Abstract
Here, we present data suggesting a novel mechanism for regulation of natural killer (NK) cell cytotoxicity through inhibitory receptors. Interaction of activation receptors with their ligands on target cells induces cytotoxicity by NK cells. This activation is under negative control by inhibitory receptors that recruit tyrosine phosphatase SHP-1 upon binding major histocompatibility class I on target cells. How SHP-1 blocks the activation pathway is not known. To identify SHP-1 substrates, an HLA-C-specific inhibitory receptor fused to a substrate-trapping mutant of SHP-1 was expressed in NK cells. Phosphorylated Vav1, a regulator of actin cytoskeleton, was the only protein detectably associated with the catalytic site of SHP-1 during NK cell contact with target cells expressing HLA-C. Vav1 trapping was independent of actin polymerization, suggesting that inhibition of cellular cytotoxicity occurs through an early dephosphorylation of Vav1 by SHP-1, which blocks actin-dependent activation signals. Such a mechanism explains how inhibitory receptors can block activating signals induced by different receptors.
In many cell types, activation induced by cell contact is controlled by inhibitory receptors that bind ligands on target cells (41, 48). The importance of this type of negative regulation is illustrated by the protection of normal cells from lysis by NK cells, of red blood cells from ingestion by macrophages (44), and of cardiomyocytes from immunoglobulin G (IgG)-mediated autoimmunity (43). A common feature of the receptors involved in these protective functions (i.e., CD158, SIRP-α, and PD-1, respectively) and of many other receptors that inhibit cellular responses is the presence of immunoreceptor tyrosine-based inhibition motifs (ITIMs) in the cytoplasmic tail (12, 48). Upon Tyr phosphorylation, ITIMs bind to the SH2 domains of protein tyrosine phosphatases (PTPases) SHP-1 and SHP-2, thereby releasing the catalytic site from autoinhibition (2, 12). Activation of human NK cell and T cell cytotoxicity is controlled by several inhibitory receptors, including the CD158 killer cell Ig-like receptors (KIR) and the lectin-like CD94/NKG2A, which bind to major histocompatibility complex (MHC) class I molecules expressed on target cells (5).
Whereas the functional consequence of SHP-1 recruitment by ITIM-containing receptors in NK cells is well established, the specific point at which SHP-1 blocks activation signals has not been defined. Engagement of inhibitory receptors by ligands on target cells blocks conjugate formation (13), Ca2+ flux (38, 52), polarization of lipid rafts in NK cells (42), and actin cytoskeleton rearrangement in T cells (26). As NK cell cytotoxicity depends on the activity of Tyr kinases (40), a number of Tyr-phosphorylated proteins are potential substrates for dephosphorylation by SHP-1. Two nonexclusive models, a “promiscuous” and a “selective” model, can be proposed for SHP-1-mediated inhibition. First, recruitment and activation of SHP-1 by inhibitory receptors at the NK-target cell interface lead to promiscuous dephosphorylation of multiple proteins at various points within the activation pathway. Alternatively, a single key substrate that controls activation is targeted for dephosphorylation. In this selective model, the specific distribution of inhibitory receptors and of signaling molecules within the NK-target cell interface (23, 53) should contribute to substrate selection by placing active SHP-1 in the proximity of particular substrates.
