Abstract
The humoral immune response to endothelium has a pivotal role in the development of atherosclerosis. Using a molecular method, we sought to identify endothelial autoantigens in carotid atherosclerosis. Immunoscreening of a HUAEC expression library with IgG from a pool of two sera from patients with carotid atherosclerosis identified a clone specific to actin. We evaluated actin-specific IgG reactivity in patients with carotid atherosclerosis and compared responses with those in patients with systemic lupus erythematosus and type 1 diabetes mellitus and in healthy subjects. Enzyme-linked immunoassay detected actin-specific IgG in a significantly higher percentage of sera from patients with atherosclerosis and systemic lupus erythematosus than from healthy subjects (16/61, 26% and 13/33, 39%versus 2/41, 5%, P = 0·012 and P < 10−4, by χ2 test). Mean optical density values were significantly higher in patients than in healthy subjects (P < 10−4 by Student's t-test). Patients with atherosclerosis and uncomplicated plaques had significantly higher serum anti-actin IgG reactivity than those with complicated plaques (P = 0·048 by Student's t-test). Our findings suggest that actin is an autoantigenic molecule of potential clinical interest in carotid atherosclerosis.
Keywords: carotid atherosclerosis, endothelium, actin, autoantigen, systemic lupus erythematosus
INTRODUCTION
Atherosclerosis is a complex and indolent histopathological process. Its development depends not on a single risk factor but on an interplay between diverse factors [1,2]. An initial event leading to atherosclerosis is endothelial damage and activation [3]. These events cause localized chronic inflammation in the vascular wall, chiefly mediated by T cells and monocytes or macrophages [4,5]. This localized immune-mediated inflammation induces a systemic immune humoral and cellular response [6,7]. Besides antibodies against oxidized low density lipoproteins, β2 glycoprotein I and heat-shock protein 60, anti-endothelial cell antibodies (AECA) have been reported in sera from patients with atherosclerosis [8–11]. Ample evidence shows an association between the vascular complications of several autoimmune diseases including systemic lupus erythematosus (SLE), rheumatoid arthritis, antiphospholipid antibody syndrome and scleroderma and elevated levels of AECA [12–14]. By activating endothelial cells, AECA up-regulate expression of adhesion molecules as well as cytokine and chemokine secretion. Until recently, few published data were available on the identity of endothelial cell autoantigens in immune disorders [15–20]. Identifying endothelial autoantigens involved in the autoimmune processes during atherosclerosis could help to explain how chronic inflammation of the vascular wall initiates and progresses.
Our primary aim in this study was to seek and characterize molecules that behave as endothelial autoantigens in carotid atherosclerosis. Screening of a cDNA library from human umbilical artery endothelial cells (HUAEC) with sera from patients with carotid atherosclerosis identified one positive strongly reactive clone encoding the protein actin. To evaluate anti-actin serum immunoreactivity we used enzyme-linked immunosorbent assay (ELISA) to measure specific IgG responses in patients with carotid atherosclerosis and compared them with responses in patients with SLE and type-1 diabetes mellitus and in healthy subjects. We also sought a possible relationship between the presence of anti-actin antibodies and the clinical characteristics of patients with carotid atherosclerosis and with SLE.
PATIENTS AND METHODS
Study population
We studied 61 consecutive patients with carotid atherosclerosis (mean age 71·3 ± 8·1 years; 33 Male, 28 Female), 33 patients with SLE (mean age 35·8 ± 7·3 years; 5 M, 28 F), 26 patients with type 1 diabetes mellitus (mean age 32 ± 13·3 years; 11 M, 15 F), and 41 healthy subjects (mean age 69·5 ± 8·7 years; 22 M, 19 F). Of the 61 patients with carotid atherosclerosis enrolled, 46 were undergoing endarterectomy at the Department of Surgical Sciences of the University of Rome, ‘La Sapienza’. Surgery was indicated for asymptomatic severe or preocclusive carotid-artery stenosis of more than 70% and evidence of haemodynamic instability on ultrasonography, especially in patients with ipsilateral signs of cerebral ischaemia on computed tomographic (CT) scan and symptomatic stenosis of 70% or even less, with or without ipsilateral signs of focal cerebral ischaemia on CT scan in patients with neurological symptoms. The 46 patients were grouped according to the macroscopic appearances of the plaques freshly removed from carotid arteries at endarterectomy. Lesions with an ulcerated fibrous cap (with or without thrombosis) or intraplaque haemorrhage (with or without swelling) were classified as complicated plaques. Lesions without these features were classified as uncomplicated. Of 46 patients enrolled, 23 patients had complicated and 23 had uncomplicated plaques. The consecutive patients with SLE, and type 1 diabetes mellitus were outpatients attending the Division of Rheumatology, University of Rome ‘La Sapienza’. All patients with SLE fulfilled the revised criteria of the American College of Rheumatology for the classification of the disease [21]. None of the patients with type I diabetes mellitus selected had signs or symptoms of vascular complications. According to the clinical records, the following risk factors for cardiovascular disease were identified in 61 patients with carotid atherosclerosis and in 33 patients with SLE: diabetes, smoking, hypertension, cardiovascular disease in relatives, body-mass index and hypercholesterolaemia (Table 1). The healthy volunteers enrolled in this study were attending the outpatient clinic at the Division of Rheumatology, University of Rome ‘La Sapienza’ and had none of the aforementioned risk factors for cardiovascular diseases. The study protocol was approved by the local Ethics Committee and informed consent was obtained from all participants before the procedures. On enrolment, peripheral blood was drawn from all participants and serum samples were stored at −20°C until assayed.
Table 1. Characteristic of the 61 patients with carotid atherosclerosis and of the 33 patients with SLE divided according to the presence of serum anti-actin IgG immunoreactivity.
| Patients with atherosclerosis | Patients with systemic lupus erythematosus | |||||
|---|---|---|---|---|---|---|
| Characteristics | Anti-actin IgG immunoreactivity (n = 16) | No anti-actin IgG immunoreactivity (n = 45) | P-value | Anti-actin IgG immunoreactivity (n = 13) | No anti-actin IgG immunoreactivity (n = 20) | P-value |
| Age (years, means ± SD) | 73·7 ± 6·1 | 68·9 ± 8·5 | NS | 34·4 ± 9·0 | 40·2 ± 12·6 | NS |
| Sex (males/females) | 10/6 | 23/22 | NS | 2/11 | 4/16 | NS |
| Hypergammaglobulinaemia | 0 | 0 | – | 3 (23%) | 4 (20%) | NS |
| Diabetes | 2 (10%) | 22 (48%) | 0·01 | 0 | 0 | – |
| Smoking | 8 (50%) | 32 (69%) | NS | 3 (23%) | 5 (25%) | NS |
| Hypertension | 11 (70%) | 28 (62%) | NS | 2 (15%) | 8 (40%) | NS |
| Cardiovascular diseases in relatives | 8 (50%) | 17 (37%) | NS | 3 (23%) | 6 (33%) | NS |
| Hypercholesterolaemia | 3 (20%) | 9 (19%) | NS | 2 (15%) | 8 (40%) | NS |
| Anti-endothelial cell antibodies | 4 (25%) | 10 (22%) | NS | 6 (46%) | 8 (40%) | NS |
| Body mass index | 27·4 ± 3·8 | 26·0 ± 4·6 | NS | 25·7 ± 3·2 | 26·2 ± 2·7 | NS |
P-values by Student's t-test and Fisher's exact test. NS indicates not significant.Diabetes is type 2 diabetes, defined as fasting glucose levels = 140 mg/dL or need for antidiabetic medications; all the diabetic patients were treated with oral hypoglycaemic agents. Smoking is defined as current smokers. Hypertension is defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or need for hypertensive medication. Hypercholesterolaemia is defined as total cholesterol >200 mg/dl or need for lipid-lowering therapy.
Immunoscreening of the cDNA expression library
A commercially available HUAEC cDNA library (Stratagene, Cambridge, UK) was used to screen for clones showing immunoreactivity with a pool of sera from two patients with carotid atherosclerosis, which in preliminary experiments resulted AECA positive in a cell-surface ELISA. The expression library was screened essentially as we previously described [22]. Pooled sera were diluted 1 : 350 in phosphate-buffered saline (PBS) containing 1% milk, 0·1% Tween-20 and supplemented with 0·02% sodium azide. To reduce nonspecific binding to Escherichia coli (XL1 blue MRF') and phage vector, diluted pool was preadsorbed three times on nonrecombinant phage plaques. For primary immunoscreening the library was plated out at 12500 pfu/140 mm plate using XL1 blue MRF' host cells according to the supplier's instructions. In brief, nitrocellulose filters were incubated with 10 mm isopropylβ-D-thiogalacto-pyranoside (IPTG) for 20 min and air-dried. Filters were then overlaid onto the plates and incubated for 4 h at 37°C. After incubation, their orientation was marked and they were carefully removed, and blocked for 1 h in 5% milk/PBS. A preadsorbed pool of sera was then added to the filters and incubated overnight at RT with gentle agitation. Membranes were then washed four times with 0·05% PBS Tween-20 over 15 min. Bound antibody was detected using 1 : 3000 dilution of mouse anti-human IgG (Bio-Rad, Richmond, CA, USA) in PBS containing 0·05% Tween-20, 1% milk for 3 h at RT. After a final four washes in 0·05% PBS Tween-20 membranes were incubated for 20 min with diaminobenzidine substrate (Sigma Aldrich, St. Louis, MO, USA). Plaques corresponding to immunoreactive regions were cored from the original plate and resuspended in suspension medium (SM) containing 10 µl chloroform. Positive plaques were re-screened with the same pool of sera to obtain the clonality.
Cloned phage showing immunoreactivity was recovered as pBluescript by single-stranded rescue using the helper phage (Stratagene) according to the supplier's instructions and used to transform SolR XL1cells. Positive recombinants were then harvested and grown overnight in 5 ml LB and plasmid DNA isolated using the Qiagen Plasmid Mini Kit (Qiagen, GmbH, Hilden, Germany). The nucleotide sequence of the cloned cDNA insertion was sequenced with automated sequencer ABI Prism 310 Collection (Applied Biosystems, Foster City, CA, USA) and sequences were then compared with the GenBank sequence database using both Fasta and Blast analysis [23,24].
Indirect immunofluorescence assay
Human umbilical vein endothelial cells (HUVEC) were isolated by collagenase perfusion from normal-term umbilical cord veins, as previously described [25], and cultured in M-199 medium (Sigma) supplemented with 20% fetal calf serum (FCS), 2 mm l-glutamine (Bio Whittaker, Verviers, Belgium), 100 U/ml penicillin, 100 mg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. HUVEC, grown to 60–70% confluency, seminated at 5 × 106 per well on glass coverslips, were fixed with acetone/methanol 1/1 (v/v) for 10 min at 4°C and then soaked in balanced salt solution (BSS) (Sigma) for 30 min at 25°C. Cells were then incubated for 30 min at 25°C in the blocking buffer (2% bovine serum albumin (BSA) in PBS, containing 5% glycerol and 0·2% Tween-20). Cells were then labelled with rabbit anti-actin polyclonal antibodies (Sigma) or with sera from healthy donors or patients with atherosclerosis and were incubated for 1 h at 4°C. After washing three times with PBS, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat polyclonal anti-rabbit IgG or with FITC-conjugated anti-human IgG in PBS 1% BSA, for 30 min at 4°C.
The fluorescence distribution was analysed with a fluorescence microscope Olympus U RFL T equipped with RT Spot power supply (Diagnostic Instruments Inc., MI, USA).
ELISA
ELISA for specific total IgG was developed essentially as described by Challacombe et al. [26]. In brief, polystyrene plates (Dynex, Germany) were coated with actin (Sigma) 0·5 µg/well in 0·05 µm NaHCO3 buffer pH 9·5. Coated plates were incubated overnight at 4°C and then washed 3 times with PBS containing 0·05% Tween-20 in an automated washer (Wellwash 4, Labsystem, Finland). Plates were blocked with PBS Tween containing 3% gelatin (Bio-Rad), 100 µl/well for 1 h at room temperature and washed as previously described. Human sera were diluted in PBS Tween-20 and 1% gelatin (1 : 100 for total IgG and 1 : 10 for IgG subclass detection) and pipetted on plates at 100 µl per well. Plates were incubated for 1 h at 20°C and washed as described. Mouse monoclonal anti-human IgG1, IgG2, IgG3 and IgG4 antibodies (BD Biosciences, Heidelberg, Germany) were diluted 1 : 1000 in PBS Tween and peroxidase-conjugated goat anti-human IgG (Bio-Rad) were diluted 1 : 3000 in the same buffer. These dilutions were used as second antibodies, and incubated (100 µl/well) for 1 h at 20°C. Peroxidase-conjugated goat anti-mouse IgG (Bio-Rad), diluted 1 : 3000 in PBS Tween, incubated for 1 h at 20°C, were used as third antibody for IgG subclass analysis. O-phenylenediamine dihydrochloride (Sigma) was used as a substrate and absorbance was measured at 490 nm. Means + 2 SD of the absorbance reading of the healthy controls were considered as cut-off levels for positive reactions. The results of unknown samples on the plate were accepted if internal controls (two serum samples, one positive and one negative) had an absorbance reading within mean ± 10% of previous readings.
Cultures of HUVEC at the third to fourth passage were used to detect AECA (IgG), using a cell-surface ELISA on living cells, as previously reported by Del Papa et al. [27]. AECA were expressed as binding index (BI) equal to 100 × (S–A)/(B–A), where S is the optical density (OD) of the sample tested, A the OD obtained with only the secondary antibody, and B the OD of a positive reference serum. AECA were considered as positive when BI was higher than the cut-off value (mean + 2 SD of a 40 healthy controls) corresponding to 46·1% of a positive reference serum from a patients with SLE.
Statistical analysis
Unless otherwise specified all values are means ± SD. Fisher's exact test and chi-square analysis were used to evaluate differences between percentages; and Student's t-test to evaluate differences between arithmetic means. P-values equal to or less than 0·05 were considered to indicate statistical significance.
RESULTS
Immunoscreening of HUAEC expression library with sera from patients with carotid atherosclerosis
Immunoscreening of the HUAEC expression library with IgG from a pool of two sera from patients with carotid atherosclerosis identified a strongly reactive clone. The amino acid sequence, predicted from the 1128 base-pair (bp) open reading frame of this clone, is 376 residues long and has 100% identity with the human γ actin.
Immunofluorescence analysis determining anti-actin reactivity in HUVEC
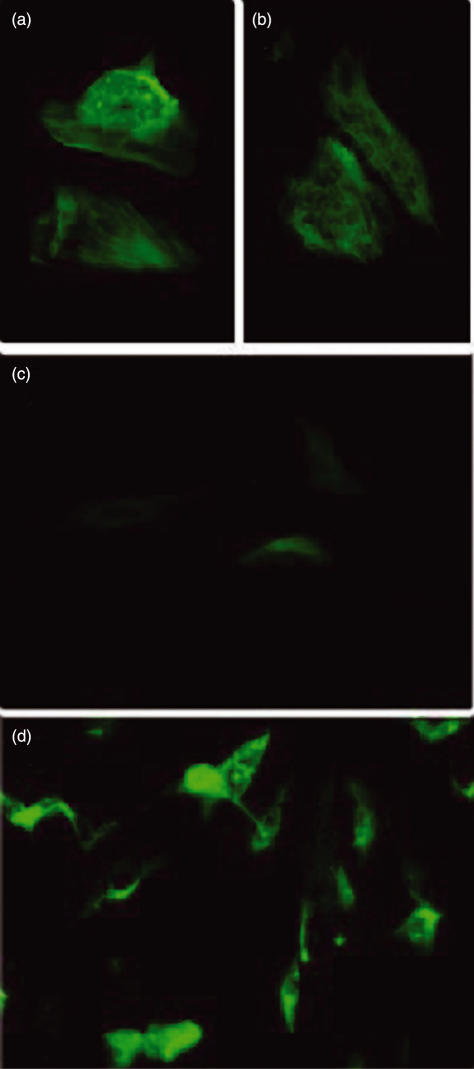
IgG in sera from the two patients with carotid atherosclerosis used in immunoscreening yielded HUVEC cytoplasmic staining with a cell distribution reminiscent of actin microfilaments (Fig. 1a,b). A rabbit polyclonal anti-actin antibody yielded virtually the same distribution pattern (Fig. 1d). These findings indicate that the sera from patients with atherosclerosis used for screening the library react with human actin in endothelial cells. Immunofluorescence analysis detected no cytoplasmic staining in HUVEC incubated with serum from five healthy donors (Fig. 1c).
Fig. 1.
Immunofluorescence analysis of the IgG reactivity pattern to HUVEC cells of serum from the two patients with atherosclerosis used in the screening (a) and (b); serum from one healthy donor (c) and rabbit anti-actin polyclonal antibodies (d).
ELISA for anti-actin IgG in serum from patients with carotid atherosclerosis, SLE and type 1 diabetes mellitus
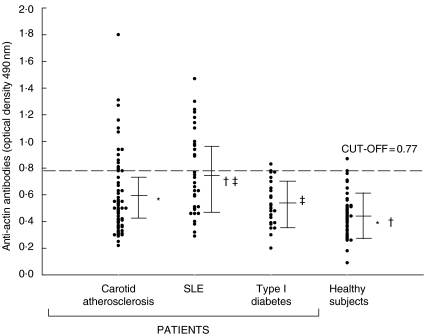
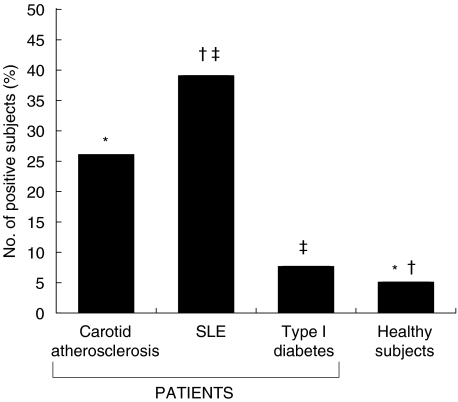
Because actin filaments within the endothelial cells showed IgG-immunoreactivity, and because preliminary experiments showed that recombinant and native actin gave equivalent serological results (data not shown), in serological tests instead of denatured recombinant actin we used native actin, which retained conformational epitopes and closely reflected in vivo conditions. The mean OD values for IgG immunoreactivity were significantly higher in patients with carotid atherosclerosis and in patients with SLE than in the healthy control group (0·59 ± 0·16 and 0·76 ± 0·30 versus 0·46 ± 0·15; P < 10−4, by Student's t-test) (Fig. 2). Mean OD values of anti-actin IgG were significantly lower in patients with type 1 diabetes than in patients with SLE (0·53 ± 0·17 versus 0·76 ± 0·30; P = 0·001). The frequency of actin-specific IgG was significantly higher in patients than in healthy subjects (atherosclerosis, 16/61 26% and SLE, 13/33, 39%versus 2/41, 5%, P = 0·012 and P < 10−4 by χ2 test); and in patients with SLE than in patients with diabetes type 1 (13/33, 39%versus 2/26, 7·6%; P = 0·01) (Fig. 3). IgG subclass analysis in the 16 patients with carotid atherosclerosis showed the presence of anti-actin IgG1 and IgG2 isotypes: 5 patients (31%) had anti-actin IgG1; 5 (31%) had IgG2; and 6 (37%) had IgG1 and IgG2. All the 13 patients with SLE had anti-actin IgG1 and IgG2.
Fig. 2.
Anti-actin reactivity in patients with carotid atherosclerosis, systemic lupus erythematosus (SLE), type 1 diabetes and in healthy subjects. Each dot represents a subject. The dotted line represents the average value plus two standard deviations of the healthy subjects. Statistically significant differences by Student's t-test: *†P < 10−4, ‡P = 0·001.
Fig. 3.
Distribution of anti-actin antibodies in patients with carotid atherosclerosis, systemic lupus erythematosus (SLE), type 1 diabetes and in healthy subjects. Statistically significant differences by χ2 test: *P = 0·012, †P < 10−4, ‡P = 0·01.
Anti-actin IgG and characteristics of patients
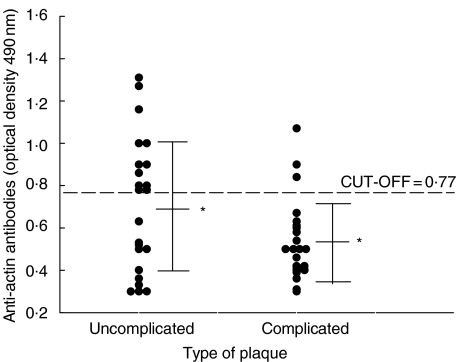
The presence of anti-actin IgG in serum of patients with atherosclerosis and SLE was independent of the presence of cardiovascular risk factors (Table 1). According to Fisher's exact test, the presence of serum anti-actin antibodies in patients with carotid atherosclerosis was inversely related to the presence of diabetes (P = 0·01). We found no significant association between serum anti-actin antibodies and the presence of AECA in patients with atherosclerosis and SLE (Table 1). The presence of serum anti-actin reactivity did not correlate with hypergammaglobulinemia in patients with SLE (Table 1). We found no significant association between vascular risk factors related to inflammation (levels of C reactive protein and the erythrocyte sedimentation rate) with the presence of anti-actin antibodies (data not shown). No significant difference was found for serum anti-actin antibodies between patients who underwent endarterectomy and those who did not (data not shown). Of the 46 patients who underwent endarterectomy, the frequency of anti-actin antibodies was higher in patients with uncomplicated plaque than in those with complicated plaque (10/23, 43%versus 3/23, 13%; P = 0·047) The mean OD value of anti-actin IgG was also significantly higher in patients with uncomplicated plaques than in patients with complicated plaques (Student's t-test: P = 0·048) (Fig. 4). In our study about 50% of the patients with carotid atherosclerosis had vascular lesions in coronary or peripheral arteries, or both, but we found no correlation between these vascular abnormalities and the presence of anti-actin antibodies (data not shown).
Fig. 4.
Anti-actin reactivity in patients with carotid atherosclerosis divided according to the type of plaque. Each dot represents a subject. The dotted line represents the average value plus two standard deviations of the healthy subjects. Statistically significant difference by Student's test: *P = 0·048.
DISCUSSION
Serum anti-actin antibodies, initially detected among patients with chronic active hepatitis in 1965 [28,29], were later identified in various autoimmune diseases including SLE, in infectious mononucleosis and in cancer including urogenital carcinomas and malignant melanoma [30–34]. In this study, after using a molecular cloning strategy to identify endothelial autoantigens, besides confirming earlier evidence of serum anti-actin antibodies in patients with SLE, we provide evidence of serum anti-actin antibody reactivity in patients with carotid atherosclerosis.
Our finding that actin is a significant autoantigen in SLE (a classic systemic autoimmune disease in which endothelial damage plays a crucial role) and atherosclerosis raises the possibility that these two diseases share immunological features. Apoptosis may play an important role in bypassing tolerance to intracellular autoantigens. In patients with SLE, several autoantigens localized in endoplasmic reticulum and ribosomes were identified in blebs of apoptotic keratinocytes [35]. In a recent study on the tumour-infiltrating B cell response, Hansen et al. reported that infiltrating B lymphoplasmacytic cells in medullary breast cancer produce antibodies specific to the self antigen actin. Medullary breast cancer cells in apoptosis may render actin immunogenic by exposing it on the cell surface [36,37]. On the contrary, a recent report describes endothelial-cell actin fibre arrangement as a result of apoptosis, without plasma membrane expression of actin molecules [38]. Many autoantigens and in particular actin act as substrates for the pro-apoptotic cysteine proteases. The novel polypeptides generated can be released into the extracellular spaces or be presented as neoantigens, thus generating an autoimmune response [39,40].Whether apoptotic endothelial cells within atherosclerotic plaque can expose actin on their surface or release actin into the extracellular spaces remains unclear. In this study, patients with atherosclerosis whose serum contained anti-actin IgG often had less advanced uncomplicated plaque, rather than the more complicated, unstable eroded plaque vulnerable to rupture. If the percentage of endothelial apoptotic cells that display actin molecules able to capture anti-actin antibodies into the plaque increases as plaques advance then this mechanism might explain why we found lower anti-actin reactivity in patients with complicated than in those with uncomplicated plaque. Support for this hypothesis comes from studies describing immunoglobulin accumulation into atherosclerotic plaques, but the antibody specificity is still unknown [41,42].
Traditional cardiovascular risk factors have no role in the induction of anti-actin IgG in sera from patients with atherosclerosis and SLE. The lack of association between hypergammaglobulinaemia and the presence of serum anti-actin IgG in patients confirms the specificity of anti-actin IgG reactivity.
Even though in this study we used an endothelial cDNA expression library, we cannot exclude the possibility that the autoimmune response we observed was generated against actin present in smooth muscle cells or other cellular populations of atherosclerotic plaque. Our finding of higher IgG reactivity to actin in patients with carotid atherosclerosis and with SLE, diseases characterized by endothelial damage, than in healthy, age-matched subjects, and in patients with type-1 diabetes without signs or symptoms of vascular complications nevertheless suggests that the autoimmune response against actin could preferentially target the endothelium. Anti-actin autoantibodies could therefore result from endothelial damage. The unanswered question is whether anti-actin antibodies activate vascular endothelium thus playing a direct pathogenetic role in atherosclerotic disease or they are an epiphenomenon caused by the endothelial damage. In fact the endothelial damage could be caused by anti-endothelial cell antibodies, but also by other causes such as infections. Systemic infection by various pathogens, including viruses and bacteria may interact with the endothelium contributing to pathological changes in the arterial walls and may result in altered coagulation, vasculitis and atherosclerosis.
We found no significant association between the classical test for detecting AECA and the presence of anti-actin antibodies by ELISA in patients with carotid atherosclerosis and SLE. The cell-surface ELISA on living cells we used to detect AECA reveals only plasma membrane antigens. Anti-endothelial cell reactivity is not restricted to plasma membrane proteins alone. Anti-endothelial-cell reactivity is also directed against cytoskeletal components such as vimentin [43]. Our data on IgG subclass profiles showed that anti-actin IgG in the serum of patients with carotid atherosclerosis and with SLE invariably belonged to the IgG1 or IgG2 subclasses or both. Previous reports showed that in autoimmune hepatitis actin-specific IgG1 and IgG3 predominated [44]. Why production of actin-specific IgG isotypes differs in autoimmune hepatitis, and atherosclerosis and SLE is hard to explain.
The lack of information about autoantibodies associated with atherosclerosis makes it difficult to understand the pathogenetic mechanism underlying the onset and progression of atherosclerotic plaque, let alone design of therapy. The presence of anti-actin autoantibodies in serum from patients with atherosclerosis suggests that actin is a new autoantigenic molecule of potential clinical importance that could lead to a deeper understanding of the specific antigen-induced mechanisms underlying immune-mediated inflammation in carotid atherosclerosis.
Acknowledgments
This work was supported by an I.S.S. grant n. 2138/RI.
REFERENCES
- 1.Greaves DR, Channon K. Inflammation and immune responses in atherosclerosis. Trends Immunol. 2002;23:535–41. doi: 10.1016/s1471-4906(02)02331-1. [DOI] [PubMed] [Google Scholar]
- 2.Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nature Med. 2002;8:1218–26. doi: 10.1038/nm1102-1218. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–9. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi K, Takeya M, Sakashita N. Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med Electron Microsc. 2002;35:179–203. doi: 10.1007/s007950200023. [DOI] [PubMed] [Google Scholar]
- 5.Lord RS, Bobryshev YV. Hallmarks of atherosclerotic lesion development with special reference to immune inflammatory mechanisms. Cardiovasc Surg. 2002;10:405–14. doi: 10.1016/s0967-2109(02)00039-x. [DOI] [PubMed] [Google Scholar]
- 6.Profumo E, Siracusano A, Ortona E, et al. Cytokine expression in circulating T lymphocytes from patients undergoing carotid endarterectomy. J Cardiovasc Surg. 2003;44:237–42. [PubMed] [Google Scholar]
- 7.Nicoletti A, Caligiuri G, Hansson GK. Immunomodulation of atherosclerosis: myth and reality. J Intern Med. 2000;247:397–405. doi: 10.1046/j.1365-2796.2000.00660.x. [DOI] [PubMed] [Google Scholar]
- 8.Shoenfeld Y, Sherer Y, George J, Harats D. Autoantibodies associated with atherosclerosis. Ann Med. 2000;32(1S):37–40. [PubMed] [Google Scholar]
- 9.George J, Meroni PL, Gilburd B, Raschi E, Harats D, Shoenfeld Y. Anti-endothelial cell antibodies in patients with coronary atherosclerosis. Immunol Lett. 2000;73:23–7. doi: 10.1016/s0165-2478(00)00192-9. [DOI] [PubMed] [Google Scholar]
- 10.Farsi A, Domeneghetti MP, Brunelli T, et al. Activation of the immune system and coronary artery disease: the role of anti-endothelial cell antibodies. Atherosclerosis. 2001;154:429–36. doi: 10.1016/s0021-9150(00)00482-2. [DOI] [PubMed] [Google Scholar]
- 11.van Haelst PL, Kobold AC, van Doormaal JJ, Tervaert JW. AECA and ANCA in patients with premature atherosclerosis. Int Rev Immunol. 2002;21:19–26. doi: 10.1080/08830180210412. [DOI] [PubMed] [Google Scholar]
- 12.Westphal JR, Boerbooms AMTH, Shalkwijk CJM, et al. Anti-endothelial cell antibodies in sera of patients with autoimmune diseases: comparison between ELISA and FACS analysis. Clin Exp Immunol. 1994;96:444–9. doi: 10.1111/j.1365-2249.1994.tb06049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Worda M, Sgonc R, Dietrich H, Niederegger H, Sundick RS, Gershwin ME, Wick G. In vivo analysis of the apoptosis-inducing effect on anti-endothelial cell antibodies in systemic sclerosis by the chorionallantoic membrane assay. Arthritis Rheum. 2003;48:2605–14. doi: 10.1002/art.11179. [DOI] [PubMed] [Google Scholar]
- 14.Hill MB, Phipps JL, Hughes P, Greaves M. Anti-endothelial cell antibodies in primary antiphospholipid syndrome and SLE. patterns of reactivity with membrane antigens on microvascular and umbilical venous cell membranes. Br J Haematol. 1998;103:416–21. doi: 10.1046/j.1365-2141.1998.00979.x. [DOI] [PubMed] [Google Scholar]
- 15.Yazici ZA, Behrendt M, Cooper D, Goodfield M, Partridge LJ, Lindsey NJ. The identification of endothelial cell autoantigens. J Autoimmun. 2000;15:41–9. doi: 10.1006/jaut.2000.0391. [DOI] [PubMed] [Google Scholar]
- 16.Frampton G, Moriya S, Pearson JD, et al. Identification of candidate endothelial cell autoantigens in systemic lupus erythematosus using a molecular cloning strategy: a role for ribosomal P protein P0 as an endothelial cell autoantigen. Rheumatology. 2000;39:1114–20. doi: 10.1093/rheumatology/39.10.1114. [DOI] [PubMed] [Google Scholar]
- 17.Meroni PL, Del Papa N, Raschi E, et al. Beta2-glycoprotein I as a ‘cofactor’ for anti-phospholipid reactivity with endothelial cells. Lupus. 1998;7(2S):S44–7. doi: 10.1177/096120339800700211. [DOI] [PubMed] [Google Scholar]
- 18.Hochleitner BW, Hochleitner EO, Obrist P, Eberl T, Amberger A, Margreiter R, Wick G. Fluid shear stress induces heat shock protein 60 expression in endothelial cells in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2000;20:617–23. doi: 10.1161/01.atv.20.3.617. [DOI] [PubMed] [Google Scholar]
- 19.Dimitriu-Bona A, Matic M, Ding W, Yang CP, Fillit H. Cytotoxicity to endothelial cells by sera from aged MRL/lpr/lpr mice is associated with autoimmunity to cell surface heparan sulfate. Clin Immunol Immunopathol. 1995;76:234–40. doi: 10.1006/clin.1995.1121. [DOI] [PubMed] [Google Scholar]
- 20.Moscato S, Pratesi F, Bongiorni F, Scavuzzo MC, Chimenti D, Bombardieri S, Migliorini P. Endothelial cell binding by systemic lupus antibodies: functional properties and relationship with anti-DNA activity. J Autoimmun. 2002;18:231–8. doi: 10.1006/jaut.2002.0583. [DOI] [PubMed] [Google Scholar]
- 21.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of SLE. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 22.Margutti P, Ortona E, Vaccari S, et al. Cloning and expression of a cDNA encoding an elongation factor 1beta/delta protein from Echinococcus granulosus with immunogenic activity. Parasite Immunol. 1999;21:485–92. doi: 10.1046/j.1365-3024.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 23.Pearson WR, Lipman DJ. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–8. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 25.Vismara A, Meroni PL, Tincani A, et al. Relationship between anti-cardiolipin and anti-endothelial cell antibody in systemic lupus erythematosus. Clin Exp Immunol. 1988;74:247–53. [PMC free article] [PubMed] [Google Scholar]
- 26.Challacombe SJ, Biggerstaff M, Greenall C, Kemeny DM. ELISA detection of human IgG subclass antibodies to Streptococcus mutans. J Immunol Meth. 1986;87:95–102. doi: 10.1016/0022-1759(86)90348-0. [DOI] [PubMed] [Google Scholar]
- 27.Del Papa N, Meroni PL, Tincani A, et al. Relationship between anti-phospholipid and anti-endothelial cell antibodies: further characterization of the reactivity on resting and cytokine-activated endothelial cells. Clin Exp Rheumatol. 1992;10:37–42. [PubMed] [Google Scholar]
- 28.Johnson GD, Holborow EJ, Glynn LE. Antibody to smooth muscle in patients with liver disease. Lancet. 1965;2:878–9. doi: 10.1016/s0140-6736(65)92505-5. [DOI] [PubMed] [Google Scholar]
- 29.Leibovitch L, George J, Levi Y, Bakimer R, Shoenfeld Y. Anti-actin antibodies in sera from patients with autoimmune liver diseases and patients with carcinomas by ELISA. Immunol Lett. 1995;48:129–32. doi: 10.1016/0165-2478(95)02456-5. [DOI] [PubMed] [Google Scholar]
- 30.Matsiota P, Druet P, Dosquet P, Guilbert B, Avrameas S. Natural autoantibodies in systemic lupus erythematosus. Clin Exp Immunol. 1987;69:79–88. [PMC free article] [PubMed] [Google Scholar]
- 31.Boulassel M-R, Tomasi J-P, Deggouj N, Gersdorff M. Identification of β-actin as a candidate autoantigen in autoimmune inner ear disease. Clin Otolaryngol. 2000;25:535–41. doi: 10.1046/j.1365-2273.2000.00416.x. [DOI] [PubMed] [Google Scholar]
- 32.Holborow EJ, Hemsted EH, Mead SV. Smooth muscle autoantibodies in infectious mononucleosis. Br Med J. 1973;3:323–5. doi: 10.1136/bmj.3.5875.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurki P, Linder E, Miettinen A, Alfthan O, Heikkinen A, Pasternack A. Tissue antibodies in malignant and benign urogenital disease. Int J Cancer. 1977;19:332–6. doi: 10.1002/ijc.2910190308. [DOI] [PubMed] [Google Scholar]
- 34.Boulila A, Hachicha J, Adyel FZ, Jlidi R, Avrameas S, Ternynck T, Ayadi H. Deposition of anti-actin antibodies in the kidney of a patient with systemic lupus erythematosus under immunosuppressive treatment. Nephrol Dial Transplant. 1996;11:2478–81. doi: 10.1093/oxfordjournals.ndt.a027218. [DOI] [PubMed] [Google Scholar]
- 35.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hansen MH, Nielsen HV, Ditzel HJ. The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc Natl Acad Sci USA. 2001;98:12659–64. doi: 10.1073/pnas.171460798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansen MH, Nielsen HV, Ditzel HJ. Translocation of an intracellular antigen to the surface of medullary breast cancer cells early in apoptosis allows for an antigen-driven antibody response elicited by tumor-infiltrating B cells. J Immunol. 2002;169:2701–11. doi: 10.4049/jimmunol.169.5.2701. [DOI] [PubMed] [Google Scholar]
- 38.Kook H, Ahn KY, Lee SE, Na HS, Kim KK. Nitric oxide-dependent cytoskeletal changes and inhibition of endothelial cell migration contribute to the suppression of angiogenesis by RAD50 gene transfer. FEBS Lett. 2003;553:56–62. doi: 10.1016/s0014-5793(03)00967-0. [DOI] [PubMed] [Google Scholar]
- 39.Kayalar C, Ord T, Testa MP, Zhong LT, Bredesen DE. Cleavage of actin by interleukin 1β-converting enzyme to reverse Dnase I inhibition. Proc Natl Acad Sci USA. 1996;93:2234–8. doi: 10.1073/pnas.93.5.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piacentini M, Colizzi V. Tissue transgutaminase: apoptosis versus autoimmunity. Immunol Today. 1999;20:130–4. doi: 10.1016/s0167-5699(98)01416-9. [DOI] [PubMed] [Google Scholar]
- 41.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–8. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 42.Sohma Y, Sasano H, Shiga R, Saeki S, Suzuki T, Nagura H, Nose M, Yamamoto T. Accumulation of plasma cells in atherosclerotic lesions of Watanabe heritable hyperlipidemic rabbits. Proc Natl Acad Sci USA. 1995;92:4937–41. doi: 10.1073/pnas.92.11.4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jurcevic S, Ainsworth ME, Pomerance A, Smith JD, Robinson DR, Dunn MJ, Yacoub MH, Rose ML. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–92. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 44.Zamanou A, Tsirogianni A, Terzoglou C, Balafas A, Economidou I, Lymberi P. Anti-smooth muscle antibodies (ASMAs) and anti-cytoskeleton antibodies (ACTAs) in liver diseases: a comparison of classical indirect immunofluorescence with ELISA. J Clin Laboratory Anal. 2002;16:194–201. doi: 10.1002/jcla.10040. [DOI] [PMC free article] [PubMed] [Google Scholar]