells (Fig. 4b). Thus, it was suggested that the RAG proteins expressed in normal circulating B cells induced secondary rearrangement within Igκ light chain gene locus.
Emergence of λ+ B cells in the highly purified κ+ B cells stimulated with SAC + IL-2
To further confirm the secondary Ig light chain gene rearrangement in normal B cells, we have conducted RT-PCR. We took advantage of the fact that λ chain genes usually rearrange after κ chain genes [22]. The highly purified B cells were further separated into surface κ+ B cells (negatively selected) and surface λ+ B cells. The κ+ B cell fraction contained more than 99% of cells expressing surface κ and less than 1% cells expressing λ chain (Fig. 4d, day 0). The κ+ B cells were stimulated with SAC + IL-2 for several days and their mRNA extracted. The κ+ B cells did not express λ chain mRNA at day 0 and day 1 (Fig. 4c). The λ chain mRNA was expressed in the surface κ+ B cells stimulated with SAC + IL-2 for 2–3 days. Thus, it was suggested that the RAG proteins expressed in normal circulating B cells induced secondary rearrangement of Ig light chain gene.
It is therefore important to clarify whether RAGs expressed in circulating B cells upon activation really mediate secondary rearrangement of Ig light chain gene and subsequent cell surface expression of Ig light chain protein on the B cells. To directly assess involvement of RAG protein expression in the emergence of surface λ chain on the purified surface κ+ B cells stimulated with SAC + IL-2, we have performed two colour staining: cytoplasmic RAG2 expression and surface λ chain expression of the B cells were analysed with flow cytometry (Fig. 4d). The expression of RAG2 protein was gradually increased and RAG2 protein was expressed in a majority of the B cells at day 3. At day 3, λ+ B cells were appeared only on RAG2+ B cells. It should be emphasized that all of the λ+ B cells emerging in the culture at day 3 expressed RAG2 simultaneously.
Outgrowth of contaminating λ+ B cells in the purified κ+ B cell population may be responsible for the emergence of λ+ B cells in the experiments. However, [1] λ+ B cells were not present at day 0 (freshly isolated) and at days 1 and 2 (activated) of the purified κ+ B cell preparation, [2]λ+ B cells did not emerge from RAG negative B cells at day 3. These results suggested that outgrowth of contaminating λ+ B cells in the purified κ+ B cell population did not occur. In addition, we have cultured the κ+ B cells and the λ+ B cells separately with SAC + IL-2 to assess their proliferative potential. The κ+ B cells and the λ+ B cells proliferated almost equally or the κ+ B cells proliferated slightly better than the λ+ B cells in response to SAC + IL-2 (data not shown). Collectively, we found that the proliferation of the λ+ B cells never exceeded that of the κ+ B cells.
When the purified B cells were stimulated with SAC + IL-2, more than a half of the B cells express RAG2 protein (55·0 + 20·3%, mean + SD of 14 normal individuals; range 21·4–92·0%). As control experiments, we also purified surface λ+ B cells, and stimulated with SAC + IL-2. We found that surface κ+ cells were never emerged from SAC + IL-2 stimulated purified surface λ+ B cells (data not shown). These findings indicated that RAG proteins expressed in normal circulating B cells were functional and promoted secondary gene rearrangement and subsequent λ chain protein expression. In addition, secondary gene rearrangement may be a common event for circulating B cells, because more than a half of normal peripheral blood B cells undergoes secondary gene rearrangement.
RAG expression in surface κ+ B cells stimulated by SAC + IL-2 accompanied apoptosis induction
It is theoretically possible that a part of the B cells that undergo secondary gene rearrangement results in successful (in-frame: productive) rearrangement, whereas remaining of the B cells that have resulted in the nonproductive (out of frame) rearrangement continue the rearrangement process until successful rearrangement. However, some of the B cells eventually die via apoptosis, because of unsuccessful (nonproductive) rearrangement throughout.
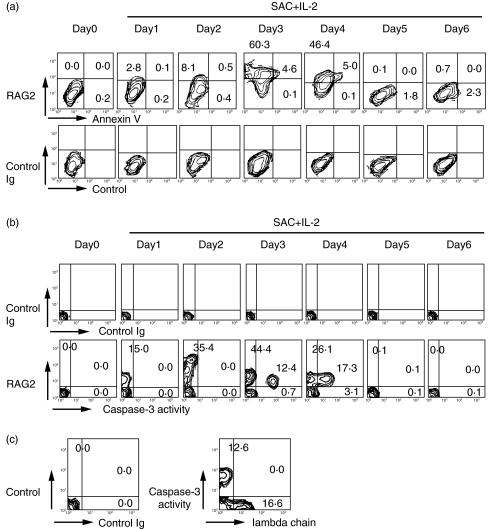
We have assessed cytoplasmic RAG2 expression and annexin V binding of the activated B cells, to test whether a part of normal activated B cells that expressed RAG eventually die via apoptosis. Annexin V binding represents unequilibrium of membrane potential, which is intimately associated with the early phase of apoptotic cell death [48,49]. As shown in Fig. 5a, RAG2 protein expression was evident at day 2–3. At days 3 and 4, annexin V+ apoptotic B cells emerged only in RAG2+ B cells. These findings were confirmed by the appearance of caspase-3-activated B cells in the RAG2+ B cells (Fig. 5b). It should be emphasized that all of the apoptotic cells simultaneously expressed RAG2. The result strongly suggested that a part of B cells expressing RAG2 died via apoptosis. Thus, the same stimulus induced both secondary gene rearrangement and apoptosis of B cells. To evaluate the relationship between apoptosis and the secondary Ig gene rearrangement followed by λ chain expression, we performed flow cytometric analysis. As shown in Fig. 5c, after 3 days of stimulation with SAC + IL-2, double staining for cell surface λ chain and caspase-3 activity demonstrated that caspase-3-activated apoptotic cells emerged only in λ chain negative B cells. These results suggest that some of the B cells eventually died via apoptosis because of unsuccessful light chain rearrangement.
Fig. 5.
Apoptosis induction during secondary gene rearrangement of normal peripheral blood B cells. a. Intracytoplasmic RAG expression and apoptosis induction of normal κ+ B cells stimulated with SAC + IL-2. Cytoplasmic RAG2 expression and annexin V binding of the B cells were studied by two colour immunofluorescence analysis. RAG2 protein expression was evident at day 3. At days 3 and 4, annexin V+ apoptotic B cells have emerged only in RAG2+ B cells. All the annexin V+ B cells expressed RAG2 simultaneously. The results shown were representatives of 6 independent experiments. b. Intracytoplasmic RAG expression and caspase-3 activity of normal κ+ B cells stimulated with SAC + IL-2. Cytoplasmic RAG2 expression and caspase-3 activity of the B cells were studied by two colour immunofluorescence analysis. At days 3 and 4, caspase-3-activated apoptotic B cells have emerged only in RAG2+ B cells. The results shown were representatives of 3 independent experiments. c. Cell surface λ chain expression and caspase-3 activity of the B cells were studied by two colour immunofluorescence analysis. At day 3, normal κ+ B cells stimulated with SAC + IL-2 were examined and caspase-3-activated cells have emerged only in λ-negative B cells.
Discussion
In this study we found that activation of normal human peripheral blood B cells provoked RAG re-expression and the RAGs were functional for secondary gene rearrangement in a majority of circulating B cells, and a minority of the B cells subsequently died via apoptosis [20]. In addition, re-expression of RAGs in human mature B cells was induced by the stimulation of SAC + IL-2 as well as the stimulation with anti-CD40 Ab and IL-4 (data not shown), suggesting that suboptimal stimulation without surface Ig receptor stimulus is enough to induce RAGs and secondary Ig gene rearrangement (receptor revision).
Re-expression of RAGs in human mature B cells has been reported, and is suggested to contribute further diversification of immune repertoire [19–25]. In some instances, secondary rearrangement induces development of pathogenic autoantibodies in certain diseases [31–33]. We have previously characterized a germline Ig Vκ gene encoding cationic anti-DNA Ab light chain in patients with systemic lupus erythematosus (SLE) [37] and found that normal B cells edited A30-Jκ2 gene, which was intimately associated with anti-DNA Ab, in their genome. Put simply, if secondary gene rearrangement would occur by inversion mechanism, productively rearranged germline A30-Jκ2 gene might be present far distant from Cκ gene with reverse orientation in the normal B cell genome. Using conventional and long distance PCR, we found that A30-Jκ2 was successfully amplified, however, A30-Cκ was not in normal B cells, suggesting that rearrangement was nonproductive, or if the DNA sequence was productive, receptor editing was occurred. On the other hand, the A30-Jκ2 and A30-Cκ fragments were amplified in genome of SLE B cells, suggesting that SLE B cells do not edit and express A30-derived mRNA and Ab. To this end, we first amplified A30-Jκ2 fragment by PCR using genomic DNA of purified normal B cell as templates and the products were further used as templates for second PCR using g87 primer and Jκ2 primer. Sequencing analysis demonstrated that those from normal B cells were productive, suggesting that A30-Jκ2 gene that is once productively rearranged may be receptor edited in normal B cells [37].
In addition, our findings suggest that receptor revision may occur at the mature stage of B cell differentiation after Ag mediated selection of the B cells with high affinity Ig receptor in normal humans. Thus, it is possible that both receptor editing and receptor revision occur in B cells at immature/mature transitional stage in bone marrow and circulating B cells in the peripheral blood.
We thus propose that RAG re-expression occurs at mature B cell stage as well to revise their surface Ig or to induce apoptosis. In accordance with our proposal, receptor editing at the mature B cell level has been suggested. Dorneret al. [50] have shown that receptor editing of Vκ in SLE occurred in the periphery after somatic hypermutation had been initiated. Brard et al. [51] have described that secondary rearrangement during clonal expansion was not surprising in view of RAG expression in germinal centres. Thus, autoAb producing B cells may have once appeared in periphery by failure of receptor editing at the immature/mature transitional stage. The autoAb expressing B cells would be stimulated by DNA or a crossreactive Ag via Ag specific manner, resulting in clonal expansion of the B cells and further acquisition of somatic mutations of their Ig V gene. The autoreactive B cells that have escaped the receptor editing at the transitional stage, would become a subject of receptor revision at the mature B cell stage (after Ag stimulation and acquisition of somatic mutations) in normal humans. We thus need to study receptor editing of human mature B cells with defined Ag specificity, such as anti-DNA Ab.
Recent studies with regard to RAG re-expression in mature B cells have suggested a role of RAG re-expression in receptor revision, that is associated with further diversification of the Ab repertories rather than receptor editing [26–28, 52, 53]. It is possible that RAGs expressed on mature B cells may act both to further diverse B cell repertoire and to prevent self-reactivity by receptor revision. Rather, distinction of receptor diversification (revision) and editing may not be important; secondary gene rearrangement may be a random process of light chain gene. In some instances, the secondary rearranged Ig gene may have lower affinity than the original Ig gene for the autoAg, then the Ig may become nonautoreactive (editing), and in other instances, the secondarily selected gene may have higher affinity for the exogenous nominal Ag (revision). In some artificial as well as pathological cases, secondary gene rearrangement leads to development of autoimmunity [31–33,51]. We found that CD5+ B cells expressed RAG preferentially (data not shown), as have been reported in mice [30]. It is thus possible that autoAb secretion by CD5+ B cells is linked to excessive RAG expression and extensive receptor revision, as reported by others [30]. It is also possible that autoAb production by CD5− B cells is free from enhanced RAG expression. Thus, deficient RAG expression in CD5-B cells with autoreactivity would contribute to the autoAb secretion. In any events, further studies addressing this issue are needed.
It has been reported that RAG+ B cells were localized within GCs and were present as apoptotic tingible body+ macrophages [18]. We suggest that some of the B cells that have expressed RAG would die via apoptosis and thus, some of the RAG+ B cells would become apoptotic tingible body+ macrophages in humans as well. Our study emphasizes that RAG expression is important for both apoptosis induction and secondary gene rearrangement (receptor revision) of human circulating B cells. We demonstrated that apoptotic cells emerged after RAG expression; however, they did not express cell surface λ chain, suggesting that the induction of apoptosis of mature B cells dues to unsuccessful light chain rearrangement during receptor revision, as observed in the first Ig gene rearrangement in immature B cells. We think that the B cells generating antiself antibody would be dangerous if they are circulating. Thus, appropriate expression of RAG in circulating B cells may be important for the prevention of autoimmunity. To this end, it is important to clarify whether artificial expression of RAGs in the autoreactive B cells would prevent autoAb production and subsequent autoimmune diseases.
Acknowledgments
The authors express our sincere appreciation to Professor Tsuyoshi Sakane, who passed away last year, for his continuous encouragement and support to this work.
This work was supported, in part, by 2001–02 grant for the promotion of the advancement of education and research in graduate schools from the promotion and mutual aid corporation for private schools of Japan, 2001–02 grant in aid for scientific research from the Ministry of Education, Culture, Sports, and Technology of Japan, 2001–02 special coordination funds of the Ministry of Education, Culture, Sports, and Technology of Japan, 2001 Comprehensive research on ageing and health of the health science research grants of the Ministry of Health, Labor, and Welfare of Japan, grant from SRF Foundation, Tokyo and grant from Regional Science Promotion Program in Kanagawa, Japan.
References
- 1.Litman GW, Anderson MK, Rast JP. Evolution of antigen binding receptors. Annu Rev Immunol. 1999;17:109–47. doi: 10.1146/annurev.immunol.17.1.109. [DOI] [PubMed] [Google Scholar]
- 2.Cedar H, Bergman Y. Developmental regulation of immune system gene rearrangement. Curr Opin Immunol. 1999;11:64–9. doi: 10.1016/s0952-7915(99)80012-0. [DOI] [PubMed] [Google Scholar]
- 3.Thompson CB. New insights into V(D)J recombination and its role in the evolution of the immune system. Immunity. 1995;3:531–9. doi: 10.1016/1074-7613(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 4.Melek M, Gellert M, Van Gent DC. Rejoining of DNA by the RAG1 and RAG2 proteins. Science. 1998;280:301–3. doi: 10.1126/science.280.5361.301. [DOI] [PubMed] [Google Scholar]
- 5.Girschick HJ, Grammer AC, Nanki T, et al. RAG1 and RAG2 expression by B cell subsets from human tonsil and peripheral blood. J Immunol. 2001;166:377–86. doi: 10.4049/jimmunol.166.1.377. [DOI] [PubMed] [Google Scholar]
- 6.Gay D, Saunders T, Camper S, et al. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radic MZ, Zouali M. Receptor editing, immune diversification, and self-tolerance. Immunity. 1996;5:505–11. doi: 10.1016/s1074-7613(00)80266-6. [DOI] [PubMed] [Google Scholar]
- 8.Hertz M, Nemazee D. Receptor editing and commitment in B lymphocytes. Curr Opin Immunol. 1998;10:208–13. doi: 10.1016/s0952-7915(98)80250-1. [DOI] [PubMed] [Google Scholar]
- 9.Pelanda R, Schwers S, Sonoda E, et al. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–75. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 10.Lang J, Jackson M, Teyton L, et al. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp Med. 1996;184:1685–97. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+IgD- bone marrow B cells in vitro. Immunity. 1997;6:429–36. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 12.Melamed D, Nemazee D. Self-antigen does not accelerate immature B cell apoptosis, but stimulates receptor editing as a consequence of developmental arrest. Proc Natl Acad Sci USA. 1997;94:9267–72. doi: 10.1073/pnas.94.17.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pewzner-Jung Y, Friedmann D, Sonoda E, et al. B cell deletion, anergy, and receptor editing in ‘knock in’ mice targeted with a germline-encoded or somatically mutated anti-DNA heavy chain. J Immunol. 1998;161:4634–45. [PubMed] [Google Scholar]
- 14.Melamed D, Benschop RJ, Cambier JC, et al. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 1998;92:173–82. doi: 10.1016/s0092-8674(00)80912-5. [DOI] [PubMed] [Google Scholar]
- 15.Casellas R, Shih TA, Kleinewietfeld M, et al. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–4. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 16.Papavasiliou F, Casellas R, Suh H, et al. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997;278:298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- 17.Han S, Zheng B, Schatz DG, et al. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B cells. Science. 1996;274:2094–7. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 18.Hikida M, Mori M, Kawabata T, et al. Characterization of B cells expressing recombination activating genes in germinal centers of immunized mouse lymph nodes. J Immunol. 1997;158:2509–12. [PubMed] [Google Scholar]
- 19.Han S, Dillon SR, Zheng B, et al. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–5. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 20.Hikida M, Ohmori H. Rearrangement of lambda light chain genes in mature B cells in vitro and in vivo: function of reexpressed recombination-activating gene (RAG) products. J Exp Med. 1998;187:795–9. doi: 10.1084/jem.187.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hertz M, Kouskoff V, Nakamura T, et al. V(D)J recombinase induction in splenic B lymphocytes is inhibited by antigen-receptor signaling. Nature. 1998;394:292–5. doi: 10.1038/28419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Retter MW, Nemazee D. Receptor editing occurs frequently during normal B cell development. J Exp Med. 1998;188:1231–8. doi: 10.1084/jem.188.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nussenzweig MC. Immune receptor editing: revise and select. Cell. 1998;95:875–8. doi: 10.1016/s0092-8674(00)81711-0. [DOI] [PubMed] [Google Scholar]
- 24.Rast JP, Litman GW. Towards understanding the evolutionary origins and early diversification of rearranging antigen receptors. Immunol Rev. 1998;166:79–86. doi: 10.1111/j.1600-065x.1998.tb01254.x. [DOI] [PubMed] [Google Scholar]
- 25.Ohmori H, Hikida M. Expression and function of recombination activating genes in mature B cells. Crit Rev Immunol. 1998;18:221–35. doi: 10.1615/critrevimmunol.v18.i3.30. [DOI] [PubMed] [Google Scholar]
- 26.Wilson PC, Wilson K, Liu YJ, et al. JD Capra. Receptor revision of immunoglobulin heavy chain variable region genes in normal human B lymphocytes. J Exp Med. 2000;191:1881–94. doi: 10.1084/jem.191.11.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kouskoff V, Nemazee D. Role of receptor editing and revision in shaping the B and T lymphocyte repertoire. Life Sci. 2001;69:1105–13. doi: 10.1016/s0024-3205(01)01219-x. [DOI] [PubMed] [Google Scholar]
- 28.Kelsoe G. V(D)J hypermutation and receptor revision: coloring outside the lines. Curr Opin Immunol. 1999;11:70–5. doi: 10.1016/s0952-7915(99)80013-2. [DOI] [PubMed] [Google Scholar]
- 29.Giachino C, Padovan E, Lanzavecchia A. Re-expression of RAG-1 and RAG-2 genes and evidence for secondary rearrangements in human germinal center B lymphocytes. Eur J Immunol. 1998;28:3506–13. doi: 10.1002/(SICI)1521-4141(199811)28:11<3506::AID-IMMU3506>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 30.Qin XF, Schwers S, Yu W, et al. Secondary V(D)J recombination in B-1 cells. Nature. 1999;397:355–9. doi: 10.1038/16933. [DOI] [PubMed] [Google Scholar]
- 31.Itoh K, Meffre E, Albesiano E, et al. Immunoglobulin heavy chain variable region gene replacement as a mechanism for receptor revision in rheumatoid arthritis synovial tissue B lymphocytes. J Exp Med. 2000;192:1151–64. doi: 10.1084/jem.192.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Wu X, Limbaugh BH, et al. Expression of recombination-activating genes and terminal deoxynucleotidyl transferase and secondary rearrangement of immunoglobulin kappa light chains in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2001;44:2275–84. doi: 10.1002/1529-0131(200110)44:10<2275::aid-art390>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 33.Meffre E, Davis E, Schiff C, et al. Circulating human B cells that express surrogate light chains and edited receptors. Nat Immunol. 2000;1:207–13. doi: 10.1038/79739. [DOI] [PubMed] [Google Scholar]
- 34.Yu W, Nagaoka H, Jankovic M, et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–7. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 35.Monroe RJ, Seidl KJ, Gaertner F, et al. RAG2: GFP knockin mice reveal novel aspects of RAG2 expression in primary and peripheral lymphoid tissues. Immunity. 1999;11:201–12. doi: 10.1016/s1074-7613(00)80095-3. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki N, Sakane T, Engleman EG. Anti-DNA antibody production by CD5+ and CD5- B cells of patients with systemic lupus erythematosus. J Clin Invest. 1990;85:238–47. doi: 10.1172/JCI114418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suzuki N, Harada T, Mihara S, et al. Characterization of a germline Vk gene encoding cationic anti-DNA antibody and role of receptor editing for development of the autoantibody in patients with systemic lupus erythematosus. J Clin Invest. 1996;98:1843–50. doi: 10.1172/JCI118985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harada T, Suzuki N, Mizushima Y, et al. Usage of a novel class of germ-line Ig variable region gene for cationic anti-DNA autoantibodies in human lupus nephritis and its role for the development of the disease. J Immunol. 1994;153:4806–15. [PubMed] [Google Scholar]
- 39.Miltenyi S, Muller W, Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11:231–8. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- 40.Brenner CA, Tam AW, Nelson PA, et al. Message amplification phenotyping (MAPPing): a technique to simultaneously measure multiple mRNAs from small numbers of cells. Biotechniques. 1989;7:1096–103. [PubMed] [Google Scholar]
- 41.Suzuki N, Kaneko S, Ichino M, et al. In vivo mechanisms for the inhibition of T lymphocyte activation by long-term therapy with tacrolimus (FK-506): experience in patients with Behcet's disease. Arthritis Rheum. 1997;40:1157–67. doi: 10.1002/art.1780400622. [DOI] [PubMed] [Google Scholar]
- 42.Nagawa F, Ishiguro K, Tsuboi A, et al. Footprint analysis of the RAG protein recombination signal sequence complex for V(D)J type recombination. Mol Cell Biol. 1998;18:655–63. doi: 10.1128/mcb.18.1.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermes I, Haanen C, Steffens-Nakken H, et al. A novel assay for apoptosis. Flowcytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J Immunol Meth. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 44.Sander B, Andersson J. Assessment of cytokines by immunofluorescence and the paraformaldehyde-saponin procedure. Immunol Rev. 1991;119:65–93. doi: 10.1111/j.1600-065x.1991.tb00578.x. [DOI] [PubMed] [Google Scholar]
- 45.Meffre E, Papavasiliou F, Cohen P, et al. Antigen receptor engagement turns off the V(D)J recombination machinery in human tonsil B cells. J Exp Med. 1998;188:765–72. doi: 10.1084/jem.188.4.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlissel M, Constantinescu A, Morrow T, et al. Double-strand signal sequence breaks in V(D)J recombination are blunt, 5′-phosphorylated, RAG-dependent, and cell cycle regulated. Genes Dev. 1993;7:2520–32. doi: 10.1101/gad.7.12b.2520. [DOI] [PubMed] [Google Scholar]
- 47.Hikida M, Nakayama Y, Yamashita Y, et al. Expression of recombination activating genes in germinal center B cells: involvement of interleukin 7 (IL-7) and the IL-7 receptor. J Exp Med. 1998;188:365–72. doi: 10.1084/jem.188.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boersma AW, Nooter K, Oostrum RG, et al. Quantification of apoptotic cells with fluorescein isothiocyanate-labeled annexin V in chinese hamster ovary cell cultures treated with cisplatin. Cytometry. 1996;24:123–30. doi: 10.1002/(SICI)1097-0320(19960601)24:2<123::AID-CYTO4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki N, Mihara S, Sakane T. Development of pathogenic anti-DNA antibodies in patients with systemic lupus erythematosus. FASEB J. 1997;11:1033–8. doi: 10.1096/fasebj.11.12.9337156. [DOI] [PubMed] [Google Scholar]
- 50.Dorner T, Farner NL, Lipsky PE. Ig lambda and heavy chain gene usage in early untreated systemic lupus erythematosus suggests intensive B cell stimulation. J Immunol. 1999;163:1027–36. [PubMed] [Google Scholar]
- 51.Brard F, Shannon M, Prak EL, et al. Somatic mutation and light chain rearrangement generate autoimmunity in anti-single-stranded DNA transgenic MRL/lpr mice. J Exp Med. 1999;190:691–704. doi: 10.1084/jem.190.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Wildt RM, Hoet RMA, van Venrooij WJ, et al. Analysis of heavy and light chain pairings indicates that receptor editing shapes the human antibody repertoire. J Mol Biol. 1999;285:895–901. doi: 10.1006/jmbi.1998.2396. [DOI] [PubMed] [Google Scholar]
- 53.Dorner T, Lipsky PE. Immunoglobulin variable-region gene usage in systemic autoimmune diseases. Arthritis Rheum. 2001;44:2715–27. doi: 10.1002/1529-0131(200112)44:12<2715::aid-art458>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]