Abstract
In order to study the interaction between a γ-herpesvirus and malaria we established a co-infection model that involves infection of mice with murine γ-herpesvirus (MHV-68) and Plasmodium yoelii non-lethal strain (PYNL). To investigate the interaction between acute malaria and the lytic stage of MHV-68, the timing of infections was chosen such that the peak virus and parasite burdens would be present at the same time. Under this condition, we observed significant mortality in co-infected mice and aggressive lung inflammation with a marked influx of neutrophils and megakaryocytes. If mice were latently infected with MHV-68 and then co-infected with malaria we noticed significantly less viral load and parasitaemia. Using MHC/peptide tetramer staining we found that acute malaria reduces the anti-MHV-68 CD8+ T cell response in the animals that develop severe disease. Our study provides important fundamental information, which will be of use when devising strategies to combat infections with more than one agent, a situation that often occurs naturally.
Keywords: CD8+ T cell response, inflammation, parasite, protection, virus
INTRODUCTION
Epidemiological studies indicate that co-infection by Epstein–Barr virus (EBV), a γ-herpesvirus, and Plasmodium falciparum[1] is common in large areas of Africa. Dual infection by these two pathogens has for many years been associated with the development of Burkitt's lymphoma [2,3]. In this report, we studied a murine co-infection model involving P. yoelii 17XNL (non-lethal strain), a murine malaria parasite, and MHV-68, a rodent γ-herpesvirus. There are significant similarities between MHV-68 and the human γ-herpesvirus in terms of biology and pathogenesis [4–6], and MHV-68 is a widely accepted small animal model for these infections. Among the murine malarial parasites, P. yoelii has been used extensively for vaccine development studies, as several aspects of the immune response to P. yoelii malaria mimic those in human malaria [7–9].
The mutual interactions between viruses and malarial parasites are poorly understood. Recently two important studies were carried out directed at understanding the impact of malarial pathogens on virus expression in hepatitis B virus transgenic (HB Tg) mice and in human immunodeficiency virus transgenic (HIV Tg) mice. HB Tg mice infected with P. yoelii 17XNL showed lower levels of hepatitis B virus RNA and DNA in the liver. The authors concluded that this heterologous protection was dependent on the interferon (IFN)-γ-induced nitric oxide (NO) response [10]. In contrast to this finding HIV Tg mice, upon infection with P. chabaudi, another murine plasmodial parasite, mediate higher expression of viral P24 antigen. The authors postulated that this augmentation was related to enhanced cellular activation (i.e. expression of CD40 and production of IFN-γ) by the malarial parasite [11]. Recent reports suggest worsening of AIDS disease in humans who were co-infected with malaria and HIV [12]. HIV-infected pregnant women appear to be more susceptible to P. falciparum[13,14], and in addition P. falciparum antigen preparations enhance viral replication in in vitro infected peripheral blood mononuclear cells (PBMC) from non-HIV-infected donors [15].
In this study, we utilized a non-transgenic system to test the hypothesis that dual infection with malaria and MHV-68 induces worse disease than either agent alone. We also addressed whether there was any disruption of the virus-specific immune response after co-infection with malaria in mice latently infected with MHV-68.
MATERIALS AND METHODS
Mice and infections
Virus
MHV-68 virus (clone G2·4) was obtained originally from Professor A. A. Nash (University of Edinburgh, UK). Virus was propagated and titred as described previously [16]. Female Balb/c mice, 6–8 weeks old, were purchased from the National Cancer Institute (Bethesda, MD, USA). Mice were infected intranasally with 400 plaque-forming units (PFU) of MHV-68 under anaesthesia with 2,2,2-tribromoethanol.
Parasite
In the present study we used the non-lethal strain of P. yoelii (17XNL). The parasite was stored in liquid nitrogen and then used to infect source mice. PRBCs obtained from source mice were suspended in RPMI-1640 and injected intraperitoneally (5 × 105 PRBCs/mouse) in all experimental mice. Parasitaemia was monitored by making blood smears and counting Giemsa-stained fields. All animal experiments were approved by the Animal Care and Use Program of Dartmouth College.
Viral titre measurement
Plaque assay for detection of free virus in lungs.
A plaque assay was used to measure free virus in acutely infected lungs, as described previously [16]. Briefly, whole lungs were homogenized in medium then serial dilutions of this homogenate were added to 3T3 monolayers in a minimal volume and left to adsorb for 1 h before overlaying with carboxymethyl cellulose. After 5 days of incubation at 37°C the assays were fixed with methanol and stained with Giemsa stain, and then the plaques were enumerated microscopically.
Quantitative fluorescent polymerase chain reaction (PCR).
DNA was extracted from spleen or lung tissue using a Qiagen (Valencia, CA, USA) DNeasy kit, and was then quantified using a UV spectrophotometer. DNA (300 ng) was subjected to quantitative fluorescent (QF)-PCR using the protocol that has been described previously [17]. Briefly, a primer set and probe complementary to the ORF50 gene of MHV-68 were added to the DNA plus PCR Supermix (Invitrogen, Carlsbad, CA, USA). Quantitative PCR was performed using an iCycler (Bio-Rad, Hercules, CA, USA).
Histological analysis
Samples of lung were removed from each mouse (n = 3 per group), fixed in 10% buffered formalin. Paraffin-embedded lung tissues were sectioned and stained with haematoxylin and eosin. Random sections of the lungs were examined histologically and then photographed. The level of inflammation was evaluated using a simplified modification of a published report [18]. The degree of inflammation was scored ranging from 0 to 3 according to the percentage of the scanned field at low power. No inflammation scored 0, up to one-third of the section affected scored 1, two-thirds scored 2 and more than two-thirds scored 3.
RNA extraction and RNase protection assay
Total RNAs from freshly isolated lung or spleen samples were extracted using the TRIzol reagent according to the manufacturer's instructions (Life Technologies). Cytokine mRNA expression in the lungs and spleens were detected using the RiboQuant multiProbe RNase Protection Assay System kit (BD PharMingen). Briefly, probes labelled with 32P (custom probes and the mCK-2b MultiProbe template set from BD PharMingen, no. 556156) used for specific cytokine mRNA detection were as follows: for interleukin (IL)-12p35, IL-12p40, IL-10, IL-1α, IL-1β, IL-Ra, IL-18, IL-6, interferon (IFN)-γ, macrophage migration inhibitory factor (MIF), L32 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH). RNA samples were digested by RNase treatment for 45 min at 30°C. Samples were loaded into a 5% acrylamide gel. The value of each hybridized probe was normalized to that of GAPDH, included as internal standard (set arbitrarily to 1). For quantification, autoradiographs were scanned and band densities were analysed by National Institutes of Health Image 1·61/ppc software (National Institutes of Health, Bethesda, MD, USA) using a Macintosh computer. Results were expressed as the percentage of the intensity of the band analysed relative to the intensity of the housekeeping (GAPDH/L32) RNA.
Carboxyfluorescein (diacetate) succinimidyl ester (CFSE) labelling and culture conditions
Spleen cells were labelled with CFSE by incubation with 0·5 µm CFSE diluted in Hanks's balanced salt solution (HBSS) for 10 min in the dark. Cells were washed subsequently with HBSS or complete tumour medium [19] before use. Spleen cells were restimulated with 5 ng/ml ORF65131−140 or M291−99 peptides plus 10 U/ml recombinant IL-2 (R&D Systems, Minneapolis, MN, USA) in 24-well plates for 4 days. Cells were harvested subsequently and stained with major histocompatibility complex (MHC)/peptide tetramers and anti-CD8 antibody as described [20].
MHC tetrameric reagents and analysis
Tetramers chosen for identifying MHV-specific CD8 T cell responses represent the only viral epitopes identified in BALB/c mice. The ORF65131-140/Dd derives from a lytic phase protein and the M291-99/Kd epitope from a latent phase protein. The construction of folded MHC class I-peptide complexes and their tetramerization have been described previously [21]. Two tetramers were used: Kd folded with peptide M291-99 (GFNKLRSTL) and Dd folded with ORF65131-140 (LGPDKSGLGF). Tetramers were stored as aliquots at −80°C. B cells were removed from spleen cell samples by panning for 1 h on plates coated overnight with 100 µg/ml goat antimouse IgG/IgM (Jackson ImmunoResearch, West Grove, PA, USA). Cells were incubated with anti-CD16/CD32 Fc block (BD PharMingen, San Diego, CA, USA) for 10 min on ice; staining with tetrameric reagents took place for 1 h at room temperature, followed by staining with anti-CD8α PerCP (clone 53–6·7) on ice for 20 min. Stained samples were analysed using a FACSCalibur flow cytometer and cellquest software (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). Control tetramers consisting of the same heavy chain folded with irrelevant peptides did not stain CD8 T cells from MHV-68 infected mice.
Statistical analysis
Results are expressed as mean ± s.d. Statistical differences between groups were analysed using Student's t-test. A value of P < 0·05 was considered significant. Fisher's exact test was used for murine survival experiments.
RESULTS
Survival, viral titre and parasitaemia in mice following co-infection with P. yoelii and MHV-68
Upon infection with non-lethal P. yoelii (PYNL), Balb/c mice develop peak parasitaemia by days 8–12 of infection (i.e. acute malaria), which will disappear around days 18–21 post-infection [22]. After experimental intranasal infection MHV-68 replicates in alveolar epithelial cells for 7–10 days, and this period represents the lytic stage of infection (MHV-68 lytic). Then by day 14 most of the replicating virus is cleared from the lungs by virus-specific T cells [16], and the virus establishes a latent infection where a low level of virus persists indefinitely with little, if any production of virus particles [16]. Our aim was to study the interactions between acute malaria and the lytic stage of MHV-68, and also between acute malaria and the latent stage of the virus. For this reason we chose the following sequence of infection: one group of mice was infected with PYNL 2 days prior to MHV-68 inoculation (PYNL + MHV-68 lytic infection) and the other group was infected first with MHV-68 for 30 days and was then exposed to PYNL (MHV-68 latent + PYNL infection). As it takes about 8–10 days for parasitaemia to peak and MHV-68 takes 7–8 days to reach peak virus load in the lungs, infecting with PYNL 2 days before MHV-68 and sampling 8 days after virus infection enabled us to examine the interaction between acute malaria and the lytic stage of MHV-68. The timing of infections for the other group (MHV-68 latent + PYNL infection) and the sampling at day 8 of PYNL p.i. allowed us to study the interaction between the latent stage of MHV-68 and acute malaria.
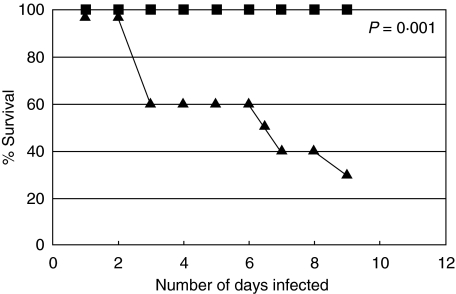
Survival was assessed in mice with single infection of MHV-68 or PYNL, and in mice dually infected with the virus and malarial parasite. Animals with PYNL + MHV-68 lytic infection showed signs of sickness, such as sluggish movement and ruffled hair, by day 4 post-virus infection. Death occurred in 40% of this group by this time-point, which reached to 70% by day 8 post-virus infection (Fig. 1). In contrast, no mice in the MHV-68 latent + PYNL group succumbed to infection by day 38 of post-viral infection. The animals in this group did not show any sign of sickness, and remained healthy until the end of the experiment. As expected, mortality was not seen in the groups of mice harbouring single infection with P. yoelii or with MHV-68 at any time-point of infection.
Fig. 1.
The survival of dually infected mice or mice with single infection with lytic or latent MHV-68 infection or mice infected with PYNL alone (n = 10/group). Filled diamonds: mice harbouring PYNL infection alone; filled squares: animals infected with lytic stages of MHV-68 alone; crosses: mice carrying latent stages of MHV-68 infection alone; filled triangles: mice infected with PYNL and 2 days later received MHV-68 inoculation; filled circles: animals infected with MHV-68 for 30 days and then infected with PYNL, MHV-68 latent + PYNL. This represents the results of two independent experiments. All points except filled triangles are at the 100% point on the graph. P-value was calculated using Fisher's exact test, and compares the PYNL + MHV-68 lytic group (triangles) with control groups.
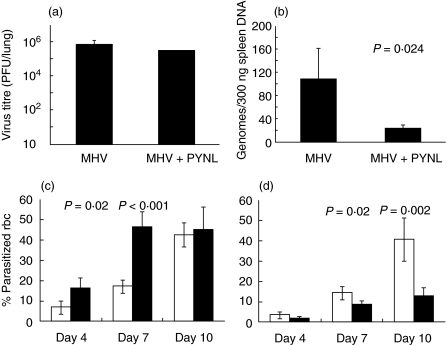
We measured the virus titre in dually infected mice and compared with that in mice with either lytic or latent MHV-68 infection. The surviving mice from the PYNL + MHV-68 lytic group appeared moribund and were killed at day 8 post-virus infection. Mice from MHV-68 latent + PYNL group were killed at day 38 post-virus infections. Spleens were collected for determining viral load. The virus titre in the lungs of PYNL + MHV-68 lytic group was determined by plaque assay and the results are shown in Fig. 2a. We did not find any significant difference in the virus titre between the MHV-68 lytic infection alone and PYNL + MHV-68 lytic infection groups.
Fig. 2.
Modulation in the growth of MHV-68 or PYNL upon dual infection. The figure shown the virus and parasite burdens in mice infected with the lytic stage of MHV-68 + PYNL (a and c) in the lungs and blood, respectively, or latent MHV-68 + PYNL (b and d) in the spleen and blood, respectively. (a) The virus titre in the lungs of mice infected with MHV-68 for 8 days, dual infected mice were infected with PYNL 2 days before MHV-68 infection (n = 4/group). (b) The latent virus burden in the spleens of mice infected with MHV-68 alone for 38 days or MHV-68 for 30 days followed by co-infection with PYNL for the final 8 days (n = 4/group). Data show the number of MHV-68 genomes per 300 ng spleen DNA as measured by quantitative PCR. Error bars show one s.d. (c and d). The levels of parasitaemia in mice (n = 4/group) carrying mixed infection with PYNL and MHV-68 (black bars) or in mice with PYNL infection (white bars) alone during lytic (c) or latent (d) MHV-68 infection. P-values were calculated using Student's t-test.
Replicating virus is not detectable in the lungs or spleens of latent MHV-68-infected mice; however, latent virus can be detected using molecular techniques. Therefore, we performed QF-PCR to measure the viral genome load in the spleens of MHV-68 latent + PYNL group. Interestingly, we observed significantly less viral DNA in the mice co-infected with MHV-68 latent + PYNL compared to mice with lone MHV-68 latent infection (P = 0·024, Fig. 2b).
We addressed the question of whether the level of parasitaemia would be affected in P. yoelii-infected mice due to dual infection with MHV-68. We found that the level of parasitaemia was significantly higher in mice with PYNL + MHV-68 lytic infection at day 4 post-parasite infection than in mice infected with PYNL alone (P = 0·02). This difference in parasitaemia between these two groups remained significant until day 7 post-parasite infection (P < 0·001). However, at day 10 of parasite infection, which is the time of peak parasitaemia, the level in the PYNL + MHV-68 lytic group became comparable to that in PYNL alone. These results are shown in Fig. 2c.
Interestingly, we observed a significant reduction in the level of parasitaemia in MHV-68 latent + PYNL mice compared to mice infected with PYNL alone. This reduction was apparent at day 4 post-parasite infection, which declined further when examined at day 7 post-infection (P = 0·02). Even at the peak of parasitaemia, i.e. at day 10 post-malarial infection, the difference in parasitaemia between MHV-68 latent + PYNL and PYNL alone remained significant (P = 0·002). These results are presented in Fig. 2d.
Mice carrying the lytic stage of MHV-68 infection and malaria develop severe lung inflammation
As the lung is the principal target site for MHV-68 replication during acute infection [16], we investigated whether pathology was induced at this site. Mice were sacrificed at day 10 post-parasite infection in the case of PYNL + MHV-68 lytic or at day 8 post-parasite infection in the MHV-68 latent + PYNL group; lung tissues were sectioned and stained with haematoxylin and eosin. Histological examination of lungs from the PYNL + MHV-68 lytic group revealed uniform thickening of the interalveolar septae with infiltration of macrophages, lymphocytes and numerous neutrophils with destruction of the alveolar wall in places. The degree of inflammation in this group was in the range of 3 (throughout the section) (Fig. 3a), whereas it was in the range of 1–2 in PYNL infection alone or the MHV-68 lytic infection alone group (Fig. 3b,c). Overall, in the PYNL + MHV-68 lytic group, neutrophil infiltration in the septal wall was strikingly more than in the MHV-68 lytic infection alone group. Haemozoin pigments were seen in the septae but were more numerous than in the PYNL alone infection group (Fig. 3a,b). Plasma cells, although rare, were present in the septal wall. These changes were consistent with the diagnosis of severe interstitial pneumonia. Higher numbers of megakaryocytes were seen in the septal wall (eight per 10 higher power field (HPF)) in the lung section from this group than in the PYNL alone group (three per 10 HPF). Of note, no megakaryocytes were noticeable in the lung section of MHV-68 lytic infection alone (Fig. 3c).
Fig. 3.
Histological changes in the lungs of Balb/c mice harbouring mixed infection with malaria and MHV-68. In the case of lytic MHV-68 + PYNL infection histological analysis was performed at day 8 post-virus infection (day 10 post-malaria infection). For latent MHV-68 + PYNL analysis was performed on day 38 post-virus infection (day 8 post-malaria infection). (a) Lytic MHV-68 + PYNL infection (H&E ×40), Right lower bottom: haemozoin pigment and megakaryocyte (H&E ×200 right upper; H&E ×400 right lower); (b) PYNL infection alone (H&E ×40 left), neutrophil in the septa, fewer numbers (H&E ×100) on the right. Inset: haemozoin pigment and megakaryocyte (H&E ×400); (c) lytic MHV-68 infection alone (H&E ×40), peribronchial (H&E ×400, right upper) and bronchial epithelial (H&E ×200, right lower) neutrophil infiltration but no megakaryocytes; (d) latent MHV-68 + PYNL infection (H&E ×40 on left), few neutrophils in the septae (H&E ×200 on right); (e) latent MHV-68 infection alone, little inflammation in the lung (H&E ×100). Sections from three mice were examined for each experimental group, and similar histological findings were present in all mice within a group.
Interestingly, lung inflammation in the MHV-68 latent + PYNL group was minimal (in the range of 1) (Fig. 3d). We observed patchy areas of alveolar septal wall thickening with infiltration by macrophages, lymphocytes and occasional neutrophils. The level of neutrophil infiltration was markedly less and much fewer haemozoin pigments were seen in this group than in the PYNL + MHV-68 lytic group (Fig. 3a). Occasional megakaryocytes were present in fewer numbers in the MHV-68 latent + PYNL group (two per 10 HPF) than in the PYNL + MHV-68 lytic group (eight per 10 HPF).
We did not find marked alterations in lung histology in animals infected with P. yoelii alone (Fig. 3b). Mice with MHV-68 latent infection alone did not present marked pathological findings (Fig. 3e). Areas of fresh haemorrhage seen in the lung section of this group were probably secondary to surgical procedure. No megakaryocytes were seen in the lungs of mice infected with MHV-68 alone, either during the lytic or latent stage of infection.
Cytokine responses in mice harbouring mixed infection of P. yoelii and MHV-68
Malarial parasites invade red blood cells and parasitized red blood cells circulate through different organs of the host. Among them, spleen is known to have an important role in protection against malaria by immunological mechanisms towards trapping infiltrated parasitized erythrocytes. We observed a marked inflammation in the lung during the interaction of acute stages of malaria and MHV-68. Having considered the above circumstances, we have investigated the cytokine response that is generated endogenously by sampling the tissues directly from these two organs by RNase protection assay (RPA). RPA permitted us to evaluate a number of cytokines that are produced simultaneously.
Acute MHV-68 infection
We measured the cytokine response in co-infected mice to investigate whether the increased lung pathology in dual infected mice correlated with an elevation in the levels of pro-inflammatory cytokines. Portions of lungs from these animals were collected and cytokine mRNA expression was measured by RNase protection assay (RPA). RPAs performed during the lytic stages of MHV-68 replication showed that dual infection resulted in higher levels of IFN-γ and IL-6 compared with either MHV-68 or PYNL infection alone. In addition, there were lower levels of MIF and IL-1Rα in dual infected mice compared with single infection alone (Fig. 4a), although the latter was not statistically significant.
Fig. 4.
Cytokine mRNA expression in the lungs (a, lytic infection) or spleens (b, latent infection) of P. yoelii malaria and MHV-68 dual infected mice and in mice with single infection with P. yoelii or MHV-68 (n = 3/group). The expression of the indicated cytokines was determined by RNase protection assay. mRNA expression was standardized to the expression of the housekeeping genes (L32/GAPDH) and are expressed in relative densitometry values. Black bars: PYNL alone; grey bars: MHV-68 alone. White bars: dual infection. Data are representative of two independent experiments with similar results. Values are mean ± s.d. from three separate measurements. **P < 0·01 and *P < 0·05 in dually infected mice, compared with the mice with single PYNL or MHV-68 infection.
Latent MHV-68 infection
Cytokine mRNAs in the samples of spleens were measured by RPA. The levels of expression of IL-10, IL-1Ra, IFN-γ and MIF was augmented significantly in mixed infection with the latent stages of MHV-68 and PYNL compared with the MHV-68 latent infection alone. Furthermore, the response of IL-12p35, IL-12p40 and IL-1β was significantly lower in dual infection than in MHV-68 latent infection alone. With regard to the expression of other cytokines such as IL-1α, IL-6 and IL-18 there was no discriminating pattern that emerged between the single infection with latent MHV-68 and the dual infection with latent MHV-68 and PYNL (Fig. 4b).
Impact of P. yoelii malaria on the MHV-68-specific CD8+ T cell response
It has been well documented that CD8+ T cells are important in controlling MHV-68 during both lytic and latent stages of the infection [20,23,24]. Therefore, we wished to determine the effect of malaria co-infection on the MHV-68-specific CD8+ T cell response.
Mice that received PYNL and were infected with MHV-68 2 days later were killed at day 8 post-virus infection. Splenocytes were harvested and labelled with CFSE then restimulated for 4 days in the presence of M2 or ORF65 peptides. Staining was performed on restimulated cultures rather than freshly isolated cells in these experiments because at 8 days post-infection the virus-specific CD8 T cell responses are relatively small [23], so we needed to expand the virus-specific CD8 T cells before detection.
Cells were then stained with MHC/peptide tetrameric reagents loaded with epitopes recognized by virus-specific CD8 T cells [25,26] and anti-CD8 antibody. As expected, we did not find detectable responses to the latent cycle M2 peptide in the MHV-68-infected mice because reactivity to the M2 epitope arises only 14–19 days post-infection. However, we did observe cells staining with a tetramer loaded with an epitope recognized in the lytic phase of the response (Db/ORF65131−140). These cells represented 0·37–0·14% of the CD8 T cell population in mice infected with MHV-68 alone (Table 1). Interestingly, these cells were reduced by approximately threefold in mice with PYNL + MHV-68 lytic infection (Table 1). This indicates that dual infection with PYNL and MHV-68 had a suppressive effect on the virus-specific CD8 T cell response.
Table 1.
Co-infected mice mount a weaker CD8 T cell response to MHV-68 antigens than mice infected with MHV-68 alone
| Samplea | % ORF65131-140/Dd+ cellsb | % M291-99/Kd cellsc |
|---|---|---|
| MHV-68 #1 | 0·37 | 0·02 |
| MHV-68 #2 | 0·17 | 0·04 |
| MHV-68 #3 | 0·14 | 0·03 |
| PYNL + MHV-68 #1 | 0·07 | 0·03 |
| PYNL + MHV-68 #2 | 0·06 | 0·08 |
BALB/c mice were infected with P. yoelii malaria parasites i.p., then infected i.n. with 400PFU MHV-68 2 days later. Mice were sacrificed 8 days following virus infection. A second group of mice received MHV-68 alone.
CFSE-labelled spleen cells cultures were restimulated in vitro with peptides as described. For analysis, samples were stained with the tetramers indicated plus anti-CD8 antibody. Figures show the percentage of live, tetramer + CFSElo cells a s a proportion of total CD8 cells.
The ORF65131-140/Dd epitope derives from a lytic phase protein and the M291-99/Kd epitope from a latent phase protein.
We then tested the effect of PYNL infection on the antiviral CD8 T cell response in mice latently infected with MHV-68. As these mice had high levels of pre-existing memory T cells to MHV-68 we performed tetramer staining directly on splenocytes from infected mice. As shown in Table 2, the proportion of M291−99/Kd-specific CD8 T cells was similar in both MHV-68 only and MHV-68 latent + PYNL groups. Responses to the ORF65131−140/Dd epitope were lower than the M2-specific response; however, both groups of mice also had comparable responses to this epitope. Therefore, PYNL infection did not appear to alter the levels of virus-specific memory CD8 T cells in latently infected mice.
Table 2.
The CD8 T cell response in latently infected mice is unaffected by malaria co-infection
| Samplea | % ORF65131-140/Dd+ cellsb | % M291-99/Kd+ cells |
|---|---|---|
| MHV-68 #1 | 0·5 | 1·2 |
| MHV-68 #2 | 0·3 | 1·6 |
| MHV-68 #3 | 0·2 | 1·0 |
| MHV-68 #4 | 0·4 | 0·8 |
| MHV-68 + PYNL #1 | 0·7 | 0·9 |
| MHV-68 + PYNL #2 | 0·2 | 1·1 |
| MHV-68 + PYNL #3 | 0·5 | 1·1 |
| MHV-68 + PYNL #4 | 0·7 | 0·7 |
Mice were infected with MHV-68 i.n. then 30 days later half the mice received P. yoelii malaria parasites i.p. Ten days after malaria infection the spleens were removed and the virus-specific CD8 T cell populations measured by staining with MHC/peptide tetramers and anti-CD8 antibody.
Figures show the percentage of tetramer+ cells as a proportion of total CD8 cells.
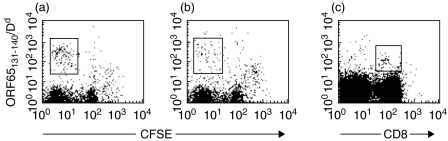
To validate enumeration of antigen-specific CD8s through tetramer staining, representative staining on restimulated and primary spleen cells is shown in Fig. 5.
Fig. 5.
Representative tetramer staining on restimulated and primary spleen cells. Representative staining on spleen cells from mice infected for 8 days with MHV-68 alone (a) or PYNL followed by MHV-68 (b) restimulated in vitro with ORF65131−140 peptide for 4 days then stained with the indicated tetramer plus anti-CD8 antibody. Representative staining with this tetramer is also shown from a mouse latently infected with MHV-68 (c); freshly isolated spleen cells were stained with tetramer and anti-CD8 antibody. The boxes represent the regions we considered positive for tetramer staining.
By assessing percentage ORF65131−140/Dd+ cells we noticed a distinct pattern in CD8+ T cell response between mixed infection and MHV-68 alone infection. Although CD8 T cell response peaked about 10 days post-infection in mice infected with MHV-68 alone, we could not use the day 10 time-point in the current study. We had to sample at day 8 after the virus infection due to the mortality of dual infected (PYNL + lytic MHV-68) mice. By this time post-infection there are few lymphocytes in the bronchial alveolar lavage (BAL) and the lymph nodes are very small (data not shown). Therefore, in order to obtain enough cells for these studies we chose to study the response in the spleen.
DISCUSSION
In this study, we describe the outcome of interactions between the malarial parasite and a γ-herpesvirus in a murine model of co-infection. Mammalian hosts are often exposed simultaneously to multiple infectious agents in nature. Interacting microorganisms may induce heterologous cross-protection or modulate the immune response to either pathogen leading to enhanced pathogenesis [27]. The interactions between a γ-herpesvirus and the malaria parasite are of particular interest, as it has been proposed that the combination of a massive early infection with EBV followed by depression of the cellular immune response in malaria infection can lead to the development of Burkitt's lymphoma [2].
In the current study we have concentrated on two timings of modelling, which allowed us to examine the interactions between (1) the acute stage of malaria and lytic MHV-68 and (2) latent MHV-68 and acute malaria. We recognize the importance of other timings of modelling, and studies are under way in our laboratory to understand the mechanisms of interaction between these pathogens under the conditions of different sequences of infection.
We did not observe Burkitt's lymphoma-like conditions in our experiments; however, there was a marked exacerbation in disease when mice were undergoing an acute infection with both agents. In mice co-infected with PYNL and MHV-68 lytic stage we observed about 70% mortality, although neither of these infections is normally fatal. In the surviving animals that became moribund, we did not find a significant increase in virus titre compared to MHV-68 lytic infection alone. We observed a significant augmentation in parasitaemia in the early stages of the dual infection compared to PYNL infection alone, which then reached normal levels at the time animals were sacrificed for virus titre determination. These results suggest that mortality/enhanced disease in the PYNL + MHV-68 co-infected mice is not due to increased viral replication or parasitaemia. We hypothesized that immune pathology induced by the simultaneous infection with two different agents may have been responsible. Therefore, we addressed the question of whether the enhanced disease seen in the PYNL + MHV-68 lytic group correlated with increased inflammation in the lung, which is the principal target organ for murine γ-herpesvirus infection during the acute stage after intranasal administration [16]. Histological analysis demonstrated clearly a marked infiltration of inflammatory cells including macrophages, lymphocytes and numerous neutrophils in the lungs during PYNL + MHV-68 lytic infection, which in places resulted in the destruction of the alveolar wall. The lungs displayed signs of severe interstitial pneumonia, with large numbers of neutrophils and also numerous megakaryocytes. Malaria pigment-carrying neutrophilic granulocytes can be detected frequently in the peripheral blood of malaria patients [28]. It is possible that the large quantity of haemozoin (malaria pigment) seen in the lungs of PYNL + MHV-68 lytic group could mediate recruitment of neutrophils [29]. Megakaryocytic cells mature adjacent to bone marrow sinus walls and subsequently release platelets within the sinusoidal space or in lung capillaries [30]. The presence of large number of megakaryocytes in the interstitium of the lung in dual infection reflects thrombocytopenia and could correlate with lung injury. Malaria has been reported to induce haematopoeitic crisis, which is often accompanied by thrombocytopenia [31]. We postulate that the combined infiltration of polymorphonuclear neutrophils and megakaryocytes could be involved in the rapid development of severe interstitial pneumonic disease in mice with PYNL + MHV-68 lytic infection.
We investigated the expression of cytokines in the lung to test whether the response of some of them would be associated with the enhanced inflammation that we observed by histological analysis in the lungs of PYNL + MHV-68 lytic co-infected mice. Our results demonstrate clearly that the aggressive lung inflammation in co-infected mice is associated with an increased expression of IFN-γ and IL-6, proinflammatory cytokines. Concurrent augmentation of IFN-γ and IL−6 and their association with severe malaria was suggested in human malaria [32]. IL-6 secretion can also participate in increased inflammation response in dual infection by mediating increased megakaryocytosis and mediating their infiltration in lung interstitium [33]. This, together with histological evidence suggests strongly that immune pathology in the lung is responsible for the mortality and morbidity observed during the acute infection with PYNL and MHV-68. As yet the reason why co-infection with these two agents induces this pathology is unknown. One possibility is that there is immune cross-reactivity between the two agents, and further investigation is needed to determine if this is the case.
Our results demonstrate that parasitaemia was diminished in the early phases of dual infection, but soon reached the level found in PYNL infection alone. We and others have shown previously that an early IFN-γ response is implicated in the reduction of parasitaemia [34,35]. Similar IFN-γ-induced pathways may be involved in controlling parasitaemia in the mixed infection.
In contrast, the MHV-68 latent + PYNL-infected mice did not develop severe respiratory disease but had markedly reduced viral load and parasitaemia. Because during the latent stage of infection MHV-68 predominantly resides in the spleen, we investigated whether protection manifested by diminished viral titre in spleen is associated with a particular pattern of cytokine response in this organ. Our results demonstrate that protection correlated with higher responses of IL-10, IL-1Ra and moderately higher expression of IFN-γ. As discussed later, dual infection of latent MHV-68 mice had no effect on the CD8 T cell response to the virus. Therefore it is possible that the enhanced levels of IFN-γ in dual infected mice lowered the viral load through a bystander effect. IFN-γ levels are known to be elevated during latent infection, although not to the levels seen in the acute infection [36]. The role of IFN-γ in MHV-68 infection is controversial. One report found that mice deficient in IFN-γ controlled the virus to the same degree as wild-type mice [37]. However, other investigators report that IFN-γ has an important role in the control of the latent infection [24,38]. It is therefore interesting that this cytokine may be involved both in the induction of pathology in the lung during lytic MHV-68 + PYNL infection but relative protection in the spleen during latent infection.
An augmented response of IL-10 and IL-1Ra may be involved in reducing aggressive inflammation. It was postulated that moderately high IFN-γ along with an augmented IL-10 response might have a protective role against severe malaria in humans [39]. Our results also indicate that this pattern of combined IFN-γ and IL-10 response could play a role in the host's defence against the erythrocytic stages of malarial parasite. These cytokines are normally considered to be mutually antagonistic. However, the relationship between these cytokines is likely to be dependent upon their relative amounts, so this may explain our detection of both within the same organ. Of note, the response of IL-12p35, IL-12p40 and IL-1β was significantly lower in the spleens of mice with mixed infection than in animals with MHV-68 latent infection alone. These cytokines are known to have proinflammatory activity and thus their diminished response may contribute to protection against aggravated pathogenesis.
In MHV-68 infection, CD8 T cells against both lytic and latent antigens are important in the control of the infection [23]. We addressed whether co-infecting with malarial parasites could modulate the virus-specific CD8 T cell response in the dual infection. By using MHC/peptide tetramer staining we found that the CD8 T cell response was diminished in mice undergoing lytic infection by MHV-68 and malaria but not in mice harbouring latent MHV-68 infection. Despite this diminished MHV-68-specific CD8 T cell response, viral replication was not increased in PYNL + MHV-68 lytic infection. This indicates that despite this decrease there were still sufficient virus-specific CD8 T cells remaining to control the virus infection. Alternatively, other mechanisms may have controlled the virus infection, such as CD4 T cells. These cells are known to play a role in the control of MHV-68 infection, and there are reports showing control the virus by CD4 T cells in the absence of CD8 T cells [40].
No difference in the virus-specific CD8 T cell response was observed in latent MHV-68 + PYNL mice. During viral latency the virus-specific CD8 T cells can be considered memory T cells. In contrast, in the lytic MHV-68 + PYNL group there was an evolving primary CD8 T cell response. Therefore PYNL infection appears to inhibit the initial activation of virus-specific CD8 T cells but not affect established memory CD8 T cells.
Interestingly, we observed a significant reduction in both the parasitaemia and level of latent virus in this group of mice. As discussed earlier, this may be due to a bystander effect involving IFN-γ. It is also possible that this malaria-induced heterologous protection is due to the cytokine-mediated NO response [10].
In conclusion, we have shown that the outcome of simultaneous infection with malaria and a murine γ-herpesvirus depends on the sequence of infection. If the parasite is present during the acute stage of virus replication then there is extensive immune pathology in the lungs, resulting in considerable morbidity or mortality. However, if the parasite is present after viral latency has been established then there is no such pathology; indeed, this leads to a lower burden of both parasite and virus. Our findings offer some insight into why some people manifest disease while others acquire immunity, despite living in the same endemic area.
Acknowledgments
A. Haque was supported by CNRS (Centre National de la Recherche Scientifique). This work is supported partially by NIH grants AI13000 (L.H.K) and AI51663 (E.U).
REFERENCES
- 1.Stiller CA, Parkin DM. Geographic and ethnic variations in the incidence of childhood cancer. Br Med Bull. 1996;52:682–703. doi: 10.1093/oxfordjournals.bmb.a011577. [DOI] [PubMed] [Google Scholar]
- 2.de The G. The etiology of Burkitt's lymphoma and the history of the shaken dogmas. Blood Cells. 1993;19:667–73. [PubMed] [Google Scholar]
- 3.de The G. Co-carcinogenic events in herpesvirus oncogenesis: a review. IARC Sci Publ. 1978:933–45. [PubMed] [Google Scholar]
- 4.Virgin HW, Speck SH. Unraveling immunity to gamma-herpesviruses: a new model for understanding the role of immunity in chronic virus infection. Curr Opin Immunol. 1999;11:371–9. doi: 10.1016/s0952-7915(99)80063-6. [DOI] [PubMed] [Google Scholar]
- 5.Nash AA, Dutia BM, Stewart JP, Davison AJ. Natural history of murine gammaherpesvirus infection. Philos Trans R Soc Lond B Biol Sci. 2001;356:569–79. doi: 10.1098/rstb.2000.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty PC, Christensen JP, Belz GT, Stevenson PG, Sangster MY. Dissecting the host response to a gamma-herpesvirus. Philos Trans R Soc Lond B Biol Sci. 2001;356:581–93. doi: 10.1098/rstb.2000.0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang R, Charoenvit Y, Daly TM, Long CA, Corradin G, Hoffman SL. Protective efficacy against malaria of a combination sporozoite and erythrocytic stage vaccine. Immunol Lett. 1996;53:83–93. doi: 10.1016/s0165-2478(96)02610-7. [DOI] [PubMed] [Google Scholar]
- 8.Kang Y, Calvo PA, Daly TM, Long CA. Comparison of humoral immune responses elicited by DNA and protein vaccines based on merozoite surface protein-1 from Plasmodium yoelii, a rodent malaria parasite. J Immunol. 1998;161:4211–9. [PubMed] [Google Scholar]
- 9.Brahimi K, Badell E, Sauzet JP, et al. Human antibodies against Plasmodium falciparum liver-stage antigen 3 cross-react with Plasmodium yoelii preerythrocytic-stage epitopes and inhibit sporozoite invasion in vitro and in vivo. Infect Immun. 2001;69:3845–52. doi: 10.1128/IAI.69.6.3845-3852.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasquetto V, Guidotti LG, Kakimi K, Tsuji M, Chisari FV. Host-virus interactions during malaria infection in hepatitis B virus transgenic mice. J Exp Med. 2000;192:529–36. doi: 10.1084/jem.192.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freitag C, Chougnet C, Schito M, et al. Malaria infection induces virus expression in human immunodeficiency virus transgenic mice by CD4 T cell-dependent immune activation. J Infect Dis. 2001;183:1260–8. doi: 10.1086/319686. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman IF, Jere CS, Taylor TE, et al. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. Aids. 1999;13:487–94. doi: 10.1097/00002030-199903110-00007. [DOI] [PubMed] [Google Scholar]
- 13.Bloland PB, Wirima JJ, Steketee RW, Chilima B, Hightower A, Breman JG. Maternal HIV infection and infant mortality in Malawi: evidence for increased mortality due to placental malaria infection. Aids. 1995;9:721–6. doi: 10.1097/00002030-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Steketee RW, Wirima JJ, Bloland PB, et al. Impairment of a pregnant woman's acquired ability to limit Plasmodium falciparum by infection with human immunodeficiency virus type-1. Am J Trop Med Hyg. 1996;55:42–9. doi: 10.4269/ajtmh.1996.55.42. [DOI] [PubMed] [Google Scholar]
- 15.Xiao L, Owen SM, Rudolph DL, Lal RB, Lal AA. Plasmodium falciparum antigen-induced human immunodeficiency virus type 1 replication is mediated through induction of tumor necrosis factor-alpha. J Infect Dis. 1998;177:437–45. doi: 10.1086/514212. [DOI] [PubMed] [Google Scholar]
- 16.Sunil-Chandra NP, Efstathiou S, Arno J, Nash AA. Virological and pathological features of mice infected with murine gamma-herpesvirus 68. J Gen Virol. 1992;73:2347–56. doi: 10.1099/0022-1317-73-9-2347. [DOI] [PubMed] [Google Scholar]
- 17.Usherwood EJ, Ward KA, Blackman MA, Stewart JP, Woodland DL. Latent antigen vaccination in a model gammaherpesvirus infection. J Virol. 2001;75:8283–8. doi: 10.1128/JVI.75.17.8283-8288.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988;41:467–70. doi: 10.1136/jcp.41.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kappler JW, Skidmore B, White J, Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981;153:1198–214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Usherwood EJ, Roy DJ, Ward K, et al. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T cells. J Exp Med. 2000;192:943–52. doi: 10.1084/jem.192.7.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altman JD, Moss PH, Goulder PR, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [PubMed] [Google Scholar]
- 22.Lucas B, Kasper LH, Smith K, Haque A. In vivo treatment with interleukin 2 reduces parasitemia and restores IFN-gamma gene expression and T-cell proliferation during acute murine malaria. C R Acad Sci III. 1996;319:705–10. [PubMed] [Google Scholar]
- 23.Stevenson PG, Belz GT, Altman JD, Doherty PC. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur J Immunol. 1999;29:1059–67. doi: 10.1002/(SICI)1521-4141(199904)29:04<1059::AID-IMMU1059>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 24.Tibbetts SA, van Dyk LF, Speck SH, Virgin HW. Immune control of the number and reactivation phenotype of cells latently infected with a gammaherpesvirus. J Virol. 2002;76:7125–32. doi: 10.1128/JVI.76.14.7125-7132.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Usherwood EJ. A new approach to epitope confirmation by sampling effector/memory T cells migrating to the lung. J Immunol Methods. 2002;266:135–42. doi: 10.1016/s0022-1759(02)00106-0. [DOI] [PubMed] [Google Scholar]
- 26.Husain SM, Usherwood EJ, Dyson H, et al. Murine gammaherpesvirus M2 gene is latency-associated and its protein a target for CD8(+) T lymphocytes. Proc Natl Acad Sci U S A. 1999;96:7508–13. doi: 10.1073/pnas.96.13.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002;2:417–26. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 28.Kramer B, Grobusch MP, Suttorp N, Neukammer J, Rinneberg H. Relative frequency of malaria pigment-carrying monocytes of nonimmune and semi-immune patients from flow cytometric depolarized side scatter. Cytometry. 2001;45:133–40. doi: 10.1002/1097-0320(20011001)45:2<133::aid-cyto1155>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 29.Graca-Souza AV, Arruda MA, de Freitas MS, Barja-Fidalgo C, Oliveira PL. Neutrophil activation by heme: implications for inflammatory processes. Blood. 2002;99:4160–5. doi: 10.1182/blood.v99.11.4160. [DOI] [PubMed] [Google Scholar]
- 30.Zucker-Franklin D, Philipp CS. Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am J Pathol. 2000;157:69–74. doi: 10.1016/S0002-9440(10)64518-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moerman F, Colebunders B, D’Alessandro U. Thrombocytopenia in African children can predict the severity of malaria caused by Plasmodium falciparum and the prognosis of the disease. Am J Trop Med Hyg. 2003;68:379. author reply 380–1. [PubMed] [Google Scholar]
- 32.Gourley IS, Kurtis JD, Kamoun M, Amon JJ, Duffy PE. Profound bias in interferon-gamma and interleukin-6 allele frequencies in western Kenya, where severe malarial anemia is common in children. J Infect Dis. 2002;186:1007–12. doi: 10.1086/342947. [DOI] [PubMed] [Google Scholar]
- 33.Kirito K, Osawa M, Morita H, et al. A functional role of Stat3 in in vivo megakaryopoiesis. Blood. 2002;99:3220–7. doi: 10.1182/blood.v99.9.3220. [DOI] [PubMed] [Google Scholar]
- 34.De Souza JB, Williamson KH, Otani T, Playfair JH. Early gamma interferon responses in lethal and nonlethal murine blood-stage malaria. Infect Immun. 1997;65:1593–8. doi: 10.1128/iai.65.5.1593-1598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haque A, Graille M, Kasper LH, Haque S. Immunization with heat-killed Toxoplasma gondii stimulates an early IFN-gamma response and induces protection against virulent murine malaria. Vaccine. 1999;17:2604–11. doi: 10.1016/s0264-410x(99)00050-x. [DOI] [PubMed] [Google Scholar]
- 36.Sarawar SR, Cardin RD, Brooks JW, Mehrpooya M, Tripp RA, Doherty PC. Cytokine production in the immune response to murine gammaherpesvirus 68. J Virol. 1996;70:3264–8. doi: 10.1128/jvi.70.5.3264-3268.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarawar SR, Cardin RD, Brooks JW, et al. Gamma interferon is not essential for recovery from acute infection with murine gammaherpesvirus 68. J Virol. 1997;71:3916–21. doi: 10.1128/jvi.71.5.3916-3921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christensen JP, Cardin RD, Branum KC, Doherty PC. CD4(+) T cell-mediated control of a gamma-herpesvirus in B cell-deficient mice is mediated by IFN-gamma. Proc Natl Acad Sci U S A. 1999;96:5135–40. doi: 10.1073/pnas.96.9.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhee MS, Akanmori BD, Waterfall M, Riley EM. Changes in cytokine production associated with acquired immunity to Plasmodium falciparum malaria. Clin Exp Immunol. 2001;126:503–10. doi: 10.1046/j.1365-2249.2001.01681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stevenson PG, Cardin RD, Christensen JP, Doherty PC. Immunological control of a murine gammaherpesvirus independent of CD8+ T cells. J Gen Virol. 1999;80:477–83. doi: 10.1099/0022-1317-80-2-477. [DOI] [PubMed] [Google Scholar]