Abstract
We have reported previously that p95c, a novel 95-kDa cytosolic protein, was the target of autoantibodies in sera of patients with autoimmune hepatic diseases. We studied 30 sera that were shown previously to immunoprecipitate a 95 kDa protein from [35S]-methionine-labelled HeLa lysates and had a specific precipitin band in immunodiffusion. Thirteen sera were available to test the ability of p95c antibodies to inhibit nuclear envelope assembly in an in vitro assay in which confocal fluorescence microscopy was also used to identify the stages at which nuclear assembly was inhibited. The percentage inhibition of nuclear envelope assembly of the 13 sera ranged from 7% to 99% and nuclear envelope assembly and the swelling of nucleus was inhibited at several stages. The percentage inhibition of nuclear assembly was correlated with the titre of anti-p95c as determined by immunodiffusion. To confirm the identity of this autoantigen, we used a full-length cDNA of the p97/valosin-containing protein (VCP) to produce a radiolabelled recombinant protein that was then used in an immunoprecipitation (IP) assay. Our study demonstrated that 12 of the 13 (93%) human sera with antibodies to p95c immunoprecipitated recombinant p97/VCP. Because p95c and p97 have similar molecular masses and cell localization, and because the majority of sera bind recombinant p97/VCP and anti-p95c antibodies inhibit nuclear assembly, this is compelling evidence that p95c and p97/VCP are identical.
Keywords: autoantibody, conformational epitope, nuclear envelope assembly, p95c, p97/VCP, primary biliary cirrhosis
INTRODUCTION
Patients with autoimmune liver diseases such as primary biliary cirrhosis (PBC), autoimmune hepatitis (AIH), autoimmune cholangiopathy (AIC) and primary sclerosing cholangitis (PSC) produce autoantibodies that differ from those found in patients with systemic rheumatic diseases such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), systemic sclerosis (SSc) and Sjögren's syndrome (SjS) [1–3]. In particular, anti-mitochondrial antibodies (AMA) have been reported in 85–90% of patients with PBC and these are among the most prevalent autoantibodies found in any autoimmune disease [4–6]. Autoantibodies that bind to components of the nuclear envelope, such as anti-gp210 and anti-p62 complex, are also important markers for the diagnosis of PBC patients with and without AMA, and for monitoring the progression of disease [7–9]. Other studies have shown that anticentromere antibodies, especially anticentromere B antibody, anti-SP100 and antibodies to high mobility group (HMG) proteins 1 and 2 may also be useful for the diagnosis of PBC [10–14]. Anti-liver kidney microsome (LKM) antibody and peripheral antineutrophil cytoplasmic antibodies (p-ANCA) are valuable for the diagnosis of type 2 AIH [15,16] and PSC [17].
In 1998, we reported a novel antibody directed against a conformational epitope on a 95-kDa protein in patients with autoimmune hepatic diseases [18]. This antibody was found in 12% of PBC and 9·7% of AIH patients, but was not detected in other autoimmune conditions without hepatic involvement. Interestingly, unlike LKM and AMA and many other autoantigens, this antigen was not detected by immunoblot. Double immunodiffusion that used antigens extracted from rat liver homogenates showed a specific precipitin line that was different from other known immune precipitin systems [18]. Based on immunoprecipitation of extracts of metabolically labelled HeLa cells, the molecular mass of this autoantigen was estimated to be 95 kDa.
Recently, p97/VCP (valosin-containing protein) was characterized and found to play an important role in nuclear envelope assembly and the formation of the endoplasmic reticulum and Golgi apparatus during the final stage of mitosis [19,20]. Of interest, antibodies to p97/VCP inhibited nuclear reassembly in vitro[21]. Based on studies and paradigms of other autoantibodies that bind to and inhibit functional domains or active sites of the cognate antigens [2], we reasoned that if autoantibodies to p95 and p97/VCP were identical that they too would reduce its biological activity and inhibit nuclear assembly. In this study, we have sought to determine whether the cognate antigen of anti-p95c and p97/VCP are identical by investigating the ability of the autoantibody to inhibit nuclear envelope assembly and to immunoprecipitate recombinant p97/VCP.
MATERIALS AND METHODS
Patients and sera
Thirty sera with antibodies to p95c were identified by immunodiffusion in a serum bank established in the Health Sciences Research Institute. The diagnosis of the patients was established according to published clinical parameters and histological features of liver biopsies [22,23]. Sufficient amounts of sera from 13 patients were available for the inhibition of nuclear envelope assembly assay and to identify anti-p95c to anti-p97/VCP by immunoprecipitation (described below). A prototype serum (I) with antibodies to p95c and normal human serum were used as controls to study the steps of nuclear assembly inhibition during the cell cycle by confocal immunofluorescence microscopy.
Indirect immunofluorescence
Antinuclear antibody (ANA) and AMA were detected by indirect immunofluorescence, as described in detail elsewhere [24]. Briefly, HEp-2 slides and cryostat sectioned rat kidney and stomach (Fluoro AID 1 test, MBL Inc., Japan) were used for ANA and AMA, respectively. The sera were incubated on the substrates and after excess antibody was washed away and they were then incubated with polyvalent anti-human immunoglobulin conjugated to fluorescein isothiocyanate. The slides were read on a fluorescence microscope (Nippon Optico, Japan).
Double immunodiffusion
The Ouchterlony double immunodiffusion (ID) method was used to demonstrate the identity of precipitin reactions between soluble antigen and serum antibodies. The antigen source was prepared from rat liver mitochondrial, microsomal and supernatant fractions as described previously [18]. The mitochondrial fraction was sonicated (Tosho Electric Company, Japan) for 45 s at full power to release antigens and the protein concentration of the soluble antigens determined as described previously [18]. Sixty mg/ml of the microsomal fraction was then used as the antigen source for the detection of anti-LKM 1 and anti-p95c antibodies. Conventional antimitochondrial antibodies (i.e. antibodies to pyruvate dehydrogenase complex) and antibodies to nuclear antigens, such as anti-U1RNP, Sjögren's syndrome antigen A (SS-A)/Ro and anti-Sm, were not detected under these experimental conditions [25].
In vitro transcription/translation and immunoprecipitation
The cDNA representing the full-length valosin-containing protein (p97/VCP: Accession number CAA78412; a gift from Dr Graham Warren, Yale University, New Haven, CT, USA) was used as a template for in vitro transcription and translation (TnT, Promega, Madison, WI, USA) in the presence of [35S]-methionine as described previously [26,27]. TnT reactions were conducted at 30°C for 1·5–2 h and the presence of translation products was confirmed by subjecting 2–5 µl samples to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) and analysis by autoradiography. The in vitro translated products were then used as the antigen source. IP reactions were prepared by combining 100 µl 10% protein A-Sepharose beads (Sigma, catalogue no. P-3391), 10 µl human serum, 500 µl NET2 buffer (50 mm Tris-HCl, pH 7·4, 150 mm NaCl, 5 mm EDTA, 0·5% Nonidet P-40, 0·5% deoxycholic acid, 0·1% SDS, 0·02% sodium azide) and 5–10 µl of labelled recombinant protein obtained from the TnT reaction described above. After 1 h of incubation at 4–8°C, the Sepharose beads were washed five times in NET2, and the proteins eluted in 10 µl of sample buffer. The proteins were analysed by 10% or 12·5% SDS-PAGE as described previously [26].
Nuclear assembly assays
Demembranated sperm chromatin was prepared as described [28] and stored at −80°C at a concentration of 40 000 units/µl. Xenopus sp. eggs were collected, the jelly layer removed and then lysed to prepare an interphase extract [29]. The nuclear envelope assembly assays were then performed essentially as described by Smythe and Newport [30]. Briefly, the Xenopus egg extracts, cytosol and membrane fractions were supplemented with an ATP regenerating system (10 mm phosphocreatine, 2 mm ATP (pH 7·0), 5 µg/ml creatine kinase), and then mixed with demembranated sperm chromatin. The standard reaction mixture consisted of 10 000 units of chromatin and 10 µl crude extract or 10 µl cytosol + 1 µl membrane. After incubation at room temperature (23°C) for 1·5 h, a 2 µl aliquot of the reaction mixture was removed and diluted with 2 µl of Hoechst in dihexylocarbocyanine iodine (DHCC) buffer (15 mm PIPES-KOH, pH 7·4, 0·2 m sucrose, 7 mm MgCl2, 80 mm KCl, 15 mm NaCl, 5 mm EDTA) containing 20 mg/ml bis-benzimide DNA dye (Hoechst 33342; Calbiochem-Novabichem), a lipid dye, 3,3′-DHCC (Aldrich, Japan), and 3·7% formaldehyde on a glass slide. The sample was mounted with a cover-slip and examined under a 100x objective lens on a phase contrast and Axioplan fluorescence microscope (Carl Zeiss) fitted with exciter barrier reflector combinations appropriate for the fluorescent dyes described above.
For confocal microscopy, DNA was visualized by staining the preparations with propidium iodide and DHCC. Images were recorded with a Radiance 2000 confocal fluorescence system (Bio-Rad, Tokyo) mounted on a Nikon E600 fluorescence microscope (Nikon, Tokyo). The rate of inhibition of nuclear assembly was calculated by applying the formula: corrected inhibition rate of nuclear assembly (%) = (inhibition rate of nuclear assembly obtained from adding patient's serum (%) − inhibition rate of nuclear assembly obtained from adding normal healthy serum (%)/nuclear assembly obtained from adding normal healthy serum (%)
RESULTS
The diagnosis of the 30 patients that IP the p95c protein included 23 with PBC and seven with AIH (Table 1). Twenty-four of the 30 (80%) patients were female. Twelve patients presented with other concurrent diseases: eight with SjS, two with Hashimoto's thyroiditis, one with RA and one with dilated cardiomyopathy. Within the group of eight SjS patients, four had an overlap syndrome manifested as SLE/SjS, SSc/SjS, mixed connective tissue disease (MCTD)/SjS or RA/SjS. Among the 13 PBC patients that had a liver biopsy, two cases were classified as Scheuer stage 4, but the remaining cases were classified as either stage 1 or 2.
Table 1.
Clinical and serological features of 30 patients with antibodies to p95c
| No. | Sex | Liver biopsy | Primary diagnosis* | Secondary diagnosis | AMA titre** | ANA titre** | ANA specificity | Anti-p95c ID** | Anti-VCP IP | INA % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | + | PBC:S1-2 | SjS | 160 | 160 | U1RNP/SS-A | 128 | + + | 82 |
| 2 | F | n.d. | AIH | SjS | – | 80 | U1RNP/SS-A | 1 | + + + | 67 |
| 3 | F | n.d. | AIH | SLE/SjS | – | 640 | U1RNP/SS-A | 8 | + + | 29 |
| 4 | F | n.a. | PBC | – | 40 | – | – | 32 | + + + | 13 |
| 5 | F | n.a. | PBC | – | – | – | – | 1024 | + + + + | 88 |
| 6 | M | + | PBC:S1 | – | 40 | 80 | – | 256 | + + + + | 59 |
| 7 | M | n.d. | PBC | – | 40 | – | – | 64 | + + + + | 64 |
| 8 | F | n.d. | PBC | – | – | 40 | – | 16 | + + + | 13 |
| 9 | F | + | AIH | – | – | – | – | 16 | + | 7 |
| 10 | F | + | PBC:S2 | – | 40 | – | – | 64 | –/+ | 99 |
| 11 | F | + | PBC:S1 | Hashimoto | 320 | 80 | – | 4 | + | 15 |
| 12 | F | + | PBC:S1 | SjS | 80 | 20 | SS-A | 32 | + + | 23 |
| 13 | F | n.d. | PBC | – | 640 | – | – | 512 | + + + + | 83 |
| 14 | M | + | PBC:S1 | – | 80 | – | – | 16 | n.d. | n.d. |
| 15 | F | + | PBC:S1 | – | 160 | – | – | 256 | n.d. | n.d. |
| 16 | F | + | PBC:S2 | – | 160 | 320 | – | 2 | n.d. | n.d. |
| 17 | F | + | BC:S2 | – | 320 | – | – | 16 | n.d. | n.d. |
| 18 | F | + | PBC:S4 | – | 640 | 20 | – | 16 | n.d. | n.d. |
| 19 | M | + | PBC:S4 | – | 640 | – | – | 8 | n.d. | n.d. |
| 20 | F | n.d. | PBC | – | 160 | 80 | – | 32 | n.d. | n.d. |
| 21 | F | n.d. | PBC | – | 640 | – | – | 128 | n.d. | n.d. |
| 22 | F | n.d. | PBC | – | 320 | – | – | 32 | n.d. | n.d. |
| 23 | F | + | PBC:S1 | Hashimoto | 320 | 80 | – | 128 | n.d. | n.d. |
| 24 | F | + | PBC:S1 | SjS/SSc | 20 | 320 | SS-A/Topo1 | 32 | n.d. | n.d. |
| 25 | F | n.d. | PBC | SjS/MCTD | – | 20480 | U1RNP/SS-A | 16 | n.d. | n.d. |
| 26 | M | n.d. | PBC | DCM | 320 | – | – | 32 | n.d. | n.d. |
| 27 | F | + | AIH | – | – | 160 | – | 256 | n.d. | n.d. |
| 28 | F | + | AIH | SjS | 320 | 320 | U1RNP/SS-A | 8 | n.d. | n.d. |
| 29 | F | n.d. | AIH | RA | – | 160 | – | 64 | n.d. | n.d. |
| 30 | F | n.d. | AIH | SjS/RA | – | 160 | SS-A | 64 | n.d. | n.d. |
AMA, ANA and p95c titres are the reciprocal of number shown; + indicates the semiquantitative reaction with recombinant VCP as determined by the autoradiogram of immunoprecipitated [35S]-labelled protein. AIH, autoimmune hepatitis; AMA, antimitochondrial antibodies; ANA, antinuclear antibodies; DCM, dilated cardiomyopathy; ID, immunodiffusion; INA, inhibition of nuclear assembly; IP, immunoprecipitation: MCTD, mixed connective tissue disease; n.a., result not available; n.d., not done; PBC, primary biliary cirrhosis; RA, rheumatoid arthritis; SjS, Sjögren's syndrome; SLE, systemic lupus erythematosus; SS-A, Sjögren's syndrome antigen A; Topo1, topoisomerase 1; U1RNP, U1 ribonucleoprotein; VCP, valosin-containing protein.
Antimitochondrial antibodies (AMA) were detected in 21 (70%) of the sera with titres ranging from 1/20 to 1/640. A positive ANA was observed in 18 patients with titres that ranged from 1/40 to 1/20 480. Eight patients had antibodies to SS-A, 5 had anti-U1RNP and one with a PBC/SjS/SSc overlap syndrome had antitopoisomerase 1.
Inhibition of in vitro nuclear envelope assembly
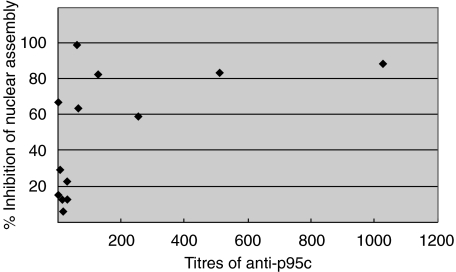
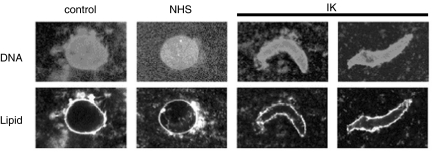
The 13 sera (PBC 10, AIH 3) with p95c antibodies demonstrated 7–99% inhibition of the envelope assembly in the in vitro assay (Table 1). When the data are plotted as percentage of inhibition rates (Fig. 1), the degree of inhibition was correlated with the titre of anti-p95c. In separate experiments, crude Xenopus egg extracts were pre-incubated with buffer alone, control normal serum and a prototype serum (IK). The degree and stage at which nuclear assembly was inhibited was examined by adding Xenopus sperm chromatin to the reaction. Pre-incubation with buffer or normal serum yielded continuous nuclear rim-staining with the phospholipid stain DHCC, indicating the presence of assembled chromatin (Fig. 2). On the other hand, pre-incubation with the prototype serum yielded discontinuous lipid-staining of the nuclear rims with some areas well covered by membrane while others were not (Fig. 2). When the surface of the chromatin was viewed under higher magnification, the nuclear envelope was not fully enclosed and the lipid-staining was reticulated.
Fig. 1.
Relationship between percentage inhibition of nuclear assembly and titres of anti-p95c antibodies in the sera of PBC patients. Sera with low titre (<1 : 128) anti-p95c antibodies exhibit less inhibition of nuclear assembly whereas sera with high titres (>1/128) show the most marked inhibition of nuclear assembly.
Fig. 2.
Confocal immunofluorescent microscopy of the nuclear reassembly assay. Nuclear envelope assembly was inhibited by the index serum (IK) but not by phosphate buffered saline or normal healthy serum. Nuclear assembly was judged to have occurred when the length of the long axis divided by the short axis was less than 2.
Immunoprecipitation of recombinant p97/VCP
The same 13 sera were then used in an IP assay that employed radiolabelled recombinant p97/VCP produced in the rabbit reticulocyte lysate system (Table 1). Twelve (93%) of the sera immunoprecipitated the ∼97 kDa recombinant protein, but normal human sera and PBC sera with antimitochondrial antibodies, but no antibodies to p95c, did not (Fig. 3). One serum (no. 10: Table 1, Fig. 3) showed an equivocal IP result.
Fig. 3.
Immunoprecipitation of p97/VCP recombinant protein with human anti-p95 sera. The p97/VCP protein was expressed as a [35S]-labelled in vitro transcription and translation (TnT) product and then immunoprecipitated with the human sera. Thirteen sera with anti-p95c antibodies (lanes 1–13) and the index anti-p95c sera (I) immunoprecipitated the ∼97 kDa recombinant protein whereas normal human serum (N) and a control serum from a patient with antimitochondrial antibodies (C) did not. The reactivity of the serum in lane 10 is weak compared to other sera but was equivocally positive on the original imaging film. Molecular weight markers are indicated on the left.
DISCUSSION
This study provides compelling evidence that the previously described p95c autoantigen and p97/VCP are the same proteins. This conclusion is based on a number of remarkable similarities between p95c and p97/VCP. First, the molecular masses and cellular localization in the cytosol are nearly identical. Secondly, all sera with anti-p95c antibodies have been shown to inhibit nuclear envelope assembly. Thirdly, all but one of the available 13 sera that had anti-p95 antibodies and inhibited nuclear assembly immunoprecipitated the recombinant p97/VCP protein.
We have shown previously that anti-p95c antibodies that were found in the sera of patients with PBC and AIH were demonstrated easily by immunodiffusion and immunoprecipitation but could not be detected by conventional immunoblotting techniques [18]. This is in contrast to most autoantibodies found in other autoimmune diseases that usually show reactivity to the cognate antigens by immunoblotting [3,31]. Based on these observations, we suggested that the epitope of the p95c autoantigen was conformational [18].
In the present study, all but one of the sera with anti-p95 antibodies bound to the recombinant p97/VCP protein in an IP assay. In this assay, certain conformational epitopes are probably present, although it is likely that post-translational modifications, which are features of the native protein, are not represented fully in the recombinant protein produced in the rabbit reticulocyte lysate system. The requirement for certain post-translational modifications may explain why the one serum did not immunoprecipitate the recombinant VCP. To gain further insight, studies are under way to map the linear and conformational epitopes on p97/VCP.
When we considered possible autoantigenic targets of the anti-p95c sera, two novel antigens in the cytosol with molecular masses each of 97 kDa came to our attention. The first was β-karyopherin (importin-β), which plays a key role in nuclear import [16]. The other was p97/VCP, which plays an important role in various membrane fusions such as Golgi and nuclear envelope assembly, and ubiquitin-dependent protein degradation [32,33]. This protein is a member of a family of AAA-ATPases, some of which (i.e. F0-ATPase) are located in the inner mito-chondrial membrane [17] and others (i.e. F1-ATPase) localized to the matrix space [34,35]. The p97/VCP complex and N-ethylmaleidemide sensitive fusion protein (NSF) share certain similarities in that they both form ring-shaped homo-hexamers (in contrast to hetero-hexamers of F1-ATPase) and are involved in biogenesis and functional activities of the Golgi and nuclear membranes [33,36]. The function of p97 can be inhibited by α-SNAP, a component of the NSF pathway, and the function of NSF can be inhibited by p47, also a component of the p97 pathway [32]. The mechanism of nuclear inhibition is thought to involve competition between α-SNAP and p47 to bind syntaxin 5, a common component of the functional p97 and NSF pathways [32].
In our study the inhibition of nuclear assembly was generally correlated with the titre of anti-p95c antibody as determined by ID, but there was a less clear-cut correlation between the nuclear inhibition assay and the semiquantitative assessment of the TnT IP results (Table 1). Therefore, it has yet to be shown conclusively that anti-p95c antibodies are indeed the factors that inhibit the nuclear assembly. A number of observations support our conclusion. First, like our observations, Hetzer and his colleagues demonstrated that anti-p97/VCP antibody inhibits nuclear envelope assembly in a Xenopus egg extract in the in vitro system [21]. Secondly, we have shown previously that AMA and anti-gp210 antibodies, which are found in sera from patients with PBC [5], and anti-U1RNP/Sm, anti-SS-A/Ro, anti-SS-B/La, which are found in sera from patients with SLE or SjS [2,3], did not inhibit nuclear assembly in the in vitro assay (data not shown). However, it remains to be determined if autoimmune sera with antibodies directed against nuclear envelope proteins such as lamin B receptor (LBR), LAP2 (lamina associated polypeptide 2), lamin A/C and lamin B might inhibit nuclear assembly. According to a recent report, some human sera might contain antibodies to other antigens, such as glyceraldehydes-3-phosphate, that also participate in nuclear assembly [28].
Hetzer et al. has reported that several steps are involved in the process of nuclear assembly and, specifically, p97–p47 is required for nuclear envelope expansion in the final steps of nuclear envelope fusion [21]. In our study, confocal immunofluorescence microscopy showed that inhibition of nuclear assembly occurred at more than one stage because some areas of chromatin were well covered by the nuclear envelope while others were not. Thus, anti-p95c antibodies may be directed against domains of p97 that are critical in different steps of nuclear envelope assembly. The antigenic domain(s) bound by anti-p95c is not known; studies are under way to define the primary conformational and linear epitopes. The assumption that human anti-p95c antibodies bind to the active site of p95/VCP and inhibit its function is based on studies of other human autoantibodies that have been shown to bind functional sites of proteins and inhibit their biological activities [37,38].
In summary, we provide evidence that the previously described p95c autoantigen and p97/VCP are identical proteins. To maintain understanding between disciplines we propose that anti-p95c antibodies be referred to as anti-p97/VCP in the future. Anti-p97/VCP was found in approximately 12·5% of patients with PBC and in 9·7% with AIH [18]. In contrast to the prevalence of AMA [5], the prevalence of anti-p95c antibody in PBC is relatively low. Therefore, the diagnostic importance of anti-p95c antibody occurs frequently in AMA-negative PBC and in ANA-negative AIH. In our study, many of the PBC or AIH patients with this antibody had overlap conditions, particularly SjS. Prospective studies of SjS patients would be important to determine if anti-p97/VCP antibodies antedate the appearance of autoimmune liver disease.
Acknowledgments
We thank Dr Gonda at Tomioka Clinic and Dr Suzuki at Defensive Medical College for supplying us with clinical information. We also thank Dr Graham Warren at Yale University for generously providing the p97/VCP cDNA, and Dr Ogura at the Division of Molecular Cell Biology of Kumamoto University and Dr Paul Enarson at the University of Calgary for assistance with preparing this manuscript. The technical assistance of Meifeng Zhang with the VCP TnT studies is greatly appreciated. This work was supported by the Canadian Institutes of Health Research (Grant MOP-57674) and supported partially by a grant for project research from Niigata University.
References
- 1.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 KD mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138:3525–31. [PubMed] [Google Scholar]
- 2.Tan EM. Antinuclear antibodies: diagnostic markers for autoimmune diseases and probes for cell biology. Adv Immunol. 1989;44:93–151. doi: 10.1016/s0065-2776(08)60641-0. [DOI] [PubMed] [Google Scholar]
- 3.von Muhlen CA, Tan EM. Autoantibodies in the diagnosis of systemic rheumatic disease. Semin Arthritis Rheum. 1995;24:323–58. doi: 10.1016/s0049-0172(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 4.Mackay IR. Autoimmunity and primary biliary cirrhosis. Bailliéres Clin Gastroenterol. 2000;14:519–33. doi: 10.1053/bega.2000.0101. [DOI] [PubMed] [Google Scholar]
- 5.Fritzler MJ, Manns MP. Anti-mitochondrial antibodies. Clin Appl Immunol Rev. 2002;3:87–113. [Google Scholar]
- 6.Strassburg CP, Obermayer-Straub P, Manns MP. Autoimmunity in liver diseases. Clin Rev Allergy Immunol. 2000;18:127–39. doi: 10.1385/CRIAI:18:2:127. [DOI] [PubMed] [Google Scholar]
- 7.Courvalin J-C, Worman HJ. Nuclear envelope protein autoantibodies in primary biliary cirrhosis. Semin Liver Dis. 1997;17:79–90. doi: 10.1055/s-2007-1007185. [DOI] [PubMed] [Google Scholar]
- 8.Miyachi K, Shibata M, Onozuka Y, Kikuchi F, Imai N, Horigome T. Primary biliary cirrhosis sera recognize not only gp210 but also proteins of the p62 complex bearing N-acetyl glucosamine residues from rat liver nuclear envelope. Anti-p62 complex antibody in PBC. Mol Biol Rep. 1996;23:227–34. doi: 10.1007/BF00351173. [DOI] [PubMed] [Google Scholar]
- 9.Miyachi K, Hankins RW, Matsushima H, et al. Profile and clinical significance of anti-nuclear envelope antibodies found in patients with primary biliary cirrhosis: a multicenter study. J Autoimmun. 2003;20:247–54. doi: 10.1016/s0896-8411(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 10.Makinen D, Fritzler MJ, Davis P, Sherlock S. Anticentromere antibodies in primary biliary cirrhosis. Arthritis Rheum. 1983;26:914–7. doi: 10.1002/art.1780260714. [DOI] [PubMed] [Google Scholar]
- 11.McHugh NJ, James IE, Fairburn K, Maddison PJ. Autoantibodies to mitochondrial and centromere antigens in primary biliary cirrhosis and systemic sclerosis. Clin Exp Immunol. 1990;81:244–9. doi: 10.1111/j.1365-2249.1990.tb03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szostecki C, Guldner HH, Will H. Autoantibodies against ‘nuclear dots’ in primary biliary cirrhosis. Semin Liver Dis. 1997;17:71–8. doi: 10.1055/s-2007-1007184. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita H, Omagari K, Whittingham S, et al. Autoimmune cholangitis and primary biliary cirrhosis − an autoimmune enigma. Liver. 1999;19:122–8. doi: 10.1111/j.1478-3231.1999.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 14.Fida S, Myers MA, Whittingham S, Rowley MJ, Ozaki S, Mackay IR. Autoantibodies to the transcriptional factor SOX13 in primary biliary cirrhosis compared with other diseases. J Autoimmun. 2002;19:251–7. doi: 10.1006/jaut.2002.0622. [DOI] [PubMed] [Google Scholar]
- 15.Manns MP, Johnson EF, Griffin KJ, Tan EM, Sullivan KF. Major anitgen of liver kidney microsomal autoantibodies in idiopathic autoimmune hepatitis if cytochrome P450db1. J Clin Invest. 1989;83:1066–72. doi: 10.1172/JCI113949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyakawa H, Kako M, Nagai K, et al. HCV-RNA in type 2 autoimmune hepatitis. Am J Gastroenterol. 1991;86:1688–9. [PubMed] [Google Scholar]
- 17.Mulder AH, Horst G, Haagsma EB, Limburg PC, Kleibeuker JH, Kallenberg CG. Prevalence and characterization of neutrophil cytoplasmic antibodies in autoimmune liver diseases. Hepatology. 1993;17:411–7. [PubMed] [Google Scholar]
- 18.Miyachi K, Matsushima H, Hankins RW, et al. A novel antibody directed against a three-dimensional configuration of a 95-kDa protein in patients with autoimmune hepatic diseases. Scand J Immunol. 1998;47:63–8. doi: 10.1046/j.1365-3083.1998.00262.x. [DOI] [PubMed] [Google Scholar]
- 19.Koller KJ, Brownstein MJ. Use of a cDNA clone to identify a supposed precursor protein containing valosin. Nature. 1987;325:542–5. doi: 10.1038/325542a0. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Shaw A, Bates PA, et al. Structure of the AAA ATPase p97. Mol Cell. 2000;6:1473–84. doi: 10.1016/s1097-2765(00)00143-x. [DOI] [PubMed] [Google Scholar]
- 21.Hetzer M, Meyer HH, Walther TC, Bilbao-Cortes D, Warren G, Mattaj IW. Distinct AAA-ATPase p97 complexes function in discrete steps of nuclear assembly. Nat Cell Biol. 2001;3:1086–91. doi: 10.1038/ncb1201-1086. [DOI] [PubMed] [Google Scholar]
- 22.Scheuer PJ. Primary biliay cirrhosis. Proc R Soc Med. 1967;60:1257–60. doi: 10.1177/003591576706001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheuer PJ. Ludwig Symposium on biliary disorders − part II. Pathologic features and evolution of primary biliary cirrhosis and primary sclerosing cholangitis. Mayo Clin Proc. 1998;73:179–83. doi: 10.4065/73.2.179. [DOI] [PubMed] [Google Scholar]
- 24.Smolen JS, Butcher B, Fritzler MJ, et al. Reference sera for antinuclear antibodies. II. Further definition of antibody specificities in international antinuclear antibody reference sera by immunofluorescence and immunoblotting. Arthritis Rheum. 1997;40:413–8. doi: 10.1002/art.1780400304. [DOI] [PubMed] [Google Scholar]
- 25.Miyachi K, Gupta RC, Dickson ER, Tan EM. Precipitating antibodies to mitochondrial antigens in patients with primary biliary cirrhosis. Clin Exp Immunol. 1980;39:599–606. [PMC free article] [PubMed] [Google Scholar]
- 26.Fritzler MJ, Lung C-C, Hamel JC, Griffith K, Chan EKL. Molecular characterization of golgin-245: a novel Golgi complex protein containing a granin signature. J Biol Chem. 1995;270:31262–8. doi: 10.1074/jbc.270.52.31262. [DOI] [PubMed] [Google Scholar]
- 27.Griffith KJ, Chan EKL, Hamel JC, Miyachi K, Fritzler MJ. Molecular characterization of a novel 97 kDa Golgi complex autoantigen recognized by autoimmune antibodies from patients with Sjögren's syndrome. Arthritis Rheum. 1997;40:1693–702. doi: 10.1002/art.1780400920. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa T, Hirano Y, Inomata A, et al. Participation of a fusogenic protein, glyceraldehyde-3-phosphate dehydrogenase, in nuclear membrane assembly. J Biol Chem. 2003;278:20395–404. doi: 10.1074/jbc.M210824200. [DOI] [PubMed] [Google Scholar]
- 29.Sasagawa S, Yamamoto A, Ichimura T, Omata S, Horigome T. In vitro nuclear assembly with affinity-purified nuclear envelope precursor vesicle fractions, PV1 and PV2. Eur J Cell Biol. 1999;78:593–600. doi: 10.1016/S0171-9335(99)80025-9. [DOI] [PubMed] [Google Scholar]
- 30.Smythe C, Newport JW. Systems for the study of nuclear assembly, DNA replication, and nuclear breakdown in Xenopus laevis egg extracts. Meth Cell Biol. 1991;35:449–68. doi: 10.1016/s0091-679x(08)60583-x. [DOI] [PubMed] [Google Scholar]
- 31.Chan EKL, Pollard KM. Detection of antibodies to ribonucleoprotein particles by immunoblotting. In: Rose NR, de Macario EC, Folds JD, Lane HC, Nakamura RM, editors. Manual of clinical laboratory immunology. Washington: American Society for Microbiology; 1997. pp. 928–34. [Google Scholar]
- 32.Kondo H, Rabouille C, Newman R, et al. p47 is a cofactor for p97-mediated membrane fusion. Nature. 1997;388:75–8. doi: 10.1038/40411. [DOI] [PubMed] [Google Scholar]
- 33.Rabouille C, Kondo H, Newman R, Hui N, Freemont P, Warren G. Syntaxin 5 is a common component of the NSF- and p97-mediated reassembly pathways of Golgi cisternae from mitotic Golgi fragments in vitro. Cell. 1998;92:603–10. doi: 10.1016/s0092-8674(00)81128-9. [DOI] [PubMed] [Google Scholar]
- 34.Stock D, Gibbons C, Arechaga I, Leslie AG, Walker JE. The rotary mechanism of ATP synthase. Curr Opin Struct Biol. 2000;10:672–9. doi: 10.1016/s0959-440x(00)00147-0. [DOI] [PubMed] [Google Scholar]
- 35.Yasuda R, Noji H, Kinosita K, Jr, Yoshida M. F1-ATPase is a highly efficient molecular motor that rotates with discrete 120 degree steps. Cell. 1998;93:1117–24. doi: 10.1016/s0092-8674(00)81456-7. [DOI] [PubMed] [Google Scholar]
- 36.Mashima J, Nagahama M, Hatsuzawa K, et al. N-ethylmaleimide-sensitive factor is associated with the nuclear envelope. Biochem Biophys Res Commun. 2000;274:559–64. doi: 10.1006/bbrc.2000.3162. [DOI] [PubMed] [Google Scholar]
- 37.Dang CV, Tan EM, Traugh JA. Myositis autoantibody reactivity and catalytic function of threonyl-tRNA synthetase. FASEB J. 1988;2:2376–9. doi: 10.1096/fasebj.2.8.2452112. [DOI] [PubMed] [Google Scholar]
- 38.Tan EM. Autoantibodies in pathology and cell biology. Cell. 1991;67:841–2. doi: 10.1016/0092-8674(91)90356-4. [DOI] [PubMed] [Google Scholar]