Abstract
Interleukin-15 (IL-15) is a neutrophil agonist that plays a role in inflammatory disorders, including a variety of pulmonary diseases. Adhesion of neutrophils onto pulmonary cells is a major event leading to development of inflammation. Recently, elevated levels of IL-15 have been associated with different pulmonary diseases. There is no clear evidence that IL-15 modulates cell surface expression of adhesion molecules in neutrophils, or that IL-15 is involved in neutrophil adhesion onto pulmonary cells. Also, it is not clear if IL-15 induces a neutrophilic inflammation in vivo. This study was aimed at elucidation of these issues. Neutrophils were treated with IL-15 and cell surface expression of CD11a, CD11b, CD11c and CD18 was monitored by flow cytometry. The human respiratory epithelial A549 cell line was used as a substrate for the neutrophil adhesion assay and cell surface expression of CD50, CD54 and CD106 was monitored in IL-15-induced A549 cells. The murine air pouch model was used for investigating potential neutrophilic inflammation induced by IL-15 in vivo. IL-15 significantly increased neutrophil cell surface expression of CD11b and CD18 and up-regulated A549 cell surface expression of CD54. Moreover, A549 cells were found to express IL-15R components and adhesion of neutrophils onto A549 cells was increased when neutrophils or A549 cells were treated with IL-15. Finally, IL-15 induced neutrophilic inflammation in vivo and concentrations of IL-6 and CXCL2/MIP-2 were increased in IL-15-induced pouches. IL-15 might participate in inflammatory pulmonary diseases by attracting neutrophils, modulating cell surface expression molecules and increasing neutrophil adhesion onto pulmonary cells.
Keywords: neutrophils, adhesion, cell adhesion molecules, interleukin-15, respiratory cells
Introduction
Interleukin-15 (IL-15) is known to mediate its biological activity through the IL-15 receptor (IL-15R), which is composed of three distinct chains, IL-15Rα, IL-2/15Rβ (CD122), and the common γc chain (CD132), shared by receptors to IL-2, IL-4, IL-7, IL-9 and IL-21. Gene and protein expressions of IL-15 have been demonstrated to be tightly regulated by complex mechanisms at the transcriptional [1–4], and secretion levels [5], suggesting that overexpression of IL-15 could be deleterious to the host. Elevated concentrations of IL-15 have been associated with several autoimmune and inflammatory diseases. Increased levels of IL-15 have been detected in the synovial fluids and in the synovium of rheumatoid arthritis patients [6,7]. IL-15 is also highly expressed in inflammatory pulmonary diseases, including tuberculosis, sarcoidosis and chronic bronchitis [8,9]. These results support the hypothesis that IL-15 expression needs to be tightly regulated and that its overexpression is associated with inflammatory diseases.
IL-15 was found to be a T-lymphocyte chemoattractant [10] and this property has been shown to contribute to rheumatoid arthritis pathogenesis [6]. IL-15 can induce the redistribution of CD50 (ICAM-3), and, to a lesser extent, ICAM-1, ICAM-2, CD43 and CD44 in uropods of T cells [11]. IL-15 produced by endothelial cells has been shown to increase the transendothelial migration of T cells in vitro and in the SCID mouse-human rheumatoid arthritis model in vivo[12]. IL-15 also cooperates in the migration of T cells through the endothelium by interacting with the LFA-1 (CD11a/CD18)/ICAM-1 (CD54) pathway [13].
Neutrophils are major players in inflammation and are known to express all components of the IL-15R [14–16]. IL-15 can induce phagocytosis, cytoskeleton rearrangement, gene expression, de novo protein synthesis and can delay apoptosis in human neutrophils [14]. Production of chemokines, cytokines and natural inhibitors is increased in IL-15-induced neutrophils, including CXCL8 (IL-8) [17,18], IL-1β, sIL-1RII and IL-1Ra [19,20]. IL-15 has also been shown to induce the redistribution of ICAM-3 and P-selectin glycoprotein ligand-1 (PSGL-1) to the uropods in neutrophil [21]. The mechanisms involved in IL-15-induced activation of human neutrophils are not fully understood. However, IL-15 was shown to activate NF-κB [18] and to induce the phosphorylation of Syk and its physical association with IL-15Rα[22].
Knowing that recruitment of neutrophils to the lungs and transmigration through the pulmonary epithelium to reach the respiratory tract are important steps in the inflammatory process, and that high levels of IL-15 are associated with different pulmonary diseases, there is curiously no data available regarding the role of IL-15 on the expression of cell surface molecules in human neutrophils, nor on its ability to modulate adhesion onto respiratory cells. Furthermore, it has never been clearly established whether IL-15 can induce a neutrophilic inflammation in vivo, as we have recently demonstrated with IL-21 [23].
The above observations prompted us to investigate the role of IL-15 in the cell surface expression of adhesion molecules in human neutrophils, as well as its role in adhesion onto respiratory cells. In addition, we were interested in answering whether or not IL-15 could induce a neutrophilic inflammation in vivo. Herein, we demonstrated that IL-15 increased cell surface expression of CD11b and CD18 in neutrophils. IL-15 was also found to up-regulate A549 cell surface expression of the intercellular adhesion molecule-1 (ICAM-1; CD54) as well as neutrophil adhesion onto these cells. Finally, using the murine air pouch model, we found that IL-15 induced a neutrophilic inflammation in vivo, based on the large recruitment of neutrophils. This neutrophil influx was associated with an increase in the concentration of the pro-inflammatory cytokine IL-6 and the chemokine CXCL2/MIP-2, two molecules important in the recruitment and activation of neutrophils.
Materials and methods
Chemicals, agonists and antibodies
Recombinant human (rh) and recombinant murine (rm) IL-15 were purchased from PeproTech Inc. (Rocky Hill, NJ, USA). Recombinant human IL-21 was kindly provided by Donald C. Foster from ZymoGenetics Inc. (Seattle, WA, USA). Recombinant human TNF-α was purchased from R & D Systems (Minneapolis, MN, USA). The FITC-rat anti-mouse F4/80 antigen (rat IgG2b), the FITC-rat anti-mouse neutrophils clone 7/4 (rat IgG2a) and the FITC-rat IgG2a negative control were from Serotec Inc. (Raleigh, NC, USA). Pure murine isotypic control antibodies IgG1 and IgG2a were purchased from BD Biosciences (Mississauga, Ontario, Canada). The mouse monoclonal anti-human CD11b clone 44 (mouse IgG1) was purchased from Sigma Chemical Company (Saint-Louis, MO, USA). The mouse IgG2a anti-human CD11a clone 38, the mouse IgG1 anti-human CD11c clone 3·9, and the mouse IgG2a anti-human CD18 clone IB4 were from Calbiochem (La Jolla, CA, USA). The mouse anti-human CD50 clone ICAM3·3, the mouse anti-human CD54 clone 15·2, and the mouse anti-human CD106 clone 1.G11B1 were purchased from Serotec. The mouse IgG1 anti-human CD25 (IL-2Rα), the mouse IgG2a anti-human CD122 (IL-2/15Rβ) and the rat IgG2b anti-human CD132 (γc) were from BD Biosciences/Pharmingen (San Diego, CA, USA). The mouse anti-human IL-15Rα clone M162 was a gift from GenMab (Utrecht, the Netherlands). Fluorescein (FITC)-conjugated goat anti-mouse IgG, F(ab′)2 fragment specific and FITC-conjugated donkey anti-rat were purchased from Jackson ImmunoResearch Laboratories Inc. (West Grove, PA, USA). Lipopolysaccharide (LPS) was purchased from Sigma.
Human neutrophil isolation
Neutrophils were isolated from venous blood of healthy volunteers by centrifugation over Ficoll-Paque (Amersham Pharmacia Biotech Inc., Baie d’Urfé, Québec, Canada) as previously described [20,22,23]. Blood donations were obtained from informed and consenting individuals according to institutionally approved procedures. Cell viability was monitored by trypan blue exclusion, and the purity (> 98%) was verified by cytology from cytocentrifuged preparations coloured by Hema-Stain staining kit.
Cell surface expression of CD11a, CD11b, CD11c, and CD18
Freshly isolated neutrophils were stimulated with rhIL-15 for 30 min. Following the incubation period, cells were suspended at 1·5 × 106 cells/ml, washed, and preincubated for 30 min at 4 °C with 20% autologous serum to prevent nonspecific binding via Fc receptors. Cells were then washed and incubated with the different antibodies (1 µg/ml) for 1 h at 4 °C. After two additional washes, cells were incubated with FITC-conjugated secondary antibody (1 µg/ml) for 1 h (4°C, light protected). Cells were then washed and fixed with paraformaldehyde (0·5%). Flow cytometric analysis (10 000 events) was performed using a FACScan (Becton Dickinson).
Epithelial cell culture
The human lung epithelial A549 cell line, known to be an excellent cell substrate for studying neutrophil adhesion onto respiratory epithelial cells [24–26] was purchased from the American Type Culture Collection (Rockville, MD, USA) and was grown in RPMI-1640 supplemented with 10% fetal calf serum and antibiotics. Cell viability was systematically evaluated before and after each treatment, and mortality never exceeded 5%.
Cell surface expression of CD50, CD54, CD106, and IL-15 receptor components on A549 cells
For these experiments, A549 cells were harvested from tissue culture flasks with the use of a rubber policeman and then transferred to 5 ml (75 × 12 mm) polypropylene tubes. Following the incubation period with PBS or rhIL-15, cells were suspended at 1·5 × 106 cells/ml, washed, and incubated with the different antibodies (1 µg/ml) for 1 h (4 °C, light protected). TNF-α was used as a positive control, as it was previously found to induce CD54 expression on A549 cells [26]. After two additional washes, cells were incubated with FITC-conjugated secondary antibody (1 µg/ml) for 1 h and were then washed. Flow cytometric analysis (10 000 events) was performed using a FACScan (Becton Dickinson). Expression of the IL-15R components on A549 cells was performed as above, but cells were not activated.
Neutrophil adherence assay
Neutrophils (treated or not with rhIL-15 for 2 h, previously found to be optimal) were labelled for 30 min with 5 µM calcein-AM (Molecular Probes, Inc., Eugene, OR, USA) and adhesion onto A549 cells was measured essentially as we have previously published [27]. The number of adherent neutrophils was calculated by counting the number of fluorescent cells from five randomly selected high-power fields (× 400) observed with a photomicroscope Leica DMRE equipped with an ebq 100 dc epifluorescent condenser. Images were taken with a Cooke Sensicam High performance camera coupled to the Image Pro-plus® (version 4·0) program. In other experiments, the neutrophil adhesion assay was performed with A549 cells activated with rhIL-15 for 24 h.
Air pouch experiments
Air pouches were created in CD−1 mice as previously published [23]. On day 6, 1 ml of rmIL-15 or the diluent (PBS) was injected into the air pouches of mice 6 h before the mice were killed by CO2 asphyxiation The air pouches were washed once with 1 ml and then twice with 2 ml of HBSS containing 10 mM EDTA, and the exudates were centrifuged at 100× g for 10 min at room temperature. Supernatants were collected and stored at −20 °C for further analysis. The cells were resuspended in 1 ml of HBSS-EDTA and counted. The cells (2 × 105) were centrifuged, spread onto microscope slides and stained with Hema-Stain to allow quantification of granulocytic and mononuclear populations. To further characterize the leucocyte subpopulations, the cells were suspended in PBS containing 5 µg/ml human IgG for 30 min at 4 °C to block Fc receptors and then stained for 30 min at 4 °C with purified rat anti-mouse 7/4 mAb directed against murine neutrophils or rat anti-mouse F4/80 antigen antibody directed against murine monocyte/macrophages [23]. Analysis was performed with a FACScan (Becton Dickinson, San Jose, CA, USA).
Detection of murine IL-6 and CXCL2
Fluids were harvested from air pouches after 6 h of treatment with buffer or IL-15. IL-6 and CXCL2 were quantified using the following commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's recommendations: Murine IL-6: (sensitivity of < 3 pg/ml, Biosource International, Camarillo, CA), Murine CXCL2 (sensitivity of < 1·5 pg/ml, R & D Systems). All samples were tested at least in duplicate.
Statistical analysis
Statistical analysis was performed with SigmaStat for Windows Version 2·03 with a one-way analysis of variance (anova). Statistical significance was established at P < 0·05.
Results
IL-15 up-regulates cell surface expression of CD11b/CD18 on human neutrophils
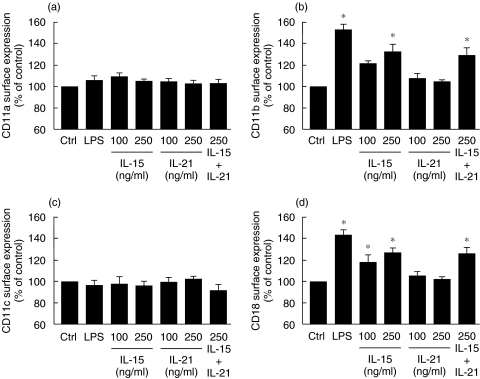
Because of the importance of IL-15 and other CD132-dependent cytokines in inflammation, and since elevated concentrations of IL-15 are associated with various inflammatory disorders, including pulmonary diseases [8,27–29], we decided to investigate the role of IL-15 on the cell surface expression of CD11a, CD11b, CD11c and CD18 in human neutrophils. As illustrated in Fig. 1, IL-15 was found to significantly increase cell surface expression of CD11b and CD18 but not of CD11a and CD11c. Only the results obtained after 30 min are illustrated, since there were no significant increases after 60 and 120 min. As expected, LPS was found to increase neutrophil cell surface expression of both CD11b and CD18. In contrast to IL-15, IL-21, a pro-inflammatory cytokine that is not a human neutrophil agonist [23], did not increase cell surface expression of the tested molecules. In addition, IL-21 did not alter the ability of IL-15 to increase cell surface expression of CD11b and CD18 in neutrophils when incubated simultaneously with IL-15. The geometric mean values for antibody binding to unstimulated cells varied from donor to donor: IgG1 (3·4–15·6); IgG2a (3·2–14·8); CD11a (53–88); CD11b (116–254); CD11c (6·3–52); CD18 (144–295).
Fig. 1.
IL-15 up-regulates cell surface expression of CD11b and CD18 in human neutrophils. Neutrophils were treated with buffer (Ctrl), LPS (1 µg/ml), IL-15, IL-21 or IL-15 + IL-21 and cell surface expression of (a) CD11a, (b) CD11b, (c) CD11c and (d) CD18 was monitored by flow cytometry as described in Methods. Results are means ± SEM (n = 5). *P < 0·05 versus control by anova.
IL-15 increases surface expression of CD54 on human respiratory epithelial A549 cells
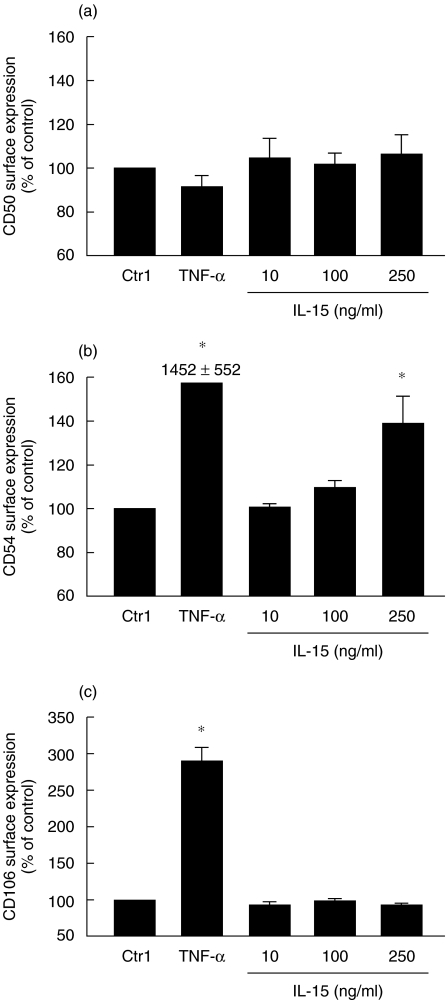
Because IL-15 up-regulated adhesion molecules expression in neutrophils, we decided to study the role of IL-15 on the expression of CD50 (ICAM-3), CD54 (ICAM-1) and VCAM-1 (CD106) in A549 cells, knowing the importance of epithelial–neutrophil interaction in airway inflammation and chronic obstructive pulmonary diseases [30]. As illustrated in Fig. 2, IL-15 increased CD54 expression in A549 cells, but had no effect on CD50 and CD106 cell surface expression after 24 h of treatment. We have also verified such expression after 1, 2 and 15 h, but the level of cell surface expression of the tested molecules was not increased after 1 and 2 h and was similar after 15 or 24 h. The geometric mean values for antibody binding to unstimulated cells varied slightly between experiments: IgG1 (3·3–6·8); CD50 (2·8–6·2); CD54 (2·9–7·7); CD106 (3·1–6·5). As expected, TNF-α increased CD54 and CD106 cell surface expression [31]. Our results suggest that, unlike human neutrophils [32], A549 cells do not strongly express CD50 on their surface, and we failed to increase its expression after stimulation with EGF, PMA, LPS, or INF-γ (data not shown).
Fig. 2.
IL-15 up-regulates cell surface expression of CD54 in human respiratory epithelial A549 cells. Cells were incubated for 24 h with buffer (Ctrl), 100 ng/ml TNF-α (A), 1000 ng/ml TNF-α (C), or IL-15. Cell surface expression of (a) CD50, (b) CD54 and (c) CD106 was monitored by flow cytometry as described in Methods. Results are means ± SEM (n = 3). *P < 0·05 versus control (Ctrl) by anova.
A549 cells expression IL-15 receptor components
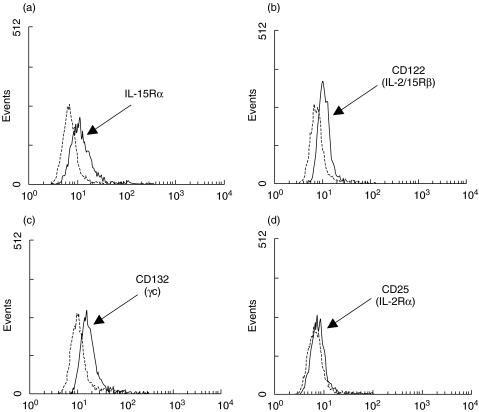
Because A549 cells respond to IL-15, and because one study reported that these cells expressed one component of the IL-15R, CD122 (IL-2/15Rβ) and weakly CD25 (IL-2Rα) [33], we next investigated cell surface expression of all IL-15R components by flow cytometry. As illustrated in Fig. 3, A549 cells express IL-15Rα, CD122 and CD132. As expected, CD25 was weakly or not expressed on these cells [33].
Fig. 3.
A549 cells express IL-15R components. Cells were cultivated, harvested and cell surface expression of (a) IL-15Rα, (b) CD122, (c) CD132 and (d) CD25 was monitored by flow cytometry as described in Methods. Results are from one experiment out of three. Dashed lines represent appropriate isotypic control.
IL-15 increased neutrophil adhesion onto respiratory epithelial A549 cells
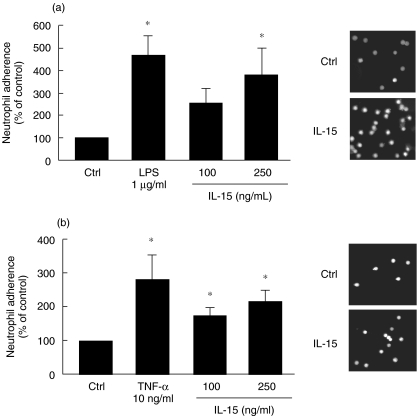
Because adhesion of neutrophils to airway epithelial cells is an important step in the inflammatory process [30], we next investigated whether or not IL-15 could increase the ability of neutrophils to adhere onto human epithelial lung A549 cells. As illustrated in Fig. 4a, the adhesion of IL-15-induced neutrophils onto A549 cells was significantly, and dose-dependently, increased when compared with untreated neutrophils, reaching an increase of 380% at 250 ng/ml IL-15. Also, treatment of A549 cells with 100 or 250 ng/ml IL-15 was found to increase the adhesion of untreated neutrophils (Fig. 4b).
Fig. 4.
IL-15 enhances adhesion of neutrophils onto A549 cells. (a) neutrophils were stimulated with buffer (Ctrl), LPS, or IL-15 for 2 h, labelled with calcein AM, incubated on confluent A549 cells for 30 min and adhesion was measured as described in Methods. Results are means ± SEM (n = 4). (b) The adhesion assay was performed as in A, except that A549 cells were stimulated with buffer (Ctrl), TNF-α or IL-15 for 24 h. Results are means ± SEM (n = 3). Pictures on the right part of the figure illustrated typical data that were plotted for the graph. *P < 0·05 versus Ctrl by anova.
IL-15 induces a neutrophilic inflammation in vivo
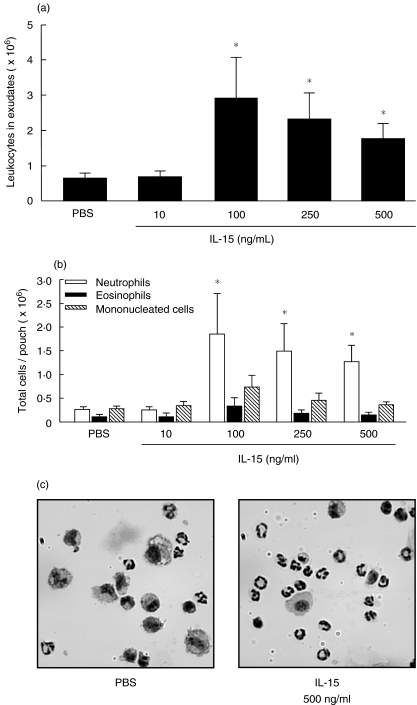
We decided to use the murine air pouch model in order to evaluate the potential pro-inflammatory effects of IL-15 in vivo, which we have recently used to establish that IL-21 is a pro-inflammatory cytokine [23]. As illustrated in Fig. 5a, IL-15 induces leucocyte accumulation into the air pouch, with a maximum of cells attracted (2·9 ± 1·1 × 106 cells/pouch) at 100 ng/ml rmIL-15. Leukocyte accumulation decreased at a concentrations of 250 ng/ml (2·3 ± 0·8 × 106 cells/pouch) or 500 ng/ml rmIL-15 (1·8 ± 0·4 × 106 cells/pouch), but the number of cells was still significantly higher than in control mice (0·7 ± 0·1 × 106 cells/pouch). Cytocentrifuged preparations were performed in order to characterize the leucocyte subpopulations migrating into the air pouch. As illustrated in Fig. 5b, low levels of neutrophils, eosinophils, and mononucleated cells were attracted in control mice as well as in mice treated with 10 ng/ml rmIL-15. Administration of 100, 250 or 500 ng/ml rmIL-15 resulted mainly in the recruitment of neutrophils. The number of eosinophils and mononucleated cells was also slightly increased after the injection of 100 ng/ml rmIL-15.
Fig. 5.
IL-15 induces a leucocyte influx in vivo. (a) air pouches were raised before injection of PBS or IL-15, exudates were harvested 6 h later and the number of leucocytes was calculated (means ± SEM (n ≥ 8)). C, representative preparations of cells harvested from concentrated PBS-injected mice and from non concentrated IL-15-injected mice. *P < 0·05 versus control by anova.
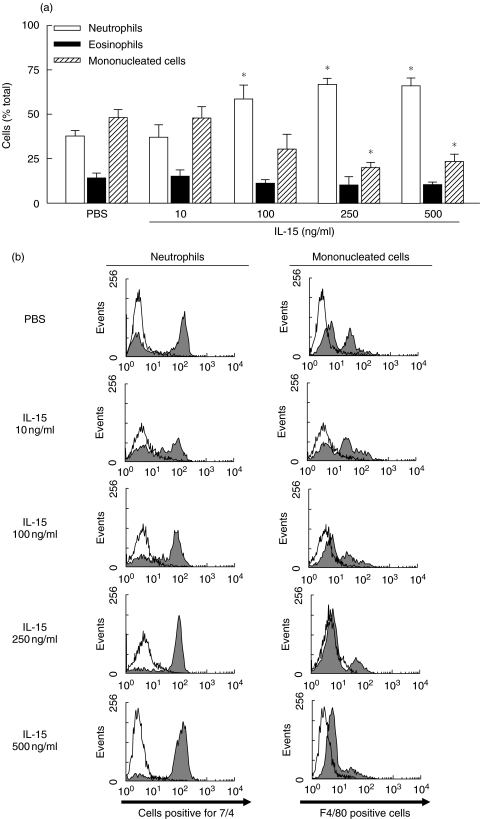
We then evaluated the percentage of each cell type attracted in the pouch. As illustrated in Fig. 6a, the injection of rmIL-15 increased the percentage of neutrophils in a concentration-dependent manner, with a maximum of ∼70% at 250 ng/ml (68·7 ± 3·6%) versus 66·0 ± 4·6% at 500 ng/ml even if the the total leucocyte number decreased when compared with 100 ng/ml. Flow cytometry analysis confirmed that the percentage of neutrophils attracted by IL-15 increased, whereas the percentage of mononucleated cells decreased in a concentration-dependent manner (Fig. 6b).
Fig. 6.
IL-15 induces a neutrophilic inflammation in vivo. (a) the percentage of each cell type attracted into PBS- or IL-15-induced pouch was evaluated (means ± SEM; n ≥ 8). (b) cells were stained with 7/4 or F4/80 antibody. Open histogram, appropriate isotypic controls. Exudates from PBS-treated mice were concentrated to obtain a good number of cells for identification. *P < 0·05 versus control by anova.
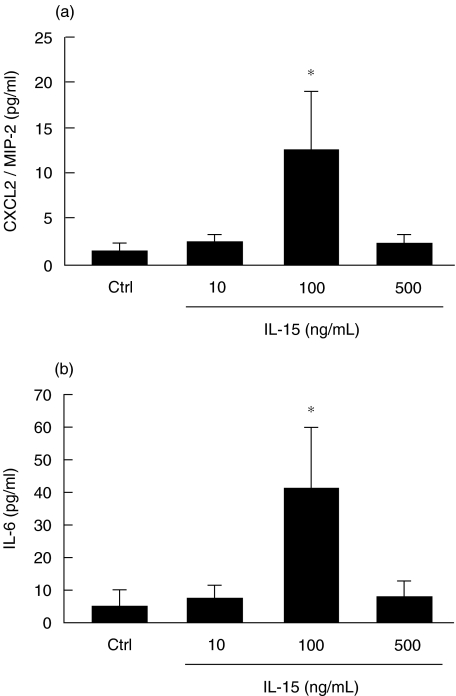
CXCL2 and IL-6 levels are increased in IL-15-induced air pouch
As we have recently documented, IL-21, unlike LPS, attracted neutrophils in vivo by a mechanism independent of IL-6, CCL3, CCL5 and CXCL2 production [23]. Because of this, we decided to measure the levels of IL-6 and CXCL2 in the IL-15-induced air pouch. We selected CXCL2, a functional equivalent of the important chemotactic factor for human CXCL8/IL-8, and the pro-inflammatory cytokine IL-6. As illustrated in Fig. 7, IL-15 increased both CXCL2 and IL-6 levels and these levels paralleled the number of total leucocytes attracted, but not the number of neutrophils that increased in a concentration-dependent response. These results strongly suggest that the production of CXCL2 and IL-6 is not performed by neutrophils. Also, these results indicate that the presence of neutrophils in IL-15-induced mice may be related to the local production of CXCL2 and IL-6 and that CXCL2 and/or IL-6 may enhance the response to IL-15 by increasing the total number of leucocytes, including neutrophils.
Fig. 7.
IL-15 increases CXCL2/MIP-2 and IL-6 production. Air pouches were created and buffer PBS (Ctrl), or IL-15 (10–500 ng/ml) were administered into pouch as described in Methods. Exudates were harvested after 6 h and CXCL2 (a) and IL-6 (b) production were measured using specific ELISA kits. Results are means ± SEM (n = 10).
Discussion
This is the first study providing evidence that IL-15 may participate in inflammatory pulmonary diseases by modulating cell surface expression of adhesion molecules, increasing neutrophil adhesion onto respiratory pulmonary cells and attracting neutrophils in vivo. Moreover, this is the first time that IL-15 has been demonstrated to induce a neutrophilic inflammation in vivo, despite the fact that this cytokine has been characterized as a neutrophil agonist and a pro-inflammatory cytokine [6,14,18].
The observations that IL-15 up-regulates adhesion between neutrophils and A549 respiratory epithelial cells and increases CD11b/CD18 expression in neutrophils and CD54 (ICAM-1) A549 cells, fit well with the participation of IL-15 in pulmonary diseases, especially knowing that elevated concentrations of IL-15 have been reported in such situations. Interestingly, activation of A549 cells by TNF-α or IL-1β was found to induce the expression of IL-15 at mRNA and protein levels [24]. However, the production of IL-15 protein was detected in the cytosol and not in the extracellular milieu. Confluent cultures of A549 cells were treated with IL-1β or IFN-γ for 72 h or TNF-α for up to 168 h and the concentration of IL-15 in the cellular supernatants never exceeded the limit of detection (∼5 pg/ml) of the ELISA kit used [24]. In another study, the serum levels of IL-15 were measured in the serum of 60 patients with systemic lupus erythematosus and 20 healthy subjects, and the levels were very low in both conditions (median 2·9 pg/ml and 1·6 pg/ml, respectively) [34]. Production and release of IL-15 is known to be under tight control suggesting that this cytokine must not be released into the external milieu [1–4]. Post-transcriptional regulation of IL-15 occurs via the 5′ untranslated region AUG triplets, 3′ regulatory elements and a putative C-terminus region regulatory site. Of interest, the existence of two isoforms of IL-15 with altered glycosylation have been reported:
an abnormally long signalling peptide of 48 amino acids representing the secreted form;
a short signalling peptide of 21 amino acids remaining inside the cell and localized to nonendoplasmic regions in both cytoplasmic and nuclear compartments [3].
Taken together, the above observations can easily explain the difficulty in detecting soluble IL-15 in biological systems and indicate why IL-15 is an important component of inflammatory disorders.
In a recent study, human apoptotic neutrophils undergoing secondary necrosis, an event occurring when apoptotic neutrophils are not eliminated by professional phagocytes like macrophages, were found to induce lung epithelial cell detachment [35]. Of interest, A549 cells were among the cell types studied. Although the molecular mechanism of A549 cell (and airway epithelial cells) detachment is complex and not fully understood, the authors clearly demonstrated that cell membrane integrity was disrupted in advance of cell detachment. Knowing that IL-15 induced ICAM-1 on A549 cells and CD11b/CD18 on neutrophils (this report), it is plausible that elevated levels of IL-15 in pulmonary diseases favour interaction between neutrophils and pulmonary epithelial cells. In addition, our results regarding the increased adhesion of IL-15-induced neutrophils onto A549 cells attest to the importance of IL-15 during pulmonary inflammation. Moreover, expression of IL-15Rα, CD122 and CD132 on A549 cell surface, as well as the fact that the adhesion of untreated neutrophils onto IL-15-activated A549 cells was increased, further support the importance of IL-15 during pulmonary inflammation.
Other CD132-dependent cytokines, in particular IL-2 and IL-4, are known to play important roles in inflammatory disorders [36]. Since neutrophils express the CD132 component on the cell surface, one can imagine that other cytokines of this family may be able to up-regulate the expression of CD11b/CD18. However, our preliminary data suggest that IL-2, IL-4, IL-7 and IL-9 do not modulate cell surface expression of CD11a, CD11b, CD11c, and CD18, when tested in the same experimental conditions as IL-15 (Martin Pelletier, unpublished observations). In addition, we have demonstrated in the present study that IL-21 does not modulate cell surface expression of these molecules. This is in agreement with our recent study which demonstrated that human neutrophils lack the IL-21Rα chain and that IL-21 is not a human neutrophil agonist [23]. Moreover, in the present study, a mixture of IL-15 and IL-21 did not alter the effect of IL-15 in increasing CD11b/CD18 expression, suggesting a lack of competition between the two cytokines, even if they are known to share CD132 as a receptor component. Taken together, the above observations suggest that IL-15 is the only CD132-dependent cytokine that increases CD11b/CD18 in human neutrophils.
CD11b/CD18 is located in the plasma membrane and secondary granules of neutrophils and, upon activation, these proteins are translocated from the granules to the plasma membrane. Different mechanisms have been suggested for the translocation and binding cycle of CD11b/CD18. Recently, BAP31, a protein regulating cellular anterograde transport, was found to specifically bind CD11b/CD18 in neutrophils [37]. In another study, Willeke et al. [38] proposed a role for Syk kinase in the binding cycle of CD11b/CD18 in neutrophils. Recently it has been suggested that engagement of β2 integrins play a role in delaying neutrophil apoptosis [39] and that β2 integrin aggregation is mediated through the IκB/NF-κB pathway. Interestingly, IL-15 was found to induce NF-κB activation and IL-8 production in human neutrophils [18] and to activate Syk kinase [22]. Whether these mechanisms or BAP31 are involved in IL-15-induced up-regulation of CD11b/CD18 is still unknown, and remain to be determined.
The fact that IL-15 induces a neutrophilic inflammation in vivo fits well with the proposed model that IL-15 participates in pulmonary inflammation. In the case of sarcoidosis, an increased number of neutrophils were found in the bronchoalveolar lavage fluids from patients, reflecting the severity of the disease and the ongoing inflammatory process, resulting in progressive loss of lung parenchyma [40]. Herein, the increased number of neutrophils in response to IL-15 corresponded with the expression of two pro-inflammatory mediators, IL-6 and CXCL2/MIP-2. Increased levels of these two molecules have been found to be highly expressed in endotoxin-challenged mouse airways, characterized by an accumulation of neutrophils [41]. Thus, our results suggest that IL-15 not only induces neutrophil influx in vivo but also induces the local production of IL-6 and CXCL2/MIP-2, which can attract at least other neutrophils. As we have recently reported for IL-21 [23], although IL-15 is known as a direct neutrophil agonist [14,15,17–20,22], we propose that IL-15 can also attract neutrophils in vivo indirectly via the production of chemokines. Since we demonstrated in this study that IL-15 can induce inflammation in vivo, it will be of great interest in future experiments to determine whether or not pro-inflammatory agents, including LPS, can increase IL-15 production in the air pouch.
In summary, we propose that the participation of IL-15 in pulmonary diseases may be linked to the following observations:
its ability to increase cell surface adhesion molecules in neutrophils and in respiratory epithelial cells;
its ability to enhance adhesion between neutrophils and respiratory cells;
its ability to attract neutrophils in vivo;
its ability to locally increase the production of at least two potent pro-inflammatory agents, namely IL-6 and CXCL2 (MIP-2).
Acknowledgments
We thank Amélie Bouchard for her technical assistance and Mary Gregory for reading this manuscript. This study was partly supported by Canadian Institutes of Health Research (MOP-89534) and Fonds de la Recherche en Santé du Québec (FRSQ). MP holds a PhD CIHR award and DG is a Scholar from FRSQ.
References
- 1.Azimi N, Brown K, Bamford R, Tagaya Y, Siebenlist U, Waldmann TA. Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-kappaB site. Proc Natl Acad Sci USA. 1998;95:2452–7. doi: 10.1073/pnas.95.5.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bamford RN, Battiata AP, Burton JD, Sharma H, Waldmann TA. Interleukin (IL) 15/IL-T production by the adult T-cell leukemia cell line HuT-102 is associated with a human T-cell lymphotrophic virus type I region /IL-15 fusion message that lacks many upstream AUGs that normally attenuates IL-15 mRNA translation. Proc Natl Acad Sci USA. 1996;93:2897–902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tagaya Y, Kurys G, Thies TA, et al. Generation of secretable and nonsecretable interleukin 15 isoforms through alternate usage of signal peptides. Proc Natl Acad Sci USA. 1997;94:14444–9. doi: 10.1073/pnas.94.26.14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaggero A, Azzarone B, Andrei C, et al. Differential intracellular trafficking, secretion and endosomal localization of two IL-15 isoforms. Eur J Immunol. 1999;29:1265–74. doi: 10.1002/(SICI)1521-4141(199904)29:04<1265::AID-IMMU1265>3.0.CO;2-V. April. [DOI] [PubMed] [Google Scholar]
- 5.Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. The 5′ untranslated region, signal peptide, and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translational control. J Immunol. 1998;160:4418–26. [PubMed] [Google Scholar]
- 6.McInnes IB, al-Mughales J, Field M, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996;2:175–82. doi: 10.1038/nm0296-175. [DOI] [PubMed] [Google Scholar]
- 7.Thurkow EW, van der Heijden IM, Breedveld FC, et al. Increased expression of IL-15 in the synovium of patients with rheumatoid arthritis compared with patients with Yersinia-induced arthritis and osteoarthritis. J Pathol. 1997;181:444–50. doi: 10.1002/(SICI)1096-9896(199704)181:4<444::AID-PATH778>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 8.Muro S, Taha R, Tsicopoulos A, et al. Expression of IL-15 in inflammatory pulmonary diseases. J Allergy Clin Immunol. 2001;108:970–5. doi: 10.1067/mai.2001.119556. [DOI] [PubMed] [Google Scholar]
- 9.Agostini C, Trentin L, Facco M, et al. Role of IL-15, IL-2, and their receptors in the development of T cell alveolitis in pulmonary sarcoidosis. J Immunol. 1996;157:910–8. [PubMed] [Google Scholar]
- 10.Wilkinson PC, Liew FY. Chemoattraction of human blood T lymphocytes by interleukin-15. J Exp Med. 1995;181:1255–9. doi: 10.1084/jem.181.3.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieto M, del Pozo MA, Sanchez-Madrid F. Interleukin-15 induces adhesion receptor redistribution in T lymphocytes. Eur J Immunol. 1996;26:1302–7. doi: 10.1002/eji.1830260619. [DOI] [PubMed] [Google Scholar]
- 12.Oppenheimer-Marks N, Brezinschek RI, Mohamadzadeh M, Vita R, Lipsky PE. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells In vitro and in the SCID mouse-human rheumatoid arthritis model In vivo. J Clin Invest. 1998;101:1261–72. doi: 10.1172/JCI1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sancho D, Yanez-Mo M, Tejedor R, Sanchez-Madrid F. Activation of peripheral blood T cells by interaction and migration through endothelium. role of lymphocyte function antigen-1/intercellular adhesion molecule-1 and interleukin-15. Blood. 1999;93:886–96. [PubMed] [Google Scholar]
- 14.Girard D, Paquet ME, Paquin R, Beaulieu AD. Differential effects of interleukin-15 (IL-15) and IL-2 on human neutrophils: modulation of phagocytosis, cytoskeleton rearrangement, gene expression, and apoptosis by IL-15. Blood. 1996;88:3176–84. [PubMed] [Google Scholar]
- 15.Girard D, Boiani N, Beaulieu AD. Human neutrophils express the interleukin-15 receptor alpha chain (IL-15Ralpha) but not the IL-9Ralpha component. Clin Immunol Immunopathol. 1998;88:232–40. doi: 10.1006/clin.1998.4576. [DOI] [PubMed] [Google Scholar]
- 16.Liu JH, Wei S, Ussery D, Epling-Burnette PK, Leonard WJ, Djeu JY. Expression of interleukin-2 receptor gamma chain on human neutrophils. Blood. 1994;84:3870–5. [PubMed] [Google Scholar]
- 17.Musso T, Calosso L, Zucca M, et al. Interleukin-15 activates proinflammatory and antimicrobial functions in polymorphonuclear cells. Infect Immun. 1998;66:2640–7. doi: 10.1128/iai.66.6.2640-2647.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald PP, Russo MP, Ferrini S, Cassatella MA. Interleukin-15 (IL-15) induces NF-kappaB activation and IL-8 production in human neutrophils. Blood. 1998;92:4828–35. [PubMed] [Google Scholar]
- 19.Jablonska E, Piotrowski L, Kiluk M, Jablonski J, Grabowska Z, Markiewicz W. Effect of IL-15 on the secretion of IL-1beta, IL-1Ra and sIL-1RII by neutrophils from cancer patients. Cytokine. 2001;16:173–7. doi: 10.1006/cyto.2001.0931. [DOI] [PubMed] [Google Scholar]
- 20.Bouchard A, Ratthe C, Girard D. Interleukin-15 delays human neutrophil apoptosis by intracellular events and not via extracellular factors. role of Mcl-1 and decreased activity of caspase-3 and caspase-8. J Leukoc Biol. 2004;75:893–900. doi: 10.1189/jlb.1103585. [DOI] [PubMed] [Google Scholar]
- 21.Alonso-Lebrero JL, Serrador JM, Dominguez-Jimenez C, et al. Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood. 2000;95:2413–9. [PubMed] [Google Scholar]
- 22.Ratthe C, Girard D. Interleukin-15 enhances human neutrophil phagocytosis by a Syk-dependent mechanism: importance of the IL-15Ralpha chain. J Leukoc Biol. 2004;76:162–8. doi: 10.1189/jlb.0605298. [DOI] [PubMed] [Google Scholar]
- 23.Pelletier M, Bouchard A, Girard D. In vivo and in vitro roles of IL-21 in inflammation. J Immunol. 2004;173:7521–30. doi: 10.4049/jimmunol.173.12.7521. [DOI] [PubMed] [Google Scholar]
- 24.Stoeck M, Kromer W, Gekeler V. Induction of IL-15 mRNA and protein in A549 cells by pro-inflammatory cytokines. Immunobiology. 1998;199:14–22. doi: 10.1016/S0171-2985(98)80060-0. [DOI] [PubMed] [Google Scholar]
- 25.Stoeck M, Schafer M, Hofmann HP, Gekeler V. Dexamethasone and cyclosporin A do not inhibit interleukin-15 expression in the human lung carcinoma cell line A549. Eur Cytokine Netw. 2000;11:414–9. [PubMed] [Google Scholar]
- 26.Chen C, Chou C, Sun Y, Huang W. Tumor necrosis factor alpha-induced activation of downstream NF-kappaB site of the promoter mediates epithelial ICAM-1 expression and monocyte adhesion. Involvement of PKCalpha, tyrosine kinase, and IKK2, but not MAPKs, pathway. Cell Signal. 2001;13:543–53. doi: 10.1016/s0898-6568(01)00171-1. [DOI] [PubMed] [Google Scholar]
- 27.Pelletier M, Lavastre V, Girard D. Activation of human epithelial lung a549 cells by the pollutant sodium sulfite: enhancement of neutrophil adhesion. Toxicol Sci. 2002;69:210–6. doi: 10.1093/toxsci/69.1.210. [DOI] [PubMed] [Google Scholar]
- 28.Agouridakis P, Kyriakou D, Alexandrakis MG, Perisinakis K, Karkavitsas N, Bouros D. Association between increased levels of IL-2 and IL-15 and outcome in patients with early acute respiratory distress syndrome. Eur J Clin Invest. 2002;32:862–7. doi: 10.1046/j.1365-2362.2002.01081.x. [DOI] [PubMed] [Google Scholar]
- 29.Zissel G, Baumer I, Schlaak M, Muller-Quernheim J. In vitro release of interleukin-15 by broncho-alveolar lavage cells and peripheral blood mononuclear cells from patients with different lung diseases. Eur Cytokine Netw. 2000;11:105–12. [PubMed] [Google Scholar]
- 30.Pettersen CA, Adler KB. Airways inflammation and COPD. epithelial–neutrophil interactions. Chest. 2002;121:142S–50S. doi: 10.1378/chest.121.5_suppl.142s. [DOI] [PubMed] [Google Scholar]
- 31.Mickelson JK, Kukielka G, Bravenec JS, et al. Differential expression and release of CD54 induced by cytokines. Hepatology. 1995;22:866–75. [PubMed] [Google Scholar]
- 32.Ratthe C, Pelletier M, Roberge CJ, Girard D. Activation of human neutrophils by the pollutant sodium sulfite: effect on cytokine production, chemotaxis, and cell surface expression of cell adhesion molecules. Clin Immunol. 2002;105:169–75. doi: 10.1006/clim.2002.5282. [DOI] [PubMed] [Google Scholar]
- 33.McMillan DN, Kernohan NM, Flett ME, et al. Interleukin 2 receptor expression and interleukin 2 localisation in human solid tumor cells in situ and in vitro: evidence for a direct role in the regulation of tumour cell proliferation. Int J Cancer. 1995;60:766–72. doi: 10.1002/ijc.2910600606. [DOI] [PubMed] [Google Scholar]
- 34.Robak E, Robak T, Wozniacka A, Zak-Prelich M, Sysa-Jedrzejowska A, Stepien H. roinflammatory interferon-gamma – inducing monokines (interleukin-12, interleukin-18, interleukin-15) – serum profile in patients with systemic lupus erythematosus. Eur Cytokine Netw. 2002;13:364–8. [PubMed] [Google Scholar]
- 35.Liu CY, Liu YH, Lin SM, Yu CT, Wang CH, Lin HC, Lin CH, Kuo HP. Apoptotic neutrophils undergoing secondary necrosis induce human lung epithelial cell detachment. J Biomed Sci. 2003;10:746–56. doi: 10.1007/BF02256327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jungel A, Distler JH, Kurowska-Stolarska M, Seemayer CA, Seibl R, Forster A. Expression of interleukin-21 receptor, but not interleukin-21, in synovial fibroblasts and synovial macrophages of patients with rheumatoid arthritis. Arthritis Rheum. 2004;50:1468–76. doi: 10.1002/art.20218. [DOI] [PubMed] [Google Scholar]
- 37.Zen K, Utech M, Liu Y, Soto I, Nusrat A, Parkos CA. Association of BAP31 with CD11b/CD18: potential role in intracellular trafficking of CD11b/CD18 in neutrophil. J Biol Chem. 2004;279:44924–30. doi: 10.1074/jbc.M402115200. [DOI] [PubMed] [Google Scholar]
- 38.Willeke T, Schymeinsky J, Prange P, Zahler S, Walzog B. A role for Syk-kinase in the control of the binding cycle of the beta2 integrins (CD11/CD18) in human polymorphonuclear neutrophils. J Leukoc Biol. 2003;74:260–9. doi: 10.1189/jlb.0102016. [DOI] [PubMed] [Google Scholar]
- 39.Yan SR, Sapru K, Issekutz AC. The CD11/CD18 (beta2) integrins modulate neutrophil caspase activation and survival following TNF-alpha or endotoxin induced transendothelial migration. Immunol Cell Biol. 2004;82:435–46. doi: 10.1111/j.0818-9641.2004.01268.x. [DOI] [PubMed] [Google Scholar]
- 40.Ziegenhagen MW, Rothe ME, Schlaak M, Muller-Quernheim J. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J. 2003;21:407–13. doi: 10.1183/09031936.03.00010403. [DOI] [PubMed] [Google Scholar]
- 41.Miyamoto M, Tomaki M, Lotvall J, Linden A. Beta-adrenoceptor stimulation and neutrophil accumulation in mouse airways. Eur Respir J. 2004;24:231–7. doi: 10.1183/09031936.04.00035204. [DOI] [PubMed] [Google Scholar]