Reduced Tyr phosphorylation of a large number of proteins upon inhibitory receptor engagement is consistent with both models because dephosphorylation of an early activation component could prevent downstream phosphorylation events. Antibody-mediated co-cross-linking of an inhibitory KIR with the activation receptor CD16 resulted in a global reduction of the Tyr phosphorylation induced by cross-linking CD16 alone (7, 8). Specifically, Tyr phosphorylation of the FcɛRI γ chain, ZAP70, PLCγ1, PLCγ2, and SLP-76 was reduced. Likewise, co-cross-linking CD94/NKG2A with CD16 reduced the Tyr phosphorylation of the FcɛRI γ chain, Syk, and Shc (45). However, in more physiological experiments, incubation of NK clones with target cells expressing a ligand for inhibitory KIR showed a more selective reduction in Tyr phosphorylation. In this experimental context, the linkers for activation of T cells (LAT) and Syk were identified as potential substrates of SHP-1 (11, 52). The linker SLP76 has also been proposed as a substrate of SHP-1 based on a far-Western blot with a trapping mutant of SHP-1 that bound to SLP-76 (7). These data suggested some selectivity in dephosphorylation by SHP-1 but did not establish whether these proteins were direct substrates or were downstream of another substrate of SHP-1. It is also unclear how LAT, Syk, ZAP70, or SLP76 could be critical targets for inhibition because NK cells from mice deficient in any one of these molecules, and lacking both Syk and ZAP70, have retained natural cytotoxic function (22, 47, 62).
We devised an experimental system to directly identify SHP-1 substrates targeted for dephosphorylation during KIR-mediated inhibition of NK cells that were in contact with resistant target cells. A chimeric receptor that contains SHP-1 in place of the KIR cytoplasmic tail was generated in order to restrict the analysis to SHP-1 substrates that were specifically targeted by KIR. While it is possible that the bypass of ITIM phosphorylation with a KIR-SHP-1 fusion could result in inhibition of earlier signals than those blocked by an ITIM-dependent SHP-1 recruitment, this scenario is unlikely because ITIM phosphorylation and that of SHP-1 occur rapidly as a result of KIR binding to its ligand on target cells, independently of actin polymerization and of integrin-mediated adhesion (29). The KIR/SHP-1 chimeric receptor behaved like wild-type KIR and inhibited adhesion of NK cells to target cells and Tyr phosphorylation of the activation receptor 2B4 (13, 58). A trapping mutation (30) was introduced in the catalytic site of KIR/SHP-1 in order to isolate substrates in NK cells. Expression of a trapping mutant of SHP-1 had been used to identify SIRP-1α as a substrate in activated macrophages (51). We show that the KIR/SHP-1-trapping mutant bound Tyr-phosphorylated Vav1 selectively in NK cells that were incubated with target cells expressing an HLA-C ligand of the inhibitory KIR.
MATERIALS AND METHODS
Cell lines and reagents.
HLA-Cw3, -Cw4, and -Cw15 transfectants of the cell line 721.221 were obtained from J. Gumperz and P. Parham (Stanford University). The human NK cell line YTS-ecoR, expressing a mouse ecotropic receptor, was obtained from G. Cohen (Massachusetts General Hospital) and was cultured in Iscove's medium supplemented with 10% fetal calf serum, l-glutamine, and 50 μM 2-mercaptoethanol. The anti-KIR2DL1 (CD158a) antibody EB6 was from Immunotech. The anti-2B4 antibody C1.7 was from Coulter Pharmaceutical (Miami, Fla.). Rabbit anti-2B4 was used for Western blotting as described previously (58). The anti-Lck antibody sc-13 and the anti-SLP76 antibody sc-9062 used for Western blotting were from Santa Cruz Biotechnology (Santa Cruz, Calif.). The antiphosphotyrosine (anti-p-Tyr) monoclonal antibody (mAb) 4G10, 4G10 agarose, and the anti-Vav1 mAb (ascites) were from Upstate Biotechnology (Lake Placid, N.Y.). The KT3 mAb was from Babco (Richmond, Calif.). Cytochalasin D was from Sigma (Saint Louis, Mo.). The mAb VV1-IG10, specific for the A33 early and intermediate vaccinia virus protein, was a gift of A. Schmaljohn (United States Army, Fort Detrick, Frederick, Md.).
Expression of KIR-SHP-1 chimeric molecules in YTS cells.
The Arg at position 459 in the SHP-1 cDNA (a gift of T. Yi, Cleveland Clinic) was changed to Met by site-directed mutagenesis to produce the mutant SHP-1(RM). The Asp at position 419 was changed to Ala to produce the mutant SHP-1(DA). The chimeric 2DL1/SHP-1 molecules and the YTS transductants were produced as described previously (13).
Cytotoxicity assays.
A total of 5 × 105 721.221 cells were labeled with bis(acetoxymethyl)2,2′:6′,2"-terpyridine-6,6"-dicarboxylate reagent (Wallac) for 30 min at 37°C. Cells were washed twice in medium containing 1 mM sulfinpyrazone (Sigma) and resuspended at 5 × 104 cells/ml. Cells were plated at 5,000 targets/well. YTS effector cells were mixed with labeled target cells in a V-bottom 96-well plate. Maximum release was determined by incubation in 1% Triton X-100. For spontaneous release, targets were incubated without effectors in medium alone. All samples were done in triplicate. After a 1-min centrifugation at 188 × g, plates were incubated for 2 h at 37°C. Plates were then shaken for 30 s and centrifuged again for 1 min at 188 × g. Twenty microliters of supernatant was harvested and mixed with 200 μl of Europium solution in a flat-bottom microtiter plate by shaking for 5 min. Plates were measured using a time-resolved fluorometer (Wallac).
NK-target cell mixing experiments.
For target cell mixing experiments, 30 × 106 effector (i.e., YTS) cells and 30 × 106 target (i.e., 221-Cw3 or 221-Cw15) cells were incubated on ice for 15 min. Target and effector cells were mixed, pelleted, and incubated for 15 min on ice to allow cells to form contacts. Cells were then incubated at 37°C for 5 min before lysis. In some experiments, the YTS cells were preincubated with 10 μM cytochalasin D in dimethyl sulfoxide (DMSO) for 30 min at 37°C. In other experiments, activated sodium orthovanadate (32) (Sigma) was added at the indicated concentrations to the cells either during the incubation on ice and at 37°C or at the time of lysis.
Vaccinia virus infection.
Recombinant vaccinia virus encoding a dominant-negative mutant of Rac1 (N17-Rac1) (4) was used to infect YTS cells for 3 h as previously described (55). Vaccinia virus infections were monitored by flow cytometry with mAb VV1-IG10. Infected cells were used for cell mixing experiments as described above or treated with 0.1 mM pervanadate for 10 min at 37°C.
Immunoprecipitations, SDS-PAGE, and Western blotting.
Cells were lysed in ice-cold lysis buffer (0.5% Triton X-100, 20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 2 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride) for 15 min on ice. Lysates were cleared by centrifugation at 20,000 × g at 4°C for 15 min. Supernatants were incubated with the anti-2DL1 antibody EB6 and protein G-agarose (Invitrogen, Carlsbad, Calif.) for 2 h at 4°C. Agarose was washed three times and boiled in reducing sodium dodecyl sulfate (SDS) sample buffer. In some cases, agarose was boiled in lysis buffer with 2% SDS. These samples were diluted with lysis buffer to a final SDS concentration of less than 0.1% and then reprecipitated with 10 μl of packed 4G10 agarose (Upstate Biotechnology). The agarose was washed and boiled in SDS sample buffer as above. SDS-polyacrylamide gel electrophoresis (PAGE) and Western blotting were performed as previously described (58).
RESULTS
Trapping SHP-1 substrates with a mutant KIR2DL1/SHP-1 chimeric receptor expressed in NK cells.
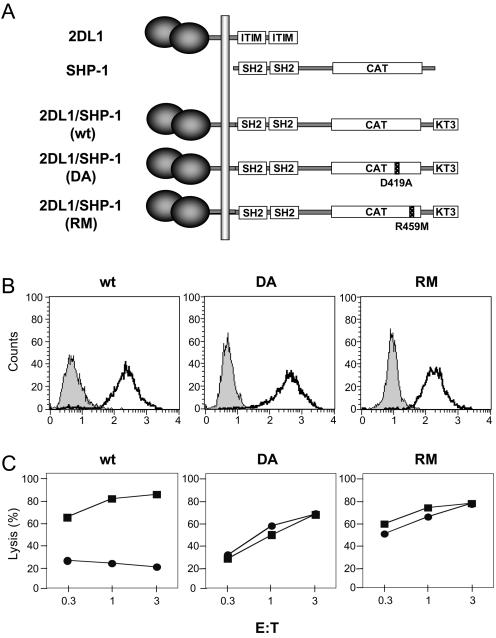
An Asp-to-Ala substitution at position 419 in SHP-1 (mutant DA) drastically reduces catalytic activity without impairing substrate binding (30, 51). To identify SHP-1 substrates in NK cells during inhibition by MHC class I on target cells, the DA trapping mutant was expressed in the cell line YTS as a KIR/SHP-1 fusion protein (Fig. 1A and B). A second mutant with an Arg-to-Met substitution at position 459 (mutant RM) that eliminates substrate binding (30, 51) served as a negative control. The KIR/SHP-1 fusion protein carrying wild-type (wt) SHP-1 was also expressed in YTS cells (Fig. 1B). All three KIR/SHP-1 receptors contain a C-terminal KT3 epitope tag. The KIR2DL1 receptor is specific for HLA-Cw4 and HLA-Cw15 but not for HLA-Cw3. The chimeric receptor 2DL1/SHP-1(wt) inhibited lysis of 221-Cw4 and 221-Cw15 but not 221-Cw3 (Fig. 1C and data not shown). YTS cells expressing 2DL1/SHP-1(DA) or 2DL1/SHP-1(RM) killed 221-Cw4 and 221-Cw3 cells equally well (Fig. 1C), showing that the two catalytically inactive SHP-1 mutants were unable to inhibit cytotoxicity.
FIG. 1.
Expression and function of chimeric 2DL1/SHP-1 molecules. (A) Chimeric 2DL1/SHP-1 molecules. Wild-type KIR2DL1 (2DL1) and SHP-1 are shown with the SH2 domains of SHP-1 aligned with the cytoplasmic ITIMs of 2DL1. The catalytic domain (CAT) of SHP-1 and the KT3 tag are boxed. (B) Staining of YTS cells expressing these chimeric constructs. Shaded profiles represent fluorescein isothiocyanate-labeled goat anti-mouse Ab only. Dark lines represent staining with the anti-2DL1 mAb EB6. (C) Lysis of 221-Cw3 (▪) or 221-Cw4 (•) by YTS-2DL1/SHP-1(wt), YTS-2DL1/SHP-1(RM), and YTS-2DL1/SHP-1(DA) cells at the effector-to-target ratios (E:T) indicated.
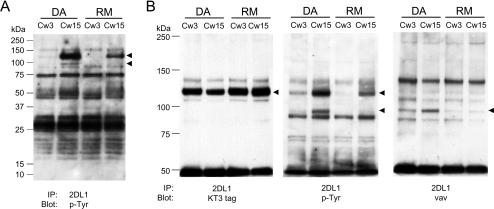
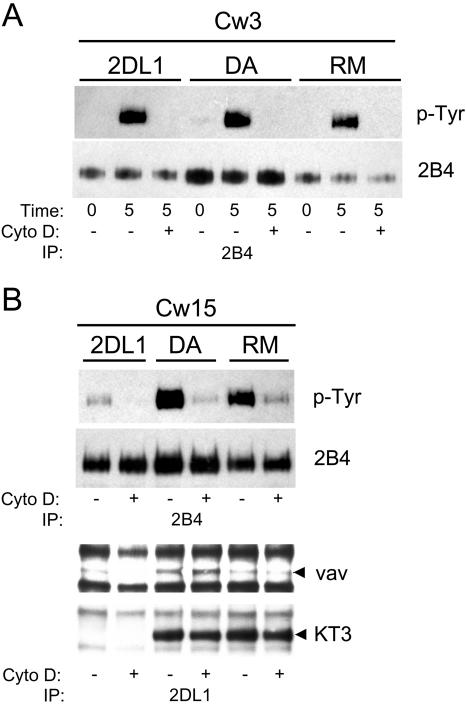
To identify proteins associated with 2DL1/SHP-1(DA) during NK-target cell interaction, clones of YTS 2DL1/SHP-1(DA) cells were mixed with 221-Cw15 cells. After lysis and immunoprecipitation of 2DL1/SHP-1, Western blotting with an anti-p-Tyr Ab revealed two proteins with molecular masses of ∼125 and ∼100 kDa, which were absent without KIR engagement (221-Cw3) (Fig. 2A and B, center). The two phosphorylated proteins were also detected after mixing with 221-Cw4 cells (data not shown). The more intense band at ∼125 kDa was also detected—but at a reduced intensity—after mixing YTS 2DL1/SHP-1(RM) cells with 221-Cw15 cells. The band at ∼100 kDa was not detectable in the 2DL1/SHP-1(RM) immunoprecipitation, consistent with the expected property of a SHP-1 substrate. Three independent clones of YTS 2DL1/SHP-1(DA) were tested. In each one, bands at ∼125 and ∼100 kDa were detected in immunoprecipitates of 2DL1/SHP-1(DA) after mixing with 221-Cw4 or 221-Cw15 cells (data not shown).
FIG. 2.
Specific association of molecules with 2DL1/SHP-1(DA) after incubation with 221-Cw15 cells. 2DL1/SHP1(DA) and 2DL1/SHP1(RM) cells were mixed for 5 min with either 221-Cw3 or 221-Cw15 cells as indicated at the top. (A) Anti-2DL1 immunoprecipitates were separated by 4 to 20% SDS-PAGE, transferred to a polyvinylidene difluoride (PVDF) membrane and Western blotted with antiphosphotyrosine mAb 4G10. Arrowheads point to two bands that are selectively enhanced in the DA-Cw15 combination. (B) Anti-2DL1 immunoprecipitates separated by 6% SDS-PAGE, transferred to a PVDF membrane, and subjected to Western blotting with antiphosphotyrosine mAb 4G10 (middle panel). After being stripped, the blot was reprobed with either an anti-Vav1 mAb (right panel) or an anti-KT3-tag mAb (left panel). Arrowheads point to the positions of tyrosine-phosphorylated proteins.
The predominant Tyr-phosphorylated protein in anti-KIR2DL1 immunoprecipitations had the expected size of the 2DL1/SHP-1 chimeric receptor itself (103 kDa plus about 20 kDa accounted for by KIR2DL1 glycosylation). Reprobing the same membrane with an anti-KT3 tag Ab revealed comigration of the 2DL1/SHP-1(DA) and 2DL1/SHP-1(RM) receptors with the ∼125-kDa protein (Fig. 2B, left). Phosphorylation of the chimeric receptors upon ligand binding was not surprising, as SHP-1 becomes Tyr phosphorylated when recruited by KIR during the inhibition of NK cells (58).
Association of Vav1 with 2DL1/SHP-1(DA) induced by receptor binding to HLA-C on target cells.
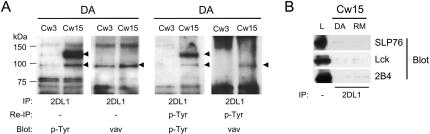
The size of the 90- to 100-kDa Tyr-phosphorylated protein detected in immunoprecipitates of 2DL1/SHP-1(DA) after incubation with 221-Cw15 cells was compatible with that of Vav1, a 95-kDa guanine nucleotide exchange factor (GEF) that regulates actin cytoskeletal rearrangements and receptor clustering through the GTPase Rac1 (46). The GEF activity of Vav1 is positively regulated by phosphorylation of several Tyr residues (1, 14). As Vav1 contributes to natural cytotoxicity (4, 18, 21, 31), its dephosphorylation could potentially prevent natural cytotoxicity. Reprobing the membrane with an anti-Vav1 mAb revealed a marked increase of Vav1 in the 2DL1/SHP-1(DA) immunoprecipitation from cells incubated with 221-Cw15 cells and also demonstrated that Vav1 was superimposable with the ∼95- kDa Tyr-phosphorylated band (Fig. 2B, right). Some constitutive association of Vav1 with 2DL1/SHP-1(DA) may also occur, given the band detected in immunoprecipitates from cells incubated with 221-Cw3 cells (Fig. 2B, right). The specific association of Vav1 with 2DL1/SHP-1(DA) induced by KIR2DL1 binding of HLA-Cw15 was quite pronounced, considering that the recovery of 2DL1/SHP-1(DA) was lowest in the sample incubated with 221-Cw15 cells, as shown in the KT3 tag reblot (Fig. 2B, left).
The Vav1 immunoblot in Fig. 2B does not imply that Vav1 associated with 2DL1/SHP-1(DA) is tyrosine phosphorylated. To test whether Vav1 was included in the 95-kDa phosphoprotein associated with 2DL1/SHP-1(DA) after incubation with 221-Cw15 cells, the immunoprecipitates of 2DL1/SHP-1(DA) were boiled in 2% SDS, diluted to a <0.1% concentration of SDS, and reprecipitated with the anti-phosphotyrosine mAb 4G10. The data obtained prior to dissociation in SDS confirmed results from previous experiments, namely, that the appearance of Tyr-phosphorylated bands at 125 and 95 kDa increased Vav1 association after incubation with target cells expressing HLA-Cw15 (Fig. 3A, left). After reprecipitation of Tyr-phosphorylated proteins, the 125- and 95-kDa bands were still detectable in a 4G10 blot (Fig. 3A, right). Furthermore, Western blotting for Vav1 after reprecipitation with anti-p-Tyr mAb revealed a clear band at ∼95 kDa, implying that the Tyr-phosphorylated 95-kDa band contained Vav1 (Fig. 3A, right). The identity of the 125-kDa band as the 2DL1/SHP-1(DA) receptor was confirmed by reprecipitation with the anti-KT3 tag mAb (data not shown). Western blotting for p-Tyr after reprecipitation with anti-p-Tyr mAb gave cleaner results than p-Tyr blotting directly after 2DL1/SHP-1(DA) immunoprecipitation, due to a marked reduction of nonspecific bands (Fig. 3A, right), in particular, those in the 10- to 50-kDa region of the gel, yet no Tyr-phosphorylated protein other than the 95-kDa band appeared to associate with 2DL1/SHP-1(DA) specifically after incubation with target cells expressing HLA-Cw15 (Fig. 3A [right] and data not shown). A direct reprecipitation of Vav1 after boiling in SDS was precluded by the poor performance of the anti-Vav1 Ab in immunoprecipitation. For the same reason, and because of the large amount of Vav1 contributed by target cells, it has not been possible to detect 2DL1/SHP-1(DA) in immunoprecipitates of Vav1. Nevertheless, our data strongly suggest that Tyr-phosphorylated Vav1 bound specifically to the trapping 2DL1/SHP-1 mutant during inhibition of YTS cells by HLA-C on target cells.
FIG. 3.
Vav1 is the only detectable tyrosine-phosphorylated protein associated with 2DL1/SHP-1(DA) after incubation with 221-Cw15 cells. 2DL1/SHP-1(DA) and 2DL1/SHP-1(RM) cells were mixed for 5 min with 221-Cw3 or 221-Cw15 cells as indicated at the top. (A) One-fourth of the immunoprecipitations with EB6 was analyzed exactly as described in the legend of Fig. 2. The remaining three-fourths was boiled in 2% SDS, diluted with lysis buffer to 0.1% SDS, reimmunoprecipitated with 4G10-agarose, and separated on a 4 to 20% SDS-polyacrylamide gel. After transfer to PVDF membranes, Western blotting was performed sequentially with anti-p-Tyr mAb 4G10 and anti-Vav1 mAb (right panels). (B) 2DL1 immunoprecipitates separated by SDS-PAGE were transferred to a PVDF membrane and subjected to Western blotting with antibodies specific for SLP76, Lck, and 2B4. The left lane is a lysate (L) of YTS cells.
Proteins previously proposed as substrates of SHP-1 during inhibition by KIR include LAT, SLP76, Syk, and 2B4 (7, 11, 52, 58). Lck was also dephosphorylated by SHP-1 when both were coexpressed transiently in 293 cells (19). Antibodies to these proteins were used for Western blotting of 2DL1/SHP-1(DA) immunoprecipitates in order to test whether they were trapped but not detected in the p-Tyr blots. Western blots for SLP76, Lck, and 2B4 showed no difference between immunoprecipitates of 2DL1/SHP-1(DA) and 2DL1/SHP-1(RM) after incubation of those transfected cells with 221-Cw15 cells (Fig. 3B). Similar blots for LAT, Cbl, Syk, PI-3K p85, Grb2, and Shc showed no specific association with 2DL1/SHP-1(DA) (data not shown). Therefore, Vav1 remained the only detectable Tyr-phosphorylated protein specifically associated with 2DL1/SHP-1(DA) during engagement of KIR by HLA-C on target cells.
Vav1 association with 2DL1/SHP-1(DA) could be due to several different types of interaction with SHP-1. First, the catalytic site of SHP-1 may bind directly to a p-Tyr of Vav1. Second, the SH2 domains of SHP-1 may bind to p-Tyr in Vav1. Third, the SH2 domain of Vav1 may bind to p-Tyr in SHP-1. Such an interaction has been detected in the mouse cell line P815 (39). Fourth, Vav1 may bind to 2DL1/SHP-1(DA) through a p-Tyr-independent interaction. Finally, Vav1 may bind indirectly by association with another protein that is itself a SHP-1 substrate. The lack of Vav1 association with 2DL1/SHP-1(RM) argued against the second and fourth possibilities.
Vav1 association with 2DL1/SHP-1(DA) is inhibited by competition for binding to the catalytic site of SHP-1.
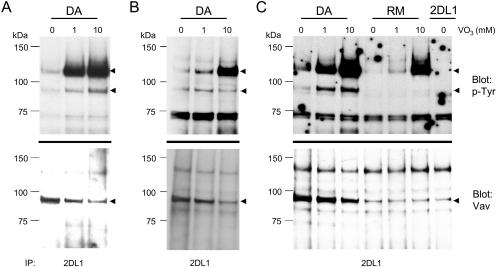
Vanadate binds with high affinity to the catalytic site of PTPases (35, 36). We exploited this property of vanadate to test whether it would elute Vav1 bound to the catalytic site of SHP-1(DA) by adding it at the time of lysis. The amount of Vav1 associated with 2DL1/SHP-1(DA) was indeed reduced by the addition of vanadate (Fig. 4A). As expected from the inhibitory effect of vanadate on PTPases, the recovery of tyrosine-phosphorylated receptor was greatly increased. Similar results were obtained by treating cells with vanadate prior to cell mixing (Fig. 4B and C). In that case, a reduced association of Vav1 with 2DL1/SHP-1(DA) was observed, despite the great enhancement of overall Tyr phosphorylation in the cells. No association of Vav1 with 2DL1/SHP-1(RM) was detected, even though Tyr phosphorylation of 2DL1/SHP-1(RM) was greatly increased (Fig. 4C). A weak 95-kDa band in the Vav1 Western blot of 2DL1/SHP-1(RM) immunoprecipitates was also present in the immunoprecipitation of wild-type, nonchimeric KIR2DL1 expressed in YTS cells mixed with 221-Cw15 cells, thus suggesting that it was nonspecific (Fig. 4C).
FIG. 4.
Competition for substrate binding with VO3. (A) 2DL1/SHP-1(DA) cells were incubated with 221-Cw15 cells and lysed in the presence of the indicated concentrations of vanadate. (B and C) 2DL1/SHP-1(DA), 2DL1/SHP-1(RM), and YTS-2DL1 cells were incubated with 221-Cw15 cells in the presence of indicated concentrations of vanadate (VO3). The cells were treated with 1 or 10 mM vanadate for 30 min prior to being mixed with 221-Cw15 cells. Immunoprecipitates with anti-2DL1 mAb EB6 were separated by SDS-PAGE, transferred to PVDF membranes, and subjected to Western blotting for phosphotyrosine (top) and Vav1 (bottom).
The decreased association of Vav1 with 2DL1/SHP-1(DA) in vanadate-treated cells rules out a p-Tyr-independent Vav1-SHP-1 interaction because neither vanadate nor Tyr-phosphorylated proteins would have competed with such an interaction. The increase in the level of Vav1 Tyr phosphorylation after vanadate treatment of either intact cells or cell lysates was only modest compared to that of 2DL1/SHP-1(DA), even after correction for the loss of associated Vav1 (Fig. 4). This result is consistent with direct binding of Vav1 to the catalytic site because a p-Tyr bound by SHP-1(DA) is already protected from dephosphorylation and does not benefit from the inhibition of phosphatases as much as an exposed p-Tyr does. These results show that association of Vav1 with 2DL1/SHP-1(DA) requires the catalytic site of SHP-1 and is not due solely to an interaction between p-Tyr and SH2 domains.
Actin cytoskeleton rearrangement is upstream of 2B4 phosphorylation and downstream of Vav1 trapping by 2DL1/SHP-1(DA).
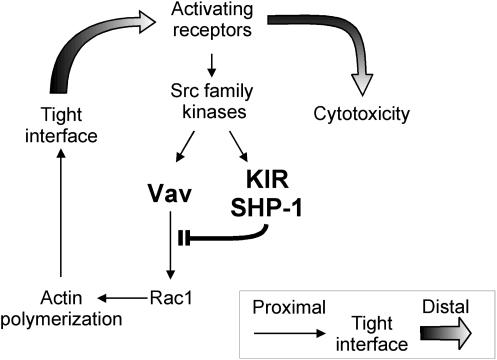
Our data suggest a model for the activation of NK cell cytotoxicity and for its inhibition by KIR (Fig. 5). In this model, early signals lead to a Tyr phosphorylation of Vav1 that precedes and controls Vav1-dependent actin cytoskeleton rearrangement. After formation of an actin-dependent interface with the target cell, receptor clustering occurs, thus resulting in an amplification of the activating signal. Binding of KIR to HLA-C on target cells induces rapid phosphorylation of KIR and of SHP-1 (29), and recruited SHP-1 dephosphorylates Vav1. Two testable predictions of this model are that dephosphorylation of Vav1 should not depend on actin polymerization, whereas phosphorylation of an activation receptor that is sensitive to KIR inhibition should depend on actin polymerization induced by a Vav1 signal.
FIG. 5.
A model for the inhibition of NK cell cytotoxicity by KIR. Early activation signals lead to Vav1-dependent actin polymerization and formation of a tight NK-target cell interface. Engagement of an inhibitory KIR by MHC class I on target cells results in rapid recruitment of SHP-1 and in Vav1 dephosphorylation prior to actin polymerization. Signals for cytotoxicity that depend on a tight interface are prevented by the inactivation of Vav1.
Tyr phosphorylation of activation receptor 2B4 induced by NK-target cell contact requires actin polymerization and lipid rafts (57) and is blocked by KIR binding to HLA-C on target cells (58). As shown here, 2B4 did not emerge as a direct substrate of SHP-1 during inhibition (Fig. 3B). A Vav1 inactivation by SHP-1 upon binding of KIR2DL1 to HLA-C on target cells that precedes Vav1-dependent actin polymerization could explain the block in 2B4 phosphorylation. We therefