Abstract
Innate immunity is important for early defence against borrelia spirochetes and should play a role in the clinical outcome of the infection. In order to study early cytokine responses, in vitro differentiated dendritic cells (DCs) and whole blood cells from 21 patients with different clinical outcomes of Lyme neuroborreliosis were stimulated with live borrelia spirochetes. The borrelia-induced secretion of interleukin (IL)-4, IL-10, IL-12p70, interferon (IFN)-γ and tumour necrosis factor (TNF)-α in DCs and IL-1β, IL-6, IL-8, IL-10, IL-12p70, TNF-α, regulated upon activation normal T cell expressed and secreted (RANTES), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β and eotaxin in whole blood cells was measured by enzyme-linked immunospot (ELISPOT) and multiplex arrays, respectively. We found increased numbers of TNF-α-secreting DCs (P = 0·018) in asymptomatic seropositive individuals compared to patients with subacute neuroborreliosis and seronegative controls. Asymptomatic individuals were also found to have elevated levels of IL-12p70 (P = 0·031) in whole blood cell supernatants compared to seronegative controls. These results are in line with previous experiments using cells of the adaptive immune response, indicating that strong T helper type 1 (Th1) proinflammatory responses might be associated with a successful resolution of Lyme disease.
Keywords: borrelia, clinical outcome, dendritic cell, IL-12p70, TNF-α
Introduction
Lyme borreliosis (LB) is a tick-borne, complex infectious disease caused by the spirochete Borrelia burgdorferi[1]. The disease is associated with several clinical manifestations, of which erythema migrans and neuroborreliosis are the most common in Europe [2]. The disease affects individuals differently depending, for example, on the various spirochete subspecies involved [3,4] and the kind of immune response generated. Some individuals remain asymptomatic [5–8] whereas others, irrespective of antibiotic treatment, are inclined to develop subacute or so-called chronic disease [9–14], the latter defined as symptoms persisting more than 6 months [11,15].
There is considerable evidence for immune mechanisms contributing to the pathogenesis of LB [16]. A number of studies have unambiguously shown a predominance of T helper type 1 (Th1) responses in LB [17–23]. A successful resolution of a borrelia infection does, however, seem to be associated with a subsequent up-regulation of antagonistic Th2 responses in both man and mouse [19,24,25].
The importance of the adaptive part of the immune system for the eradication of the borrelia spirochetes is well established. However, early defence mechanisms against the spirochete are supplied by the innate immunity, which are most probably of significant importance for the clinical outcome itself, as well as by initiating and directing the adaptive response [26–32]. Dendritic cells (DCs) play a unique role in the innate immune system, constituting a link between innate and adaptive immunity [33,34]. DCs have been shown to be able to phagocytose and process live spirochetes in vitro and upon activation secrete innate cytokines, such as interleukin (IL)-8 and IL-12 [35,36]. Furthermore, splenic mouse DCs pulsed in vitro with live borrelia spirochetes induced a protective immune response against tick-transmitted spirochetes following transfer into naive syngeneic mice [37]. However, so far no studies have focused on possible individual differences in DC-generated cytokine secretion patterns in LB patients in response to live spirochetes.
Our working hypothesis is that chronic neuroborreliosis (NB) may be due to a dysregulation of the initial innate immune response, which in turn affects the following adaptive response. This dysregulation may be inherited or acquired, and would most probably be an inbuilt disposition in certain individuals. To avoid the confounding of an ongoing immune response, we here studied the innate responses, independent of memory, in subjects with a history of different clinical outcomes of LB.
The aim of the study was to find out whether differences in innate immune responses, elicited by live B. garinii spirochetes, between individuals with a history of LB could partially explain different clinical outcomes concerning the disease. For this purpose, DCs were differentiated in vitro from peripheral blood monocytes from patients with a history of chronic NB (n = 6), acrodermatitis chronicum atrophicans (ACA) (n = 1), subacute NB (n = 7), asymptomatic seropositive individuals (n = 7) and seronegative, healthy controls (n = 7). Live borrelia spirochete-induced secretion of IL-4, IL-10, IL-12p70, interferon (IFN)-γ and tumour necrosis factor (TNF)-α was determined with enzyme-linked immunospot (ELISPOT). Furthermore, whole blood samples from patients were stimulated with live borrelia spirochetes and the secretion of different innate cytokines and chemokines (IL-1β, IL-6, IL-8, IL-10, IL-12p70, TNF-α, regulated upon activation normal T cell expressed and secreted (RANTES, monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, eotaxin) were detected with multiplex cytokine arrays.
Patients, materials and methods
Patients
A total of 21 patients with a history of different clinical outcomes of LB were included in the study (Table 1); patients with chronic disease, including patients with NB (n = 6, mean age 63 years, range 37–83) and acrodermatitis chronicum atrophicans (ACA) (n = 1; age 77 years), subacute NB patients (n = 7; mean age 51 years, range 25–67) and asymptomatic seropositive subjects (n = 7; mean age 47 years, range 39–74). Seronegative individuals, who all were staff at the hospital, were used as controls (n = 7; mean age 29 years, range 23–37). All the patients were diagnosed by one of the authors (P.F.). NB was diagnosed according to the European clinical case definitions for LB [10]: the presence of lymphocytic pleocytosis in cerebrospinal fluid (CSF) in the acute phase, intrathecal production of antiborrelia IgM/IgG antibodies, as indicated by an elevated CSF antibody index [38], possible oligoclonal bands in CSF and relevant clinical symptoms, such as headache, neck stiffness, facial palsy, meningitis and radiculitis. Subacute disease was defined as symptoms with a duration of less than 6 months, whereas a persistence of more than 6 months was considered chronic [10,11]. One of the patients in the chronic group (no. 5) had ACA and lacked intrathecal antibody production. One patient with chronic NB (no. 4), from whom blood samples were taken late in the disease course, had no pleocytosis but had antiborrelia IgG antibodies in CSF. The asymptomatic individuals were recruited at the Department of Transfusion Medicine, University Hospital, Linköping, by screening blood donors for the presence of antiborrelia IgG antibodies. They were characterized by the following: no known history of LB, no empirical course of antibiotic treatment for LB, lack of relevant clinical symptoms and the presence of antiborrelia IgG antibodies in serum. Peripheral blood mononuclear cells (PBMC) from these individuals were stimulated with an outer surface protein (Osp)-enriched fraction of borrelia, called OF, and the number of IL-4 and IFN-γ-secreting PBMC was measured with the ELISPOT assay [7]. Those individuals with the strongest cytokine responses, which were consistently of Th1-type, were included in the study. Thus, the asymptomatic individuals showed a similar polarization of the immune response to patients with a clinical borrelia infection [6].
Table 1. Characteristics of patients with chronic and subacute neuroborreliosis.
| No. | Age | Sex | Diagnosis | Disease course | Symptoms | Duration of symptoms | Borrelia IgG serum (±) | Borrelia IgM serum (±) | CSF-IgG Borrelia antibody indexa (±) | CSF-IgM Borrelia antibody indexa (±) | CSF-MNC- pleocytosisb (±) | CSF- albumin quotientc (±) | CSF-IgG synthesis indexd (±) | CSF- oligo- clonal bands (±) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | F | NB | Chronic | Back- and neck pain, headache, cognitive impairment | 5 years | + | + | + | + | + | + | + | + |
| 2 | 83 | F | NB | Chronic | Memory deficit, numbness, muscle weakness right arm, nausea, vertigo | 6 months | + | − | + | − | + | − | + | + |
| 3 | 70 | M | NB | Chronic | Radiculitis, paresthesia, numbness, thoracic neuralgia | 11 months | + | + | + | − | + | + | + | + |
| 4 | 71 | M | NB | Chronic | Radiculitis, arthritis, headache, neck stiffness | 5 years | + | − | + | − | − | − | + | + |
| 5 | 77 | M | ACA | Chronic | Headache, vertigo, radiculitis | 4 years | + | − | − | − | − | + | − | − |
| 6 | 37 | M | NB | Chronic | Arthralgia, neck- and back pain, headache, paresthesia | 3 years | + | + | + | − | + | − | − | n.d. |
| 7 | 68 | M | NB | Chronic | Headache, radiculitis, facial palsy, fatigue, vertigo | 10 months | n.d. | n.d. | + | − | + | + | − | n.d. |
| 8 | 50 | M | NB | Subacute | Tremor, vertigo, myalgia, radiculitis, back pain | 2 months | − | + | + | + | + | + | + | − |
| 9 | 25 | M | NB | Subacute | Headache, facial palsy | 2 months | + | + | + | + | + | + | + | n.d. |
| 10 | 46 | M | NB | Subacute | Facial palsy, memory deficits, numbness, hypoesthesia | 5 months | + | + | + | + | + | n.d. | n.d. | n.d. |
| 11 | 55 | M | NB | Subacute | Facial palsy, diplopia | 1·5 months | + | + | + | + | + | + | + | + |
| 12 | 67 | M | NB | Subacute | Facial palsy, headache, radiculitis, numbness | 1 month | + | + | + | + | + | + | + | n.d. |
| 13 | 49 | M | NB | Subacute | Headache, weight loss, fatigue, eye dazzle | 2 months | − | − | + | + | + | + | + | n.d. |
| 14 | 67 | F | NB | Subacute | Arthralgia, facial palsy, radiculitis, nausea | 5 months | − | − | − | + | + | + | − | + |
NB, neuroborreliosis; ACA, acrodermatitis chronicum atrophicans; chronic > 6 months’ duration; subacute < 6 months’ duration; CSF, cerebrospinal fluid; MNC, mononuclear cell; n.d., not done.
Borrelia-specific CSF antibody index was defined according to Hansen and Lebech, 1991.
Mononuclear pleocytosis in CSF was defined as positive if ≥ 5·0 × 106 mononuclear cells/l.
CSF albumin quotient was defined as positive if > 8·1 × 103.
IgG synthesis index in CSF was defined as positive if >1·25.
Culture of autologous dendritic cells
Heparinized peripheral blood from all the patients was separated on Lymphoprep® (Nycomed Pharma, Oslo, Norway) according to Boyum [39], followed by washing twice with RPMI supplemented with l-glutamine (RPMI-1640) (Gibco, Auckland, New Zealand). The mononuclear cells were resuspended in 5% pooled, heat-inactivated human serum (PHS), consisting of RPMI-1640, 100% pooled, heat-inactivated human serum (Department of Transfusion Medicine, University Hospital, Linköping, Sweden) and 1 M HEPES buffer (Invitrogen, Paisley, UK). The cells were cultured undisturbed in six-well culture plates (Costar 3524, Corning Inc., NY, USA), 1 × 106cells/ml (5 ml/well), for 1 h at 37°C, 5% CO2 with 95% humidity. Adherent cells were scraped off and washed with sterile phosphate-buffered saline (PBS), pH 7·4 (EC Diagnostics AB, Uppsala, Sweden) containing 0·5% human serum albumin (HSA) (Pharmacia Biotech, Stockholm, Sweden). Further purification of the mononuclear cells was performed according to the manufacturer's instructions, using monocyte-negative depletion with magnetic beads (monocyte negative isolation kit®, Dynal Biotech, Oslo, Norway). The isolated monocytes were resuspended in 1% human plasma, consisting of RPMI-1640, 100% heat-inactivated human plasma and 1 M HEPES buffer (1% plasma) (Department of Transfusion Medicine, University Hospital, Linköping, Sweden). The culture medium was supplemented with IL-4 (52 µg/ml, R&D Systems, Minneapolis, CA, USA) and granulocyte macrophage-colony stimulating factor (GM-CSF) (50 ng/ml, BD Biosciences, Pharmingen, San Diego, CA, USA). The cells were cultured in a strictly sterile milieu in 24-well culture-plates (Costar 3524, Corning Inc., NY, USA) at 37°C, 5% CO2 with 95% humidity for 7 days, with exchange of fresh medium and cytokines every third day, in order to obtain semimature DCs. No antibiotics were added to the culture medium, as their effect on the cells and spirochetes was considered disadvantageous. The typical morphological changes seen during differentiation were evaluated every third day, using an inverted microscope (Nicon TMS, Tokyo, Japan).
Expression of cell surface markers on dendritic cells
The expression of characteristic cell surface markers on unstimulated (day 7) DCs was analysed by flow cytometry (BD, San José, CA, USA). Random patient samples of DCs were chosen. The antibodies were added according to the manufacturer's instructions. The following directly conjugated monoclonal antibodies were used: anti-CD1a-FITC, anti-CD80-PE, anti-HLA-DR-PerCP, anti-CD11c-PE, anti-CD3-PerCP, anti-CD19-PE, anti-CD14-PerCP (BD Biosciences Pharmingen, San José, CA, USA), anti-CD86-FITC, anti-CD83-FITC (Caltag Laboratories, Burlingame, CA, USA) and the corresponding isotype controls (BD). Analysis was performed using CellQuest Pro (BD), where a minimum of 10 000 cells were counted.
In vitro stimulation of dendritic cells with live spirochetes
On day 7 of culture, DCs were counted, assessed for viability with the Trypan blue exclusion method and resuspended in 1% plasma to a concentration of 2 × 105 cells/ml. The B. garinii Ip90 strain, which was isolated from an Ixodes persulcatus tick from the Khabarovsk territory in Russia [40], was used for stimulation of the DCs. Live B. garinii spirochetes, suspended in Barbour Stoenner Kelly II (BSK II) medium and stored at 6°C [obtained from one of the authors (S.B.)] were activated in a 34°C waterbath and washed with PBS. The spirochetes were stimulated with 3% rabbit serum (Sigma Aldrich, St Louis, MO, USA) in 10% human serum (diluted with RPMI-1640) in a volume of 2 ml for 10 min. Live spirochetes were mixed with DCs in a ratio of 10 : 1 in 1% plasma. The optimal ratio of DCs to spirochetes (1 : 5, 1 : 10, 1 : 20), as well as the immunogenicity of live versus ultrasonicated spirochetes or Osp-fractions of borrelia spirochetes (prepared according to Bergström et al. 1991 [41]) were previously evaluated thoroughly. Live spirochetes were considered to be the most powerful activators of cytokine production in DCs and were therefore chosen as antigens (data not shown).
ELISPOT detection of borrelia-specific cytokine secretion in dendritic cells
The ELISPOT assay was performed essentially according to Czerkinsky et al. [42], as described previously [19]. The method was slightly modified and optimized for detection of cytokine secretion in DCs. The optimal density of DCs per well (20 000 DCs/well) and the incubation time were previously evaluated carefully (data not shown). DCs stimulated with purified protein derivative of Mycobacterium tuberculosis (PPD) (20 µg/ml, Statens Serum Institut, Copenhagen, Denmark) and lipopolysaccharide (LPS) (20 ng/ml, Sigma Aldrich, St Louis, MO, USA) were used as positive controls. Unstimulated DCs, as well as live borrelia spirochetes suspended solely in 1% plasma, were used as negative controls. The borrelia-stimulated DCs were added to the precoated ELISPOT-plates in triplicate, incubated undisturbed for 48 h at 37°C, 5% CO2 with 95% humidity and analysed for the secretion of IL-4, IL-10, IL-12p70, IFN-γ and TNF-α (Mabtech AB, Stockholm, Sweden). The spots were counted by a single person (J.S.), using the AID ELISpot Reader System (AID, Strasberg, Germany). The median value (number of spots) of the triplicates was calculated. In order to obtain the borrelia-specific cytokine secretion, the value of the unstimulated DCs (the spontaneous secretion) was subtracted from the values of the borrelia-stimulated DCs. When comparing the immunogenicity of whole versus ultrasonicated spirochetes, no differencies were observed. However, when Osps were used instead, a reduction in the number of cytokine-secreting DCs, measured with the ELIPSOT-assay, was noted (data not shown).
In vitro stimulation of patient whole blood cells with live spirochetes
Heparinized and undisturbed peripheral blood was washed twice with sterile PBS in order to study immediate immune responses in blood cells, i.e. mainly the macrophage response. Live B. garinii (Ip90) spirochetes, suspended in BSK II medium and stored at 6°C, were activated in a 34°C waterbath, followed by washing with PBS and stimulation for 10 min with 3% rabbit serum in 2 ml 10% human serum (diluted with RPMI-1640). The live spirochetes were added (20 × 106/well) to the whole blood samples, suspended to the double volume with 10% human serum (diluted with RPMI-1640), followed by dispension (2 ml/well) into six-well culture plates and incubation undisturbed at 37°C, 5% CO2 with 95% humidity, for 24 h. As controls, unstimulated whole blood cells from asymptomatic individuals and seronegative controls were cultured in parallel. The following day, after 24 h, the blood samples were centrifuged at 200 g, for 10 min at room temperature (RT) before the supernatants were collected immediately and frozen at −70°C. In addition, patient whole blood was co-cultured with 10% human serum (diluted with RPMI-1640) containing 3% rabbit serum in a volume of 2 ml and analysed in the same manner as the patient samples. When comparing supernatants of unstimulated whole blood with and without rabbit serum, undetectable (below the lowest value of the standard curve) cytokine levels were measured by the multiplex arrays and no differences in the cytokine responses were found (data not shown).
Detection of innate cytokines in patient whole blood cell supernatants post-stimulation with live spirochetes
The previously frozen, borrelia-stimulated whole blood cell supernatants from all patients where thawed at RT and analysed immediately for the presence of IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF-α using the cytometric bead array (CBA) (BD Pharmingen, San Diego, CA, USA). Beads of six diverse fluorescent intensities, each coated with a single anti-cytokine specificity, were mixed with the supernatants. The analysis was carried out following the manufacturer's instructions and analysed by flow cytometry (FACSCalibur, BD Biosciences) to detect the six innate cytokines simultaneously. In the majority of samples the concentration of IL-6 and IL-8 were above the highest value of the standard curve and these samples were therefore stored undisturbed at +6°C overnight (12 h) and re-analysed the next day, following a 10-fold dilution. Values below the lowest value of the standard curve were all set at 0 pg/ml, whereas values above the highest value were defined as 50 000 pg/ml. The latter concerned mainly IL-6 and IL-8.
Detection of chemokines in patient whole blood cell supernatants post-stimulation with live spirochetes
The previously frozen borrelia-stimulated whole blood cell supernatants from all patients where thawed at RT and analysed immediately with the ‘Human Chemokine 5-plex’ kit (Biosource International, Inc., CA, USA) for the presence of the following chemokines: RANTES, MCP, MIP-1α, MIP-1β and eotaxin. A minimum of 100 beads per analyte and well were analysed. Ten per cent plasma, with and without 3% rabbit serum, served as a control. The analysis was performed according to the manufacturer's instructions. The LuminexTM 100 System was used for calculations of the mean fluorescent intensities (MFI). The different concentrations were read with StarStation software (Applied Cytometry Systems, Sheffield, UK). Concerning MIP-1α, all values above the highest value of the standard curve were defined as 40 000 pg/ml and all values below the lowest value as 0 pg/ml. For MIP-1β, all values above the highest value were defined as 30 000 pg/ml. No corrections were needed for the lower values. The corresponding lowest values for MCP-1 and eotaxin were 0 pg/ml, respectively.
Statistics
For comparison of the number of cytokine-secreting cells and cytokine and chemokine levels between the groups, the Kruskal—Wallis test and Mann—Whitney U-test were performed using SPSS® 11·5 for Windows (SPSS Inc., Chicago, IL, USA). The GraphPad Prism version 4·00 for Windows (GraphPad Software, San Diego, CA, USA) was used for figure design. A P-value < 0·05 was considered significant.
Ethics
The study protocol was approved by the Local Ethics Committee of Linköping University Hospital, Sweden. Informed consent was obtained from all the patients and controls.
Results
Expression of cell surface markers on dendritic cells
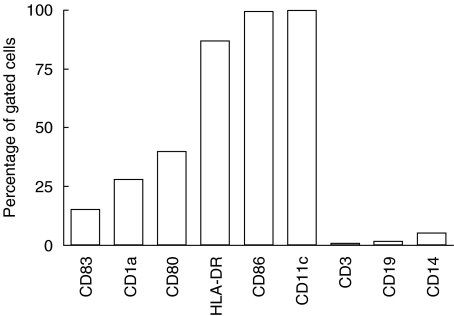
DCs were cultured successfully and shown to express surface markers characteristic of semimature DCs [43]. The expression of CD19 and CD3 in the total cell population (ungated cells) was 6·4% and 0·2%, respectively (based on six experiments), which indicates a lack of contaminating T and B cells (data not shown). Among the DCs (gated cells, according to size and granularity) the expression of CD14, CD11c and HLA-DR was 5·2%, 99·9% and 86·8%, respectively (Fig. 1).
Fig. 1.
The expression of surface markers on unstimulated in vitro differentiated dendritic cells (DCs) on day 7 of culture. Data are given in percentage of gated cells. The gate was defined according to size and granularity of the cells. A minimum of 10 000 cells was counted.
ELISPOT detection of borrelia-specific cytokine secretion in dendritic cells
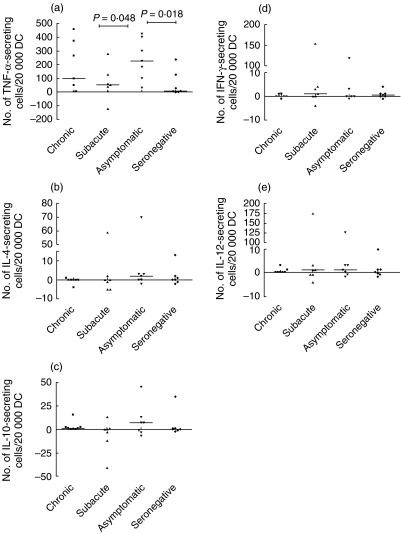
All cytokines were detectable with the ELISPOT assay. However, the number of DCs secreting IL-4, IL-10, IFN-γ and IL-12p70 in response to borrelia was unexpectedly low overall (Fig. 2b–e). In contrast, the number of borrelia-induced TNF-α-secreting DCs was high overall (Fig. 2a). The asymptomatic borrelia-seropositive individuals showed significantly increased numbers of borrelia-induced TNF-α-secreting DCs compared with the seronegative controls and the subacute patients (Kruskal—Wallis test between all groups: P = 0·08, Mann—Whitney U-test: P = 0·018 and P = 0·048, respectively). Despite a lower mean age among the controls, no statistical significance was observed when correlating age to the number of TNF-α-secreting DCs in all patient groups (data not shown). The chronic patients showed a tendency to subgrouping into two different populations; one with high numbers of TNF-α-secreting cells (>200 cells) and one with low (< 100 cells) (Fig. 2a). The borrelia-induced secretion of IL-4, IL-10, IFN-γ and IL-12p70 did not differ between the groups (Fig. 2b–e). Three outliers, one each in the subacute, asymptomatic and seronegative group showed a prominent increase in borrelia-specific cytokine responses throughout all cytokine measurements in the assay. These individuals did not differ in any way from the rest of the patients concerning clinical data.
Fig. 2.
The median number of live Borrelia garinii-specific (a) tumour necrosis factor (TNF)-α-, (b) interleukin (IL)-4-, (c) IL-10-, (d) interferon (IFN)-γ- and (e) IL-12p70-secreting cells per 20 000 in vitro differentiated dendritic cells (DCs) from patients with a history of different clinical outcomes of Lyme borreliosis (LB): chronic (including one patient with acrodermatitis chronicum atrophicans (ACA) and subacute neuroborreliosis, asymptomatic seropositive individuals and seronegative, healthy controls. The borrelia-specific cytokine secretion was obtained by subtracting the number of spots in unstimulated wells from the number in the borrelia-stimulated wells. P-values (Mann—Whitney U-test) from comparison between patient groups are shown. Note the different scales on the y-axes.
Detection of innate cytokines in patient whole blood cell supernatants post-stimulation with live spirochetes
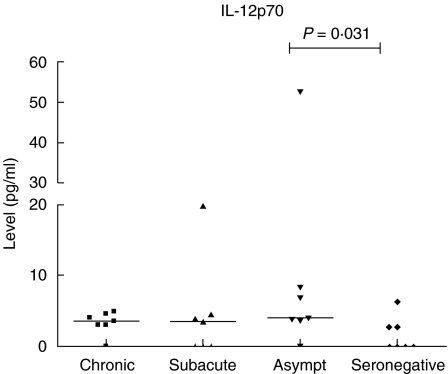
All six innate cytokines (IL-1β, IL-6, IL-8, IL-10, IL-12p70, TNF-α) were detectable with the CBA kit in supernatants from borrelia-stimulated whole blood cells (Table 2). The level of IL-12p70 was increased significantly among the asymptomatic individuals compared to the seronegative controls (Mann—Whitney U-test: P = 0·031) (Fig. 3), whereas the patients with a history of clinical borrelia infection, i.e. the chronic and the subacute patients, showed no such increase. There was no correlation between age and IL-12p70 levels (data not shown). No differences between the groups concerning IL-1β, IL-6, IL-8, IL-10 or TNF-α were found. Supernatants from unstimulated control cultures had nearly undetectable (below the lowest value of the standard curve) values concerning all cytokines (Table 2).
Table 2. Cytokine and chemokine levels in whole blood cell supernatants in different patient groups [chronic neuroborreliosis (NB), subacute NB, asymptomatic seropositive individuals and seronegative controls] following stimulation with live borrelia spirochetes.
| Factor | Chronic | Subacute | Asymptomatic | Seronegative |
|---|---|---|---|---|
| IL-1β | 2046 (60–3266) | 342 (13–4532) | 1289 (25–2865) | 838 (54–1388) |
| IL-6 | 15 934 (102–24 679) | 6558 (90–30 153) | 4177 (231–29 866) | 17 030 (1059–27 407) |
| IL-8 | 22 228 (69–50 000) | 4707 (86–50 000) | 6494 (80–50 000) | 25 042 (526–50 000) |
| IL-10 | 387 (6–977) | 70 (4–848) | 68 (13–588) | 155 (20–610) |
| IL-12p70 | 4 (0–5) | 4 (0–20) | 4 (0–53) | 0 (0–6) |
| TNF-α | 216 (2–2055) | 36 (0–2974) | 231 (2–1783) | 348 (3–1502) |
| MCP-1 | 6106 (1218–29 682) | 3744 (105–9229) | 697 (180–9972) | 4233 (51–6999) |
| Eotaxin | 46 (0–56) | 28 (0–68) | 0 (0–81) | 34 (0–73) |
| MIP-1α | 8395 (95–14 738) | 1258 (19–40 000) | 220 (18–11 359) | 4799 (0–11 255) |
| MIP-1β | 15 873 (2450–24 931) | 10 921 (269–30 000) | 2789 (874–30 000) | 12 069 (634–15 957) |
Median values (range in parenthesis) in pg/ml are given. IL: interleukin; TNF: tumour necrosis factor; MCP: monocyte chemoattractant protein; MIP: macrophage inflammatory protein.
Fig. 3.
Interleukin (IL)-12p70 levels in whole blood cell supernatants, following stimulation of whole blood cells from patients with a history of different clinical outcomes of Lyme borreliosis (LB) with live Borrelia garinii spirochetes. The patient groups consisted of chronic [including one patient with acrodermatitis chronicum atrophicans (ACA)] and subacute neuroborreliosis, asymptomatic seropositive individuals and seronegative, healthy controls. Median values are given. P-value (Mann—Whitney U-test) from comparison between the groups is shown.
Detection of chemokines in patient whole blood cell supernatants post-stimulation with live spirochetes
Due to technical difficulties, the concentrations of RANTES were not interpretable in different patient groups. No significant differences were found in the different patient groups concerning levels of MIP-1α, MIP-1β, MCP-1 and eotaxin when analysed with Kruskal—Wallis and Mann—Whitney U tests (Table 2). The controls (10% plasma, with and without 3% rabbit serum) had nearly undetectable (below the lowest value of the standard curve) values concerning all chemokines (data not shown).
Discussion
In this study we report increased numbers of TNF-α-producing DCs in asymptomatic individuals in response to live borrelia spirochetes, compared to patients with subacute NB and seronegative controls. Asymptomatic seropositive subjects, compared with seronegative controls, were also found to have elevated levels of IL-12p70 in whole blood supernatants following stimulation with live borrelia spirochetes.
TNF-α and IL-12 are both important innate cytokines with proinflammatory and antibacterial effects. IL-12, in addition, induces Th1-responses [44,45]. B. burgdorferi is well known to elicit TNF-α[26,46] and IL-12 production in several cell types [35,47]. TNF-α has previously been shown to be necessary for the elimination of borrelia spirochetes in mice, as disease susceptibility in these mice was associated with inability to produce TNF-α[48]. In humans, high TNF-α levels in CSF in early NB has been associated with a non-chronic disease course [32]. The present data on increased numbers of borrelia-induced TNF-α secreting DCs in asymptomatic subjects support the significance of TNF-α in the early eradication of borrelia. The presence of both antibodies and T-cell responses to borrelia in the asymptomatic individuals strongly indicates a previous challenge [7] and the absence of clinical symptoms of LB suggests an optimal eradication of the spirochetes.
Whether or not the production of TNF-α in DCs is unique for the borrelia spirochete is unfortunately not known. However, the activation of DCs by different antigens (including borrelia) is known to be specific with respect to pathogen associated molecular patterns (PAMPs) detected, e.g. by Toll-like receptors [49]. Therefore, the cytokine production in dendritic cells in our experiments was induced most probably by borrelia, but it could not be excluded that other agents expressing identical PAMPs also would induce similar cytokine secretion patterns.
The number of TNF-α-secreting DCs did not differ between patients with a history of chronic NB and asymptomatic subjects. Assuming that early TNF-α production is beneficial for the outcome, this is a somewhat surprising finding. The chronic patients may, however, have a defect at another level of the immune response, e.g. in the down-regulation of the inflammatory response, which leads in turn to chronic inflammation and disease. Interestingly, the number of DCs secreting IL-10, a regulatory cytokine, was very low or absent in all patients with chronic NB.
The reason for differences in the number of cytokine secreting dendritic cells following stimulation with either live spirochetes, sonicated spirochetes or outer surface protein fractions (Osp) of the borrelia spirochete might only be speculated on. Proteins in live and sonicated spirochetes are likely to be more native compared to those in an antigen preparation and would, thus, better reflect the in vivo situation in the initiation of immune responses.
Early Th1-responses may be of major importance for an optimal eradication of borrelia, as suggested previously by studies in both mice and men [24,25]. IL-12 is the major factor polarizing naive T-cells towards Th1 [50]. Thus, early supply of IL-12 is significant for establishment of strong Th1-responses. The administration of IL-12 antibodies to mice with severe combined immunodeficiency (SCID) increases the severity of acute Lyme arthritis in contrast to immunocompetent mice, in which IL-12 antibodies cause reduction of the disease [51]. This suggests that anti-IL-12 down-regulates the innate immune response in an environment without T or B cells, in turn indicating that IL-12, besides playing a significant role in the polarization of T cell responses, also has an important role in the early innate effector response. The reduction of disease in immunocompetent mice after administration of anti-IL-12-antibodies may mirror the delicate balance between eradication and immune-mediated host tissue injury, i.e. Th1-inflammation eradicates borrelia, but does also cause symptoms of disease.
The present finding of increased borrelia-induced IL-12p70 secretion in whole blood cells from asymptomatic subjects compared with seronegative individuals, but no such increase in patients with a history of clinical borreliosis, supports the significance of early IL-12 secretion for optimal eradication of borrelia. However, the number of borrelia-induced IL-12-secreting DCs remained low in our experiments, which may be explained by the fact that IL-12 secretion is enhanced by T cell-derived IFN-γ[52]. T cells were present in the whole blood stimulations, whereas in DC-stimulations the only source of IFN-γ was, in fact, the DCs. Thus, the interpretation of the current results concerning IL-12p70 in whole blood cell supernatants should be taken with caution.
In a recently published report [53], the dynamics of different pro- and anti-inflammatory cytokines in PBMC were measured following stimulation with live borrelia spirochetes. The results showed an overall synthesis of IL-1β, IL-6, IL-10, IL-12, TNF-α and IFN-γ in PBMC and more specifically, that TNF-α increased immediately after the stimulation, reaching a peak within 4 h and then remaining stable during the 24-h test. IL-12, on the other hand, started to increase after 8 h without reaching a peak within 24 h. In accordance with this, we were also able to measure detectable levels of IL-1β, IL-6, IL-8, IL-10, IL-12p70 and TNF-α in whole blood cell supernatants from all the patients, even though no differences were found between the groups, except concerning IL-12p70.
In conclusion, asymptomatic borrelia-seropositive individuals may have an enhanced innate immune activation, demonstrated as strong TNF-α and IL-12 responses to live spirochetes. Our findings support the view that proinflammatory and Th1-inducing responses in the early disease course results in an optimal resolution of LB. The results should, however, be confirmed in a larger study.
Acknowledgments
We thank Lise-Lott Lindvall, Petra Cassel and Jeannette Svartz, the Clinic for Infectious Diseases, University Hospital, Linköping, the Division of Clinical Immunology, Department of Molecular and Clinical Medicine, Faculty of Health Sciences, Linköping University and the Department of Transfusion Medicine and the Division of Clinical Immunology, University Hospital, Linköping, for help with collection of patient samples and excellent laboratory assistance. This work was supported by grants from the Swedish Research Council in Medicine, the Foundation for Strategic Research, the County Council of Östergötland and Linköping University.
References
- 1.Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease − a tick-borne spirochetosis? Science. 1982;216:1317–19. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 2.Weber K. Aspects of Lyme borreliosis in Europe. Eur J Clin Microbiol Infect Dis. 2001;20:6–13. doi: 10.1007/s100960000412. [DOI] [PubMed] [Google Scholar]
- 3.Balmelli T, Piffaretti JC. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res Microbiol. 1995;146:329–40. doi: 10.1016/0923-2508(96)81056-4. [DOI] [PubMed] [Google Scholar]
- 4.Lagal V, Postic D, Ruzic-Sabljic E, Baranton G. Genetic diversity among Borrelia strains determined by single-strand conformation polymorphism analysis of the ospC gene and its association with invasiveness. J Clin Microbiol. 2003;41:5059–65. doi: 10.1128/JCM.41.11.5059-5065.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berglund J, Eitrem R, Ornstein K, et al. An epidemiologic study of Lyme disease in southern Sweden. N Engl J Med. 1995;333:1319–27. doi: 10.1056/NEJM199511163332004. [DOI] [PubMed] [Google Scholar]
- 6.Ekerfelt C, Forsberg P, Svenvik M, Roberg M, Bergstrom S, Ernerudh J. Asymptomatic Borrelia-seropositive individuals display the same incidence of Borrelia-specific interferon-gamma (IFN-gamma)-secreting cells in blood as patients with clinical Borrelia infection. Clin Exp Immunol. 1999;115:498–502. doi: 10.1046/j.1365-2249.1999.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekerfelt C, Masreliez C, Svenvik M, Ernerudh J, Roberg M, Forsberg P. Antibodies and T-cell reactivity to Borrelia burgdorferi in an asymptomatic population: a study of healthy blood donors in an inland town district in the south-east of Sweden. Scand J Infect Dis. 2001;33:806–8. doi: 10.1080/00365540110077376. [DOI] [PubMed] [Google Scholar]
- 8.Steere AC, Sikand VK, Schoen RT, Nowakowski J. Asymptomatic infection with Borrelia burgdorferi. Clin Infect Dis. 2003;37:528–32. doi: 10.1086/376914. [DOI] [PubMed] [Google Scholar]
- 9.Nocton JJ, Steere AC. Lyme disease. Adv Intern Med. 1995;40:69–117. [PubMed] [Google Scholar]
- 10.O’Connell S Stanek G, Cimmino M. European Union concerted action on risk assessment in Lyme borreliosis: clinical case definitions for Lyme borreliosis. Wien Klin Wochenschr. 1996;108:741–47. [PubMed] [Google Scholar]
- 11.Oschmann P, Dorndorf W, Hornig C, Schafer C, Wellensiek HJ, Pflughaupt KW. Stages and syndromes of neuroborreliosis. J Neurol. 1998;245:262–72. doi: 10.1007/s004150050216. [DOI] [PubMed] [Google Scholar]
- 12.Treib J, Fernandez A, Haass A, Grauer MT, Holzer G, Woessner R. Clinical and serologic follow-up in patients with neuroborreliosis. Neurology. 1998;51:1489–91. doi: 10.1212/wnl.51.5.1489. [DOI] [PubMed] [Google Scholar]
- 13.Berglund J, Stjernberg L, Ornstein K, Tykesson-Joelsson K, Walter H. 5-Year follow-up study of patients with neuroborreliosis. Scand J Infect Dis. 2002;34:421–25. doi: 10.1080/00365540110080421. [DOI] [PubMed] [Google Scholar]
- 14.Vrethem M, Hellblom L, Widlund M, et al. Chronic symptoms are common in patients with neuroborreliosis − a questionnaire follow-up study. Acta Neurol Scand. 2002;106:205–8. doi: 10.1034/j.1600-0404.2002.01358.x. [DOI] [PubMed] [Google Scholar]
- 15.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–31. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 16.Lengl-Janssen B, Strauss AF, Steere AC, Kamradt T. The T helper cell response in Lyme arthritis: differential recognition of Borrelia burgdorferi outer surface protein A in patients with treatment-resistant or treatment-responsive Lyme arthritis. J Exp Med. 1994;180:2069–78. doi: 10.1084/jem.180.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsberg P, Ernerudh J, Ekerfelt C, Roberg M, Vrethem M, Bergström S. The outer surface proteins of Lyme disease borrelia spirochetes stimulate T cells to secrete interferon-gamma (IFN-γ): diagnostic and pathogenic implications. Clin Exp Immunol. 1995;101:453–60. doi: 10.1111/j.1365-2249.1995.tb03134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oksi J, Savolainen J, Pene J, Bousquet J, Laippala P, Viljanen MK. Decreased interleukin-4 and increased gamma interferon production by peripheral blood mononuclear cells of patients with Lyme borreliosis. Infect Immun. 1996;64:3620–23. doi: 10.1128/iai.64.9.3620-3623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekerfelt C, Ernerudh J, Bunikis J, et al. Compartmentalization of antigen specific cytokine responses to the central nervous system in CNS borreliosis. secretion of IFN-gamma predominates over IL-4 secretion in response to outer surface proteins of Lyme disease Borrelia spirochetes. J Neuroimmunol. 1997;79:155–62. doi: 10.1016/s0165-5728(97)00118-5. [DOI] [PubMed] [Google Scholar]
- 20.Yin Z, Braun J, Neure L, et al. T cell cytokine pattern in the joints of patients with Lyme arthritis and its regulation by cytokines and anticytokines. Arthritis Rheum. 1997;40:69–79. doi: 10.1002/art.1780400111. [DOI] [PubMed] [Google Scholar]
- 21.Ekerfelt C, Ernerudh J, Forsberg P, Bergström S. Augmented intrathecal secretion of interferon-gamma in response to Borrelia garinii in neuroborreliosis. J Neuroimmunol. 1998;89:177–81. doi: 10.1016/s0165-5728(98)00136-2. [DOI] [PubMed] [Google Scholar]
- 22.Widhe M, Ekerfelt C, Forsberg P, Bergström S, Ernerudh J. IgG subclasses in Lyme borreliosis: a study of specific IgG class distribution in an interferon-gamma-predominated disease. Scand J Immunol. 1998;47:575–81. [PubMed] [Google Scholar]
- 23.Grusell M, Widhe M, Ekerfelt C. Increased expression of the Th1-inducing cytokines interleukin-12 and interleukin-18 in cerebrospinal fluid but not in sera from patients with Lyme neuroborreliosis. J Neuroimmunol. 2002;131:173–78. doi: 10.1016/s0165-5728(02)00255-2. [DOI] [PubMed] [Google Scholar]
- 24.Kang I, Barthold SW, Persing DH, Bockenstedt LK. T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun. 1997;65:3107–11. doi: 10.1128/iai.65.8.3107-3111.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widhe M, Jarefors S, Ekerfelt C, et al. Borrelia-specific interferon-gamma and interleukin-4 secretion in cerebrospinal fluid and blood during Lyme borreliosis in humans: association with clinical outcome. J Infect Dis. 2004;189:1881–91. doi: 10.1086/382893. [DOI] [PubMed] [Google Scholar]
- 26.Radolf JD, Norgard MV, Brandt ME, Isaacs RD, Thompson PA, Beutler B. Lipoproteins of Borrelia burgdorferi and Treponema pallidum activate cachectin/tumor necrosis factor synthesis. Analysis using a CAT reporter construct. J Immunol. 1991;147:1968–74. [PubMed] [Google Scholar]
- 27.Hurtenbach U, Museteanu C, Gasser J, Schaible UE, Simon MM. Studies on early events of Borrelia burgdorferi-induced cytokine production in immunodeficient SCID mice by using a tissue chamber model for acute inflammation. Int J Exp Pathol. 1995;76:111–23. [PMC free article] [PubMed] [Google Scholar]
- 28.Isogai H, Kimura K, Hayashi S, et al. Levels of endogenous interleukin-1, interleukin-6, and tumor necrosis factor in congenic mice infected with Borrelia garinii. Microbiol Immunol. 1997;41:427–30. doi: 10.1111/j.1348-0421.1997.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 29.Pachner AR, Amemiya K, Delaney E, O'Neill T, Hughes CA, Zhang WF. Interleukin-6 is expressed at high levels in the CNS in Lyme neuroborreliosis. Neurology. 1997;49:147–52. doi: 10.1212/wnl.49.1.147. [DOI] [PubMed] [Google Scholar]
- 30.Talkington J, Nickell SP. Borrelia burgdorferi spirochetes induce mast cell activation and cytokine release. Infect Immun. 1999;67:1107–15. doi: 10.1128/iai.67.3.1107-1115.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harjacek M, Diaz—Cano S, Alman BA, et al. Prominent expression of mRNA for proinflammatory cytokines in synovium in patients with juvenile rheumatoid arthritis or chronic Lyme arthritis. J Rheumatol. 2000;27:497–503. [PubMed] [Google Scholar]
- 32.Widhe M, Grusell M, Ekerfelt C, Vrethem M, Forsberg P, Ernerudh J. Cytokines in Lyme borreliosis: lack of early tumour necrosis factor-alpha and transforming growth factor-beta1 responses are associated with chronic neuroborreliosis. Immunology. 2002;107:46–55. doi: 10.1046/j.1365-2567.2002.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–96. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 34.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 35.Filgueira L, Nestle FO, Rittig M, Joller HI, Groscurth P. Human dendritic cells phagocytose and process Borrelia burgdorferi. J Immunol. 1996;157:2998–3005. [PubMed] [Google Scholar]
- 36.Suhonen J, Komi J, Soukka J, Lassila O, Viljanen MK. Interaction between Borrelia burgdorferi and immature human dendritic cells. Scand J Immunol. 2003;58:67–75. doi: 10.1046/j.1365-3083.2003.01284.x. [DOI] [PubMed] [Google Scholar]
- 37.Mbow ML, Zeidner N, Panella N, Titus RG, Piesman J. Borrelia burgdorferi-pulsed dendritic cells induce a protective immune response against tick-transmitted spirochetes. Infect Immun. 1997;65:3386–90. doi: 10.1128/iai.65.8.3386-3390.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen K, Lebech AM. Lyme neuroborreliosis: a new sensitive diagnostic assay for intrathecal synthesis of Borrelia burgdorferi− specific immunoglobulin G, A, and M. Ann Neurol. 1991;30:197–205. doi: 10.1002/ana.410300212. [DOI] [PubMed] [Google Scholar]
- 39.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- 40.Kriuchechnikov VN, Korenberg EI, Shcherbakov SV, et al. [Identification of Borrelia isolated in the USSR from Ixodes persulcatus Schulze ticks] Zh Mikrobiol Epidemiol Immunobiol. 1988;12:41–4. [PubMed] [Google Scholar]
- 41.Bergström S, Sjöstedt A, Dotevall L, et al. Diagnosis of Lyme borreliosis by an enzyme immunoassay detecting immunoglobulin G reactive to purified Borrelia burgdorferi cell components. Eur J Clin Microbiol Infeat Dis. 1991;10:422–7. doi: 10.1007/BF01968022. [DOI] [PubMed] [Google Scholar]
- 42.Czerkinsky C, Nilsson LA, Ouchterlony O, Tarkowski A, Gretzer C. Detection of single antibody-secreting cells generated after in vitro antigen-induced stimulation of human peripheral blood lymphocytes. Scand J Immunol. 1984;19:575–9. doi: 10.1111/j.1365-3083.1984.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 43.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 44.Pasparakis M, Alexopoulou L, Douni E, Kollias G. Tumour necrosis factors in immune regulation: everything that's interesting is…new! Cytokine Growth Factor Rev. 1996;7:223–39. doi: 10.1016/s1359-6101(96)00031-7. [DOI] [PubMed] [Google Scholar]
- 45.Trinchieri G, Pflanz S, Kastelein RA. The IL-12 family of heterodimeric cytokines. new players in the regulation of T cell responses. Immunity. 2003;19:641–4. doi: 10.1016/s1074-7613(03)00296-6. [DOI] [PubMed] [Google Scholar]
- 46.Haupl T, Landgraf S, Netusil P, et al. Activation of monocytes by three OspA vaccine candidates: lipoprotein OspA is a potent stimulator of monokines. FEMS Immunol Med Microbiol. 1997;19:15–23. doi: 10.1111/j.1574-695X.1997.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 47.Radolf JD. Treponema pallidum and the quest for outer membrane proteins. Mol Microbiol. 1995;16:1067–73. doi: 10.1111/j.1365-2958.1995.tb02332.x. [DOI] [PubMed] [Google Scholar]
- 48.Ramachandra RN, Berczi A, Sehon AH, Berczi I. Inhibition of lipid A- and lipopolysaccharide-induced cytokine secretion, B cell mitogenesis, and lethal shock by lipid A-specific murine monoclonal antibodies. J Infect Dis. 1993;167:1151–9. doi: 10.1093/infdis/167.5.1151. [DOI] [PubMed] [Google Scholar]
- 49.Netea MG, van der Graaf C, Van der Meer JW, Kullberg BJ. Toll-like receptors and the host defense against microbial pathogens: bringing specificity to the innate-immune system. J Leukoc Biol. 2004;75:749–55. doi: 10.1189/jlb.1103543. [DOI] [PubMed] [Google Scholar]
- 50.Murphy E, Shibuya K, Hosken N, et al. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–13. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anguita J, Samanta S, Barthold SW, Fikrig E. Ablation of interleukin-12 exacerbates Lyme arthritis in SCID mice. Infect Immun. 1997;65:4334–6. doi: 10.1128/iai.65.10.4334-4336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flesch IE, Hess JH, Huang S, et al. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon gamma and tumor necrosis factor alpha. J Exp Med. 1995;181:1615–21. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jansky L, Reymanova P, Kopecky J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol Res. 2003;52:593–8. [PubMed] [Google Scholar]