Abstract
In the present study, we elucidated the effect of synthetic CpG-containing oligodeoxynucleotides (ODN) on pulmonary and disseminated infection caused by Cryptococcus neoformans. CDF-1 mice were inoculated intratracheally with a highly virulent strain of this pathogen, which resulted in massive bacterial growth in the lung, dissemination to the brain and death. Administration of CpG-ODN promoted the clearance of C. neoformans in the lungs, decreased their dissemination to brain and prolonged the survival of infected mice. These effects correlated well with the enhanced production of interleukin (IL)-12 and interferon (IFN)-γ and attenuated secretion of IL-4 in bronchoalveolar lavage fluids (BALF) and promoted development of Th1 cells, as indicated by the increased production of IFN-γ by paratracheal lymph node cells upon restimulation with cryptococcal antigens. The IFN-γ synthesis in BALF was inhibited by depletion of CD8+ and CD4+ T cells on days 7 and 14 after infection, respectively, but not by depletion of NK and γδ T cells. Consistent with these data, intracellular expression of IFN-γ was detected predominantly in CD8+ and CD4+ T cells in the lung on days 7 and 14, respectively. The protective effect of CpG-ODN, as shown by the prolonged survival, was completely and partially inhibited by depletion of CD4+ or CD8+ T cells, respectively, but not by depletion of other cells. Finally, TNF-α was markedly induced by CpG-ODN, and the protective effect of this agent was strongly inhibited by neutralizing anti-TNF-α MoAb. Our results indicate that CpG-ODN alters the Th1–Th2 cytokine balance and promotes host resistance against infection with C. neoformans.
Keywords: CpG-DNA, Cryptococcus neoformans, lung, Th1–Th2 balance
Introduction
Cryptococcus neoformans, a ubiquitous fungal pathogen, infects via an airborne route and causes a life-threatening infectious disease in the central nervous system in hosts with severely compromised immune responses, such as patients with acquired immunodeficiency syndrome [1]. Meningoencephalitis caused by this pathogen is often refractory to chemotherapy under these conditions, and development of a novel immune-based strategy is required. The host defence against C. neoformans is mediated largely by cellular immune responses [2], in which type-1 helper T (Th1) cells act as a critical population by producing interferon (IFN)-γ, while Th2 cells play a negative regulatory role [3]. Recent studies found that mice with a genetic disruption of Th1-related cytokines, such as IFN-γ, interleukin (IL)-12, IL-18 and tumour necrosis factor (TNF)-α, are highly susceptible to cryptococcal infection [4–8], while the infection was less severe in mice that did not synthesize Th2 cytokines, IL-4 and IL-10 [4,9]. Consistent with these observations, administration of IFN-γ, IL-12, IL-18 and TNF-α helps in the protection against infections caused by C. neoformans [10–13].
In earlier studies, it was found that purified deoxynucleotides (DNA) from Mycobacterium bovis bacille Calmette–Guérin (BCG) possessed immune stimulatory effects, including the activation of natural killer (NK) cells and production of type-1 and type-2 IFN in vitro and the promotion of tumour regression in vivo [14–16]. Other investigators demonstrated that purified bacterial DNA induced B cell proliferation and immunoglobulin secretion, while vertebrate DNA did not [17]. Although the mechanisms of these effects had not been understood, Krieg and coworkers discovered that it was ascribed to an unmethylated CpG motif [18,19]. The oligo-DNA (ODN) containing this motif activate murine dendritic cells (DC) to produce IL-12 and expression of co-stimulatory molecules such as CD40, which results in the development of a pattern of Th1-like immune activation [20–22]. Indeed, in vivo injections of CpG-ODN induced systemic or local Th1-biased immune responses, including the synthesis of IL-12 and IFN-γ [23–25].
Based on the immune stimulatory activities, many investigations have addressed the therapeutic application of CpG-ODN in infections, malignancies and allergic diseases [19]. Administration of this agent was found to protect mice from infections by intracellular microbial pathogens, including Listeria monocytogenes [25], Francisella tularensis [26], Leishmania major [27,28 ] and Plasmodium yoelii [29]. In the present study, we examined the effect of CpG-ODN on the clinical course of infection caused by C. neoformans and the protective immune responses against this fungal pathogen. We show here that the beneficial effects of this treatment in protecting mice are related to the promotion of antigen-specific Th1-biased immune responses rather than the activation of innate immune lymphocytes, such as NK cells and γδ T cells.
Materials and methods
Mice
CDF-1 mice were purchased from Charles River Breeding Laboratories (Osaka, Japan) and used at 8–15 weeks of age. These mice were bred in a pathogen-free environment in the Laboratory Animal Center for Biomedical Science, University of the Ryukyus. All experimental protocols described in the present study were approved by the Ethics Review Committee for Animal Experimentation of our university.
Microorganisms
A serotype A-encapsulated strain of C. neoformans, designated as YC-11, was recovered from a patient with pulmonary cryptococcosis. We established a mouse model of pulmonary cryptococcosis by directly instilling the yeast cells through the trachea, because in most cases this pathogen is acquired by inhalation. In this model, the infection was fatal with dissemination to the central nervous system [11]. The yeast cells were cultured on potato dextrose agar (PDA) plates for 2–3 days before use. Mice were anaesthetized by intraperitoneal injection of 70 mg/kg of pentobarbital (Abbott Laboratory, North Chicago, IL, USA) and restrained on a small board. Live C. neoformans (1 × 105 cells) were inoculated in 50 µl per mouse by insertion of a 25-gauge blunt needle into and parallel to the trachea.
CpG- and CNT-ODN
All ODN were synthesized at Hokkaido System Science (Sapporo, Japan). The sequence of CpG-ODN was TCC ATG ACG TTC CTG ACG TT, and that of the control (CNT)-ODN was similar, except that the CpG motif (underlined) was replaced with GpC (TCC ATG AGC TTC CTG AGC TT). All ODN were phosphorothioated and purified by HPLC. The endotoxin content measured by Limulus amoebocyte lysate assay was less than 10 pg/ml.
Enumeration of viable C. neoformans
Mice were sacrificed 3 weeks after infection and lungs and brains were dissected carefully and excised, then homogenized separately in 10 and 2 ml of distilled water, respectively, by teasing with a stainless mesh at room temperature. The homogenates, diluted appropriately with distilled water, were inoculated at 100 µl on PDA plates, cultured for 2–3 days followed by counting the number of colonies.
Preparation of BALF
Mice were sacrificed on days 3, 7 and 14 after infection and samples of bronchoalveolar lavage fluid (BALF) were collected as described below. Briefly, after bleeding under anaesthesia with ether, the chest was opened and the trachea was cannulated with the outer sheath of 24G intravenous catheter/needle unit (BD Vascular Access, Sandy, UT, USA), followed by lavage of the lung twice with 0·5 ml of chilled normal saline.
In vitro stimulation of lymph node cells
Paratracheal lymph node (LN) cells were prepared from four mice on day 14 after infection with C. neoformans and cultured at 2 × 106/ml in flat-bottomed culture plates (Falcon no. 3047, Becton Dickinson, Franklin Lakes, NJ, USA) with various doses of viable organisms or purified protein derivatives (PPD: purchased from Nihon BCG Co., Tokyo, Japan) for 48 h. The culture supernatants were collected and kept at −70°C before use.
Measurement of cytokines
Murine IL-12p40, IFN-γ, IL-4 and TNF-α were measured by enzyme-linked immunosorbent assay (ELISA) kits (BioSource International, Inc., Camarillo, CA, USA for IL-12p40; Endogen, Inc., Cambridge, MA, USA for IFN-γ and IL-4; R&D Systems, Inc., Minneapolis, MN, USA for TNF-α). The detection limits of assays for IL-12p40, IFN-γ, IL-4 and TNF-α were 2, 15, 5 and 5·1 pg/ml, respectively.
Preparation of pulmonary intraparenchymal leucocytes
Pulmonary intraparenchymal leucocytes were prepared as described previously [30]. Briefly, the chest of the mouse was opened, and the lung vascular bed was flushed by injecting 3 ml of chilled physiological saline into the right ventricle. The lungs were then excised and washed in physiological saline. The lungs, teased with a stainless steel mesh, were incubated in RPMI1640 (Gibco BRL: Grand Island, NY, USA) with 5% of fetal calf serum (FCS, Cansera: Rexdale, Ontario, Canada), 100 U/ml penicillin G, 100 µg/ml streptomycin, 10 m m 4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (HEPES), 50 µm 2-mercaptoethanol, and 2 mm l-glutamine, containing 20 U/ml collagenase (Sigma Chemical Co., St Louis, MO, USA) and 1 µg/ml DNaseI (Sigma). After incubation for 60 min at 37°C with vigorous shaking, the tissue fragments and the majority of dead cells were removed by passing through the 50 µm-nylon mesh. After centrifugation, the cell pellet was resuspended in 4 ml of 40% (v/v) Percoll (Pharmacia, Uppsala, Sweden) and layered onto 4 ml of 80% (v/v) Percoll. After centrifugation at 600 g for 20 min at 15°C, the cells at the interface were collected, washed three times and counted with a haemocytometer. The obtained cells contained lymphocytes, macrophages and neutrophils.
Analysis of intracellular IFN-γ expression
The lung leucocytes were cultured at 1 × 106/ml with 5 ng/ml phorbol 12-myristate 13-acetate (PMA), 500 ng/ml ionomycin and 2 m m monencin (Sigma) in RPMI-1640 medium supplemented with 10% FCS for 4 h. The cells were washed three times in phosphate-buffered saline (PBS) containing 1% FCS and 0·1% sodium azide and then stained with phycoerythrin (PE)-conjugated anti-CD4 or -CD8 MoAb (clone GK1·5 or 53–6·7, respectively; BD Pharmingen, San Diego, CA, USA). After washing twice, the cells were incubated in the presence of cytofix/cytoperm (BD Biosciences, San Jose, CA, USA), washed twice in BD perm/wash solution and stained with fluorescein isothiocyanate (FITC)-conjugated anti-IFN-γ MoAb (clone XMG1·2, BD Pharmingen) or control IgG (clone R3-34, BD Pharmingen). The stained cells were analysed using an EPICS XL flow cytometer (Beckman Coulter, Inc., Fullerton, CA, USA). Data were collected from 15 000–20 000 individual cells using parameters of forward-scatter and side-scatter to set a gate on lymphocyte population.
Antibodies
Monoclonal anti-T cell receptor (TCR)-γδ (hamster IgG), -CD4, -CD8 and -TNF-α (rat IgG) antibodies were purified by using a protein G column kit (Kirkegaard & Perry Laboratory, Gaithersburg, MD, USA) from the culture supernatants of hybridomas (clone UC7–13D5, GK1·5, 53–6·72 and MP6-XT2·2–11, respectively; ATCC, Manassas, VA, USA). Asialo GM1 (ASGM1) antibody was purchased from Wako Pure Chemical Industries (Osaka, Japan). To delete NK, γδ T, CD4+ or CD8+ cells, mice were injected intraperitoneally with anti-ASGM1 antibody at 200 µg or -TCR-γδ, -CD4 or -CD8 MoAb at 400 µg on days −3, 0, + 3, + 7 and + 14 after infection. Rabbit IgG (Wako Pure Chemical Industries), hamster IgG (Organon Teknika Co., Durham, NC, USA) and rat IgG (ICN Pharmaceuticals, Inc., Aurora, OH, USA) were used as the control antibodies. We confirmed that treatment with each antibody greatly reduced the specific cell population in lung intraparenchymal leucocytes when lymphocyte populations were gated on the forward- and side-scatter profiles in a flow cytometric analysis: 22·5% to 1·1% in CD4+ T cells; 9·1% to 0·7% in CD8+ T cells; 1·6% to 0·2% in γδ T cells; and 18·2% to 0·9% in ASGM1+ cells. To block endogenously synthesized TNF-α, mice were injected intraperitoneally with MoAb at 400 µg on days −1, 0, + 3 and every 7 days post-infection. Rat IgG (ICN Pharmaceuticals, Inc.) was used as a control antibody. This MoAb completely neutralized the cytotoxic activity against L929 cells of 0·1 ng/ml recombinant murine TNF-α at 0·78 µg/ml.
Statistical analysis
Data were analysed using Statview II software (Abacus Concept, Inc., Berkeley, CA, USA) on a Macintosh computer. Data are expressed as mean ± standard deviation (s.d.). Differences between groups were examined for statistical significance using one-way analysis of variance (anova) with a post-hoc analysis [Fisher's partial least squares difference (PLSD) test]. Survival data were analysed using the generalized Wilcoxon test. A P-value less than 0·05 was considered significant.
Results
Effect of CpG-ODN on the host defence to cryptococcal infection
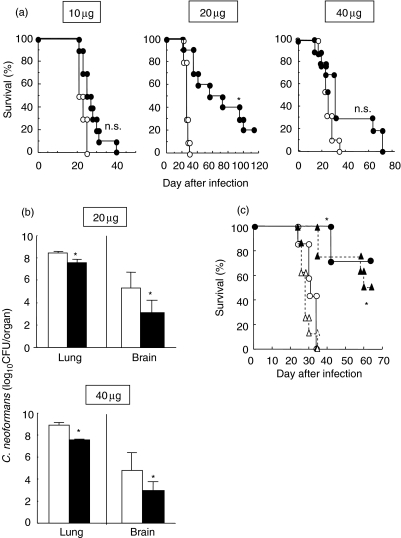
Initially, we elucidated the effects of CpG-ODN treatment on the clinical course of C. neoformans infection. Mice received multiple doses of CpG- or CNT-ODN (10, 20 or 40 µg/mouse) on days −3, 0, 3, and every 7 days after infection. As shown in Fig. 1a, all the infected mice died within 5 weeks when they were treated with CNT-ODN irrespective of the dose. Administration of CpG-ODN at 20 µg, but not at 10 µg, significantly prolonged the survival time, although 80% of these mice died 14 weeks after infection. Such an effect also tended to occur when CpG-ODN was administered at 40 µg. Furthermore, we tested the effect of these treatments on the number of live microorganisms in the lung and brain 3 weeks after infection. As shown in Fig. 1b, administration of CpG-ODN at 20 or 40 µg significantly reduced the fungal burdens in both lung and brain, compared with the same dose of CNT-ODN. To examine the therapeutic effect, treatment with 20 µg CpG- or CNT-ODN per mouse was begun at 3 days after infection. As shown in Fig. 1c, the therapeutic treatment significantly prolonged the survival of infected mice as efficiently as did the preventive treatment. These results indicate that CpG-ODN protects mice against fatal and disseminated infection with C. neoformans.
Fig. 1.
Effect of CpG-oligodeoxynucleotides (ODN) on the clinical course of cryptococcal infection (a and b). Mice infected intratracheally with Cryptococcus neoformans were treated with 10, 20 or 40 µg/mouse of CpG- or CNT-ODN on days −3, 0, 3, 7 and every 7 days after infection. (a) The number of live mice was noted daily. Open circles, CNT-ODN (n = 10); closed circles, CpG-ODN (n = 10). (b) The numbers of live colonies in lung and brain were counted on day 21 post-infection. No live colonies were detected in the brain of one mouse treated with 20 µg CNT-ODN, which was not included when the mean value was calculated. Open bars, CNT-ODN; closed bars, CpG-ODN. Data are mean ± s.d. of six mice. (c) Mice were treated with 20 µg/mouse CpG- or CNT-ODN on the same schedule above (preventive) or on days 3, 7 and every 7 days after infection (therapeutic), and the number of live mice was noted daily. Open symbols, CNT-ODN; closed symbols, CpG-ODN; circles and solid lines, preventive; triangles and doted lines, therapeutic (n = 8 each). The experiments were repeated twice with similar results. *P < 0·05, compared with CNT-ODN.
Modulation of Th1–Th2 balance by CpG-ODN treatment in mice infected with C. neoformans
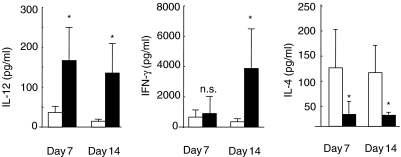
CpG-ODN directly activates DC, which results in preferential expression of Th1-related cytokines within a few hours [19]. Therefore, we next elucidated the effects of CpG-ODN treatment on the production of IL-12 and IFN-γ, as Th1-related cytokines, and IL-4, as a Th2 cytokine, in the lungs after infection with C. neoformans. For this purpose, the levels of these cytokines in BALF were compared in mice treated with CpG- or CNT-ODN at 20 µg on days 7 and 14 post-infection. As shown in Fig. 2, IL-12 and IFN-γ were detected at a marginal level in the BALF of CNT-ODN-treated mice at both time points, and administration of CpG-ODN significantly enhanced the production of IL-12 on days 7 and 14 and of IFN-γ on day 14. In contrast, the levels of IL-4 in BALF were significantly lower in mice treated with CpG-ODN than those in CNT-ODN-treated mice on both days 7 and 14 post-infection. These results indicate that CpG-ODN modulates Th1–Th2 balance towards Th1-dominance at the primary site of cryptococcal infection.
Fig. 2.
Effect of CpG-oligodeoxynucleotides (ODN) on the production of cytokines in bronchoalveolar lavage fluids (BALF) after cryptococcal infection. Mice infected intratracheally with Cryptococcus neoformans were treated with 20 µg/mouse of CpG- or CNT-ODN on days −3, 0, 3, 7 and every 7 days after infection. The concentrations of interleukin (IL-12), interferon (IFN)-γ and IL-4 in BALF were measured on days 7 and 14 post-infection. Open bars, CNT-ODN; closed bars, CpG-ODN. Data are mean ± s.d. of six mice. The experiments were repeated twice with similar results. n.s., not significant; *P < 0·05.
Effect of CpG-ODN treatment on Th1 and Th2 cell development in mice infected with C. neoformans
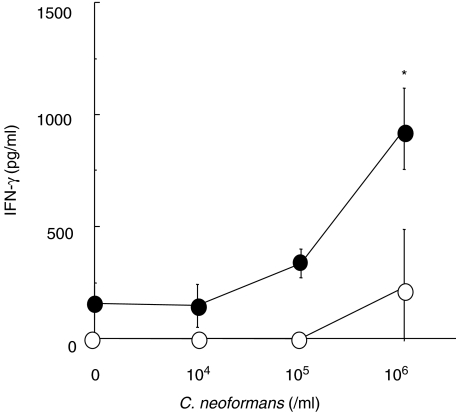
To elucidate the effect of CpG-ODN on the development of fungus-specific Th1 and Th2 cells, on day 14 after cryptococcal infection paratracheal LN cells were prepared from mice treated with CpG- or CNT-ODN at 20 µg, and synthesis of IFN-γ and IL-4 by these cells upon restimulation with live microorganisms was measured. As shown in Fig. 3, unstimulated LN cells from infected and CNT-ODN-treated mice did not produce IFN-γ and only the highest amount of antigens (1 × 106 yeast cells/ml) induced the synthesis of this cytokine. In contrast, LN cells from infected and CpG-ODN-treated mice produced a considerable amount of IFN-γ even in the absence of cryptococcal antigens, and administration of live yeast cells promoted the synthesis of IFN-γ in a dose-dependent manner. The production upon restimulation with the highest dose of antigens was significantly higher in mice treated with CpG-ODN than in CNT-ODN-treated mice. The IFN-γ production was not detected when stimulated with PPD (data not shown). On the other hand, IL-4 synthesis by restimulated LN cells was almost undetectable in both CNT-ODN- and CpG-ODN-treated mice (data not shown). Thus, development of Th1 cells specific for C. neoformans was induced by administration of CpG-ODN.
Fig. 3.
Effect of CpG-oligodeoxynucleotides (ODN) on the development of Th1 cells after cryptococcal infection. Mice infected intratracheally with Cryptococcus neoformans were treated with 20 µg/mouse of CpG- or CNT-ODN on days −3, 0, 3, 7 and every 7 days after infection. The paratracheal lymph node (LN) cells obtained on day 14 post-infection were cultured with indicated doses of live yeast cells for 48 h, and the concentration of interferon (IFN)-γ in the culture supernatants was measured. Open circles, CNT-ODN; closed circles, CpG-ODN. Data are mean ± s.d. of triplicate cultures. The experiments were repeated twice with similar results. *P < 0·05.
Lymphocyte subsets contribute to the protective response caused by CpG-ODN
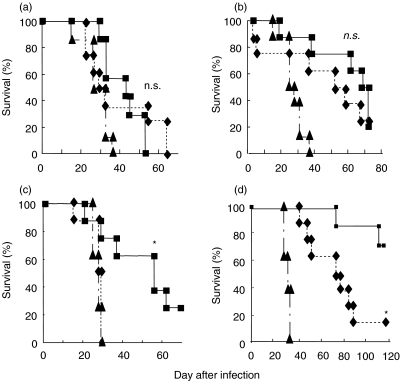
To identify the lymphocyte subsets that contribute to the protective effect of CpG-ODN, we examined the effect of depletion of NK, γδ T, CD4+ T and CD8+ T cells on the survival of mice infected with C. neoformans. As shown in Fig. 4a and b, survival of CpG-ODN-treated mice was significantly longer than that of CNT-ODN-treated mice after cryptococcal infection, and the protective effect was not affected significantly by the depletion of either NK cells or γδ T cells. In contrast, the protective effect of CpG-ODN treatment was completely abrogated in CD4+ T cell-depleted mice (Fig. 4c). Administration of anti-CD8 MoAb partially, but significantly, suppressed the protective effect of CpG-ODN on the survival of infected mice, compared with control IgG (Fig. 4d). These results indicate that CD4+ T cells, rather than NK, γδ T and CD8+ T cells, play a critical role in the CpG-ODN-induced host protection against lethal infection with C. neoformans.
Fig. 4.
Lymphocyte subsets responsible for CpG-oligodeoxynucleotides (ODN)-induced host protection. Mice infected intratracheally with Cryptococcus neoformans were treated with 20 µg/mouse of CpG- or CNT-ODN on days −3, 0, 3, 7 and every 7 days after infection. The CpG-ODN-treated mice received antibodies against Asialo GM1 (ASGM1) (a), T cell receptor (TCR)-γδ (b), CD4 (c) or CD8 (d) or respective control IgG. The number of live mice was noted daily. Each group consists of seven to eight mice. The experiments were repeated twice (a, b and c) and four times (d) with similar results. Triangles, CNT-ODN; squares, CpG-ODN + control IgG; diamonds, CpG-ODN + specific antibody. n.s., not significant; *P < 0·05, compared between control IgG and specific antibody.
Next, we elucidated the lymphocyte subsets responsible for the production of IFN-γ in CpG-ODN-treated mice after cryptococcal infection. For this purpose, the effect of the depletion of NK, γδ T, CD4+ T and CD8+ T cells was examined on days 3, 7 and 14 post-infection. As shown in Table 1, on day 3 the BALF levels of IFN-γ in infected and CpG-ODN-treated mice were not reduced significantly by depletion of any lymphocyte subsets. On day 7, IFN-γ production was inhibited significantly in CD8+ T cell-depleted mice, but not in mice depleted of other lymphocyte subsets, compared with control IgG-treated mice. In contrast, depletion of CD4+ T cells strongly inhibited the production of IFN-γ in infected and CpG-ODN-treated mice on day 14 post-infection, whereas no influence was noted for depletion of other lymphocyte subsets.
Table 1.
Effect of lymphocyte subset depletion on IFN-γ production.a
| IFN-γ (pg/ml) in BALF | |||
|---|---|---|---|
| Day 3 | Day 7 | Day 14 | |
| Rabbit IgG | 1176 ± 114 | 9714 ± 1167 | 6053 ± 3167 |
| aASGM1 antibody | 1622 ± 229b | 4557 ± 6284c | 5654 ± 1617c |
| Hamster IgG | 705 ± 417 | 7451 ± 4842 | 11371 ± 816 |
| α γδ T antibody | 1296 ± 232c | 8636 ± 2785c | 11238 ± 3432c |
| Rat IgG | 1141 ± 478 | 3680 ± 2018 | 4820 ± 2071 |
| αCD4 antibody | 1451 ± 143c | 2317 ± 1850c | ″573 ± 134b |
| Rat IgG | 1296 ± 232 | 3680 ± 2018 | 5527 ± 3906 |
| αCD8 antibody | 1249 ± 101c | 1083 ± 226b | 5761 ± 1566c |
Infected and CpG-oligodeoxynucleotides (ODN)-treated mice received control IgG or specific antibody, and the concentrations of interferon (IFN)-γ in bronchoalveolar lavage fluids (BALF) (pg/ml) were measured at each time point. The values are the mean ± s.d. of four to five mice. The experiments were repeated twice with similar results.
P < 0·05, compared to control IgG-treated mice.
Not significant, compared to control IgG-treated mice.
Induction of intracellular IFN-γ expression in CD4+ T and CD8+ T cells in lung by CpG-ODN
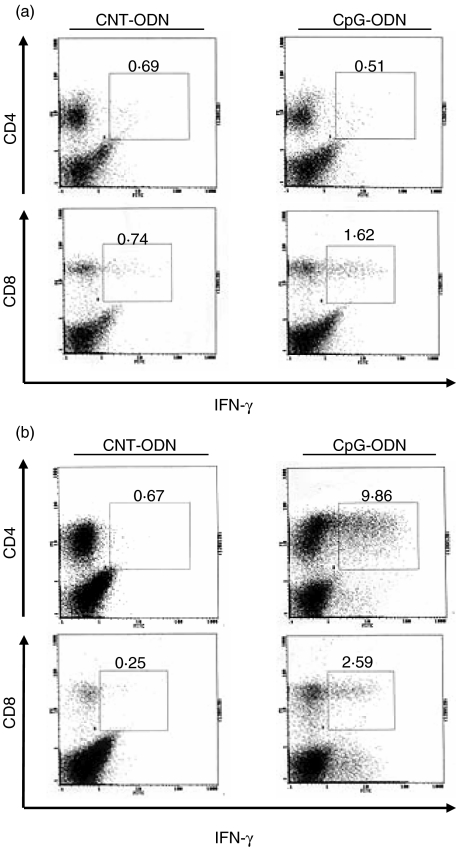
In further experiments, we examined the expression of intracellular IFN-γ in CD4+ T and CD8+ T cells in the lung on days 7 and 14 after infection with C. neoformans. As shown in Fig. 5a, intracellular IFN-γ was detected at a small level in CD4+ T and CD8+ T cells from CNT-ODN-treated mice on day 7 post-infection. Administration of CpG-ODN did not alter the expression of this cytokine in CD4+ T cells compared with CNT-ODN-treated mice. In contrast, such expression was considerably augmented in CD8+ T cells although the magnitude was not pronounced (0·74% and 1·62% in CNT- and CpG-ODN, respectively). On day 14 post-infection, IFN-γ synthesis was not detected in CD4+ T and CD8+ T cells from CNT-ODN-treated mice, and the expression of this cytokine was induced in both T cell subsets by treatment with CpG-ODN. Interestingly, CpG-ODN induction of IFN-γ synthesis was detected at a higher level in CD4+ T cells than in CD8+ T cells (9·86% versus 2·59%) (Fig. 5b). These results indicate that CpG-ODN treatment stimulates the synthesis of IFN-γ predominantly in CD4+ T cells rather than in CD8+ T cells.
Fig. 5.
CpG-oligodeoxynucleotides (ODN) stimulates intracellular interferon (IFN)-γ expression. Mice infected intratracheally with Cryptococcus neoformans were treated with 20 µg/mouse of CpG- or CNT-ODN on days −3, 0, 3, 7 and every 7 days after infection. The lung leucocytes prepared on days 7 (a) and 14 (b) post-infection were stained with fluoroscein isothyocyanate (FITC)-anti-IFN-γ MoAb and phycoerythrin (PE)-anti-CD4 or -CD8 MoAb and analysed by flow cytometry. Each number indicates the proportion of each subset. The experiments were repeated twice with similar results.
Involvement of TNF-α in the protective effect of CpG-ODN
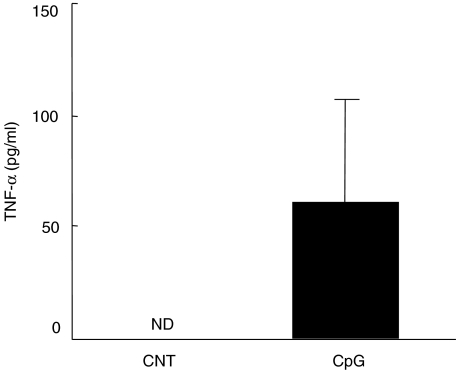
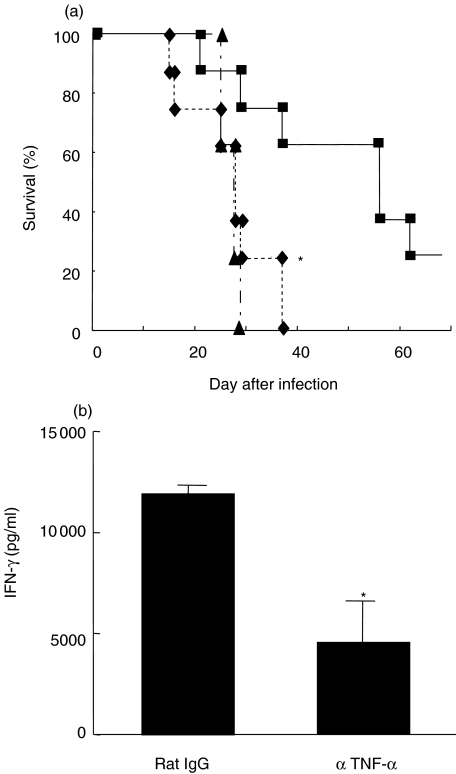
Finally, we elucidated the contribution of TNF-α to CpG-ODN-induced host protection against a lethal infection with C. neoformans. For this purpose, we examined the effect of CpG-ODN on the synthesis of TNF-α in the lung. As shown in Fig. 6, TNF-α levels were under detection limit in the BALF of CNT-ODN-treated mice on day 14 post-infection, whereas CpG-ODN markedly induced such levels. In the next experiments, we examined the effect of neutralizing anti-TNF-α MoAb on the host protective responses to cryptococcal infection caused by CpG-ODN. As shown in Fig. 7a, CpG-ODN treatment significantly extended the survival of infected mice compared with CNT-ODN-treated mice, and this protective effect was abrogated almost completely by administration of anti-TNF-α MoAb. In addition, anti-TNF-α MoAb significantly suppressed CpG-ODN-stimulated IFN-γ production in the infected lungs (Fig. 7b).
Fig. 6.
Effect of CpG-oligodeoxynucleotides (ODN) on the production of tumour necrosis factor (TNF)-α in bronchoalveolar lavage fluids (BALF) after cryptococcal infection. Mice infected intratracheally with Cryptococcus neoformans were treated with 20 µg/mouse of CpG- or CNT-ODN on days −3, 0, 3, 7 and every 7 days after infection. The concentrations of TNF-α in BALF were measured on day 14 post-infection. Data are mean ± s.d. of five mice. The experiments were repeated twice with similar results. n.d., not detected.
Fig. 7.
Effect of anti-tumour necrosis factor (TNF)-α MoAb on the host protective responses stimulated by CpG-oligodeoxynucleotides (ODN). Mice infected intratracheally with Cryptococcus neoformans were treated with 20 µg/mouse of CpG- or CNT-ODN on days −3, 0, 3, 7 and every 7 days after infection. The CpG-ODN-treated mice received anti-TNF-α MoAb or control rat IgG. (a) The number of live mice was noted daily. Each group consists of eight mice. Triangles, CNT-ODN; squares, CpG-ODN + control IgG; diamonds, CpG-ODN + specific antibody. *P < 0·05, compared between control IgG and anti-TNF-α MoAb. (b) The concentrations of IFN-γ in bronchoalveolar lavage fluids (BALF) were measured on day 14 post-infection. Data are mean ± s.d. of five mice. The experiments were repeated twice with similar results. *P < 0·05, compared with control IgG and anti-TNF-α MoAb.
Discussion
The present study shows that CpG-ODN treatment protected mice against infection with C. neoformans by promoting local clearance of this fungal pathogen and preventing its dissemination to the central nervous system. This beneficial effect was associated with alteration in the Th1–Th2 cytokine balance toward a Th1-dominant condition. In our earlier studies [31], aggravation of cryptococcal infection was associated with a Th2-biased cytokine balance in which Th1-related cytokines, IL-12, IL-18 and IFN-γ, were hardly detected in the infected lung tissues, compared with overproduction of Th2 cytokines, IL-4 and IL-10. Administration of recombinant IL-12 results in strong Th1-like immune responses and reduced mortality by this infection [11]. Similar data were obtained when CpG-ODN was administered in these mice. The synthesis of IL-12 and IFN-γ was induced strongly in the infected lungs by this treatment, whereas IL-4 production was significantly inhibited. CpG-ODN is well known to polarize the immune response towards Th1 over Th2 by promoting IL-12 synthesis and expression of co-stimulatory molecules by DC [20–22]. Similar observations are shown also in previous reports addressing the effect of CpG-ODN in other infectious diseases [25–29].
Bacterial DNA was found to activate NK cell production of IFN-γ[14–16]. The major source of initial IFN-γ production in CpG-ODN-stimulated mouse spleen cells is supposed to be NK cells [32]. Recently, Iho and colleagues reported that CpG-ODN directly activate human NK cells [33]. In our study, by contrast, the role of NK cells in CpG-ODN-induced IFN-γ synthesis as well as host protection against cryptococcal infection could not be confirmed from the experiments in which this lymphocyte subset was depleted. In addition, we did not obtain any evidences indicating the contribution of γδ T cells in these processes, although there is no report that described the involvement of this particular lymphocyte subset. We also found no difference in the protective effect of CpG-ODN between control C57BL/6 mice and Jα18 gene-disrupted mice [mice lacking invariant natural killer T (iNKT) cells] (unpublished data). These observations demonstrated that the contribution of innate immune lymphocytes to the CpG-ODN effects on cryptococcal infection was limited, although we could not exclude the possibility that these subsets were directly or indirectly activated at an earlier phase by this treatment. In fact, there are many investigations indicating the contribution of NK cells to elimination of this fungal pathogen in a direct or indirect manner [34–37]. In other studies, we reported the roles of iNKT cells and γδ T cells in the host defence to cryptococcal infection [38,39] and the contribution of NK cells and γδ T cells to IFN-γ synthesis in the lung caused by combined treatment with IL-12 and IL-18 [40]. Further studies are necessary to define the relationship to the present observations.
Generally, CpG-ODN does not have a direct stimulatory effect on resting T cells [19,41–43], although direct activation of purified human T cells by this agent has been reported recently [33]. CpG-ODN-induced T cell activation would be conducted in an indirect way by antigen-presentation and expression of cytokines and co-stimulatory molecules by DC, as reviewed by Krieg [19]. This notion is thoroughly consistent with our unpublished data showing complete abrogation of CpG-ODN induction of host defence to cryptococcal infection by anti-CD11c MoAb that depletes DC. Previous studies reported the contribution of both CD4+ and CD8+ T cells to IFN-γ production induced by CpG-ODN treatment of different animal models, although their contribution varied from one model to another [44,45]. In our hands, using intracellular cytokine analysis we found that the major source of IFN-γ production was CD8+ T cells rather than CD4+ T cells in lung on day 7 after infection, although the overall proportion of intracellular IFN-γ+ CD8+ T cells was not very high (2·6%). In contrast, on day 14 CD4+ T cells became the major IFN-γ-producing cells instead of CD8+ T cells, as shown by intracellular cytokine production ratio in each subset (CD8+ IFN-γ+ 2·59% versus CD4+ IFN-γ+ 9·86%). These findings were consistent with the results showing that IFN-γ synthesis detected at a protein level in BALF was significantly inhibited in CD8+ T cell-depleted, but not CD4+ T cell-depleted mice on day 7 and vice versa on day 14 after infection with C. neoformans. These findings indicate that CD8+ T cells are the major source of IFN-γ production stimulated by CpG-ODN, although not large production, at an earlier phase and CD4+ T cells are the main IFN-γ producer at a later phase of infection. In further experiments, the protective effects of CpG-ODN treatment against fatal infection were abrogated completely by depletion of CD4+ T cells and only partially affected by depletion of CD8+ T cells. Thus, in our model, the development of C. neoformans-specific Th1 cells, rather than type-1 cytotoxic T cells (Tc1), contributes more profoundly to the induction of host protective immune responses caused by CpG-ODN.
TNF-α is known to play a critical role in the host defence to intracellular microorganisms [46]. Mice with a genetic disruption of this cytokine are highly susceptible to infection with C. neoformans [8]. In a series of studies by Huffnagle and colleagues [47,48], TNF-α was shown to function at an early phase by initiating the accumulation of inflammatory leucocytes and development of Th1-based immune responses to C. neoformans. Here, we demonstrated that TNF-α was an important cytokine in the CpG-ODN induction of IFN-γ synthesis and host protection against this infectious pathogen. Although the cellular source remains to be elucidated, DC or macrophages may be directly involved in TNF-α production caused by CpG-ODN, as reported in earlier investigations [20,49].
In conclusion, we demonstrated in the present study that CpG-ODN protects mice against infection with C. neoformans by promoting Th1-mediated immune responses. Recently, clinical trials using CpG-ODN in cancer therapy, treatment of allergic diseases and in vaccination against infectious diseases has been reported [50,51], suggesting that this treatment can be conducted without any serious adverse effects. Thus, CpG-ODN could be a candidate therapeutic agent against refractory cryptococcal infection in patients with compromised immune responses. However, our data identifying CD4+ T cells as a major subset of IFN-γ production may limit the usefulness of this agent, because the compromised immune response associated with cryptococcosis patients is a severe reduction in this T cell subset [1]. Further investigations will be necessary to make the CpG-ODN treatment useful in such clinical settings.
Acknowledgments
This work was supported in part by a Grant-in-Aid for Science Research (C) (KAKENHI12670261) from the Ministry of Education, Culture, Sports, Science and Technology and by Grants of Research on Health Sciences focusing on Drug Innovation (SA14806) from the Japan Health Sciences Foundation and by Grants of Research on HIV/AIDS (HIS-AIDS-005) from the Ministry of Health, Labor and Welfare, Japan.
References
- 1.Stevens DA. Fungal infections in AIDS patients. Br J Clin Pract Suppl. 1990;71:11–22. [PubMed] [Google Scholar]
- 2.Lim TS, Murphy JW. Transfer of immunity to cryptococcosis by T-enriched splenic lymphocytes from Cryptococcus neoformans-sensitized mice. Infect Immun. 1980;30:5–11. doi: 10.1128/iai.30.1.5-11.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koguchi Y, Kawakami K. Cryptococcal infection and Th1–Th2 cytokine balance. Int Rev Immunol. 2002;21:423–38. doi: 10.1080/08830180213274. [DOI] [PubMed] [Google Scholar]
- 4.Yuan RR, Casadevall A, Oh J, Scharff MD. T cells cooperate with passive antibody to modify Cryptococcus neoformans infection in mice. Proc Natl Acad Sci USA. 1997;94:2483–8. doi: 10.1073/pnas.94.6.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decken K, Kohler G, Palmer-Lehmann K, et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawakami K, Koguchi Y, Qureshi MH, et al. Reduced host resistance and Th1 response to Cryptococcus neoformans in interleukin-18 deficient mice. FEMS Microbiol Lett. 2000;186:121–6. doi: 10.1111/j.1574-6968.2000.tb09092.x. [DOI] [PubMed] [Google Scholar]
- 7.Kawakami K, Koguchi Y, Qureshi MH, et al. IL-18 contributes to host resistance against infection with Cryptococcus neoformans in mice with defective IL-12 synthesis through induction of IFN-γ production by NK cells. J Immunol. 2000;165:941–7. doi: 10.4049/jimmunol.165.2.941. [DOI] [PubMed] [Google Scholar]
- 8.Rayhane N, Lortholary O, Fitting C, et al. Enhanced sensitivity of tumor necrosis factor/lymphotoxin-α-deficient mice to Cryptococcus neoformans infection despite increased levels of nitrite/nitrate, interferon-γ, and interleukin-12. J Infect Dis. 1999;180:1637–47. doi: 10.1086/315061. [DOI] [PubMed] [Google Scholar]
- 9.Blackstock R, Buchanan KL, Adesina AM, Murphy JW. Differential regulation of immune responses by highly and weakly virulent Cryptococcus neoformans isolates. Infect Immun. 1999;67:3601–9. doi: 10.1128/iai.67.7.3601-3609.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakami K, Tohyama M, Teruya K, Kudeken N, Xie Q, Saito A. Contribution of interferon-γ in protecting mice during pulmonary and disseminated infection with Cryptococcus neoformans. FEMS Immunol Med Microbiol. 1996;13:123–30. doi: 10.1016/0928-8244(95)00093-3. [DOI] [PubMed] [Google Scholar]
- 11.Kawakami K, Tohyama M, Xie Q, Saito A. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin Exp Immunol. 1996;104:208–14. doi: 10.1046/j.1365-2249.1996.14723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawakami K, Qureshi MH, Zhang T, Okamura H, Kurimoto M, Saito A. IL-18 protects mice against pulmonary and disseminated infection with Cryptococcus neoformans by inducing IFN-γ production. J Immunol. 1997;159:5528–34. [PubMed] [Google Scholar]
- 13.Kawakami K, Qifeng X, Tohyama M, Qureshi MH, Saito A. Contribution of tumour necrosis factor-α (TNF-α) in host defence mechanism against Cryptococcus neoformans. Clin Exp Immunol. 1996;106:468–74. doi: 10.1046/j.1365-2249.1996.d01-870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tokunaga T, Yamamoto H, Shimada S, et al. Antitumor activity of deoxyribonucleic acid fraction from Mycobacterium bovis BCG. I. Isolation, physicochemical characterization, and antitumor activity. J Natl Cancer Inst. 1984;72:955–62. [PubMed] [Google Scholar]
- 15.Yamamoto S, Kuramoto E, Shimada S, Tokunaga T. In vitro augmentation of natural killer cell activity and production of interferon-α/beta and -γ with deoxyribonucleic acid fraction from Mycobacterium bovis BCG. Jpn J Cancer Res. 1988;79:866–73. doi: 10.1111/j.1349-7006.1988.tb00049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tokunaga T, Yamamoto T, Yamamoto S. How BCG led to the discovery of immunostimulatory DNA. Jpn J Infect Dis. 1999;52:1–11. [PubMed] [Google Scholar]
- 17.Messina JP, Gilkeson GS, Pisetsky DS. Stimulation of in vitro murine lymphocyte proliferation by bacterial DNA. J Immunol. 1991;147:1759–64. [PubMed] [Google Scholar]
- 18.Krieg AM, Yi AK, Matson S, et al. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995;374:546–9. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- 19.Krieg AM. CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol. 2002;20:709–60. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 20.Jakob T, Walker PS, Krieg AM, Udey MC, Vogel JC. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides. a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042–9. [PubMed] [Google Scholar]
- 21.Sparwasser T, Koch ES, Vabulas RM, et al. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur J Immunol. 1998;28:2045–54. doi: 10.1002/(SICI)1521-4141(199806)28:06<2045::AID-IMMU2045>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Schulz O, Edwards AD, Schito M, et al. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–62. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- 23.Klinman DM, Yi AK, Beaucage SL, Conover J, Krieg AM. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc Natl Acad Sci USA. 1996;93:2879–83. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipford GB, Sparwasser T, Zimmermann S, Heeg K, Wagner H. CpG-DNA-mediated transient lymphadenopathy is associated with a state of Th1 predisposition to antigen-driven responses. J Immunol. 2000;165:1228–35. doi: 10.4049/jimmunol.165.3.1228. [DOI] [PubMed] [Google Scholar]
- 25.Krieg AM, Love-Homan L, Yi AK, Harty JT. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J Immunol. 1998;161:2428–34. [PubMed] [Google Scholar]
- 26.Elkins KL, Rhinehart-Jones TR, Stibitz S, Conover JS, Klinman DM. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J Immunol. 1999;162:2291–8. [PubMed] [Google Scholar]
- 27.Zimmermann S, Egeter O, Hausmann S, et al. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J Immunol. 1998;160:3627–30. [PubMed] [Google Scholar]
- 28.Walker PS, Scharton-Kersten T, Krieg AM, et al. Immunostimulatory oligodeoxynucleotides promote protective immunity and provide systemic therapy for leishmaniasis via IL-12- and IFN-γ-dependent mechanisms. Proc Natl Acad Sci USA. 1999;96:6970–5. doi: 10.1073/pnas.96.12.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gramzinski RA, Doolan DL, Sedegah M, Davis HL, Krieg AM, Hoffman SL. Interleukin-12- and gamma interferon-dependent protection against malaria conferred by CpG oligodeoxynucleotide in mice. Infect Immun. 2001;69:1643–9. doi: 10.1128/IAI.69.3.1643-1649.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawakami K, Kohno S, Morikawa N, Kadota J, Saito A, Hara K. Activation of macrophages and expansion of specific T lymphocytes in the lungs of mice intratracheally inoculated with Cryptococcus neoformans. Clin Exp Immunol. 1994;96:230–7. doi: 10.1111/j.1365-2249.1994.tb06547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawakami K, Tohyama M, Qifeng X, Saito A. Expression of cytokines and inducible nitric oxide synthase mRNA in the lungs of mice infected with Cryptococcus neoformans: effects of interleukin-12. Infect Immun. 1997;65:1307–12. doi: 10.1128/iai.65.4.1307-1312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowdery JS, Chace JH, Yi AK, Krieg AM. Bacterial DNA induces NK cells to produce IFN-γin vivo and increases the toxicity of lipopolysaccharides. J Immunol. 1996;156:4570–5. [PubMed] [Google Scholar]
- 33.Iho S, Yamamoto T, Takahashi T, Yamamoto S. Oligodeoxynucleotides containing palindrome sequences with internal 5′-CpG-3′ act directly on human NK and activated T cells to induce IFN-γ production in vitro. J Immunol. 1999;163:3642–52. [PubMed] [Google Scholar]
- 34.Murphy JW, MacDaniel DO. In vitro reactivity of natural killer (NK) cells on Cryptococcus neoformans. J Immunol. 1982;128:1577–83. [PubMed] [Google Scholar]
- 35.Lipscomb MF, Alvarellos T, Toews GB, et al. Role of natural killer cells in resistance to Cryptococcus neoformans infections in mice. Am J Pathol. 1991;128:354–61. [PMC free article] [PubMed] [Google Scholar]
- 36.Hidore MR, Nabavi N, Sonleitner F, Murphy JW. Murine natural killer cells are fungicidal to Cryptococcus neoformans. Infect Immun. 1991;59:1747–54. doi: 10.1128/iai.59.5.1747-1754.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawakami K, Koguchi Y, Qureshi MH, et al. NK cells eliminate Cryptococcus neoformans by potentiating the fungicidal activity of macrophages, rather than by directly killing them, upon stimulation with IL-12 and IL-18. Microbiol Immunol. 2000;44:1043–50. doi: 10.1111/j.1348-0421.2000.tb02601.x. [DOI] [PubMed] [Google Scholar]
- 38.Kawakami K, Kinjo Y, Uezu K, et al. Monocyte chemoattractant protein-1-dependent increase of Vα14 NKT cells in lungs and their roles in Th1 response and host defense in cryptococcal infection. J Immunol. 2001;167:6525–32. doi: 10.4049/jimmunol.167.11.6525. [DOI] [PubMed] [Google Scholar]
- 39.Uezu K, Kawakami K, Miyagi K, et al. Accumulation of γδ T cells in the lungs and their regulatory roles in Th1 response and host defense against pulmonary infection with Cryptococcus neoformans. J Immunol. 2004;172:7629–34. doi: 10.4049/jimmunol.172.12.7629. [DOI] [PubMed] [Google Scholar]
- 40.Qureshi MH, Zhang T, Koguchi Y, et al. Combined effects of IL-12 and IL-18 on the clinical course and local cytokine production in murine pulmonary infection with Cryptococcus neoformans. Eur J Immunol. 1999;29:643–9. doi: 10.1002/(SICI)1521-4141(199902)29:02<643::AID-IMMU643>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 41.Sun S, Zhang X, Tough DF, Sprent J. Type I interferon-mediated stimulation of T cells by CpG DNA. J Exp Med. 1998;188:2335–42. doi: 10.1084/jem.188.12.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lipford GB, Bauer M, Blank C, Reiter R, Wagner H, Heeg K. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur J Immunol. 1997;27:2340–4. doi: 10.1002/eji.1830270931. [DOI] [PubMed] [Google Scholar]
- 43.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–68. doi: 10.1016/s0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 44.Rhee EG, Mendez S, Shah JA, et al. Vaccination with heat-killed leishmania antigen or recombinant leishmanial protein and CpG oligodeoxynucleotides induces long-term memory CD4+ and CD8+ T cell responses and protection against leishmania major infection. J Exp Med. 2002;195:1565–73. doi: 10.1084/jem.20020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim TY, Myoung HJ, Kim JH, et al. Both E7 and CpG-oligodeoxynucleotide are required for protective immunity against challenge with human papillomavirus 16 (E6/E7) immortalized tumor cells: involvement of CD4+ and CD8+ T cells in protection. Cancer Res. 2002;62:7234–40. [PubMed] [Google Scholar]
- 46.Havell EA. Role of TNF in resistance to bacteria. Immunol Series. 1992;56:341–63. [PubMed] [Google Scholar]
- 47.Huffnagle GB, Toews GB, Burdick MD, et al. Afferent phase production of TNF-α is required for the development of protective T cell immunity to Cryptococcus neoformans. J Immunol. 1996;157:4529–36. [PubMed] [Google Scholar]
- 48.Herring AC, Lee J, McDonald RA, Toews GB, Huffnagle GB. Induction of interleukin-12 and gamma interferon requires tumor necrosis factor alpha for protective T1-cell-mediated immunity to pulmonary Cryptococcus neoformans infection. Infect Immun. 2002;70:2959–64. doi: 10.1128/IAI.70.6.2959-2964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon HJ, Lee KW, Yu SH, Han JH, Kim DS. NF-κB-dependent regulation of tumor necrosis factor-α gene expression by CpG-oligodeoxynucleotides. Biochem Biophys Res Commun. 2003;31:129–38. doi: 10.1016/j.bbrc.2003.09.168. [DOI] [PubMed] [Google Scholar]
- 50.Shoda LK, Kegerreis KA, Suarez CE, Mwangi W, Knowles DP, Brown WC. Immunostimulatory CpG-modified plasmid DNA enhances IL-12, TNF-α, and NO production by bovine macrophages. J Leukoc Biol. 2001;70:103–12. [PubMed] [Google Scholar]
- 51.Agrawal S, Kandimalla ER. Medicinal chemistry and therapeutic potential of CpG DNA. Trends Mol Med. 2002;8:114–21. doi: 10.1016/s1471-4914(01)02264-x. [DOI] [PubMed] [Google Scholar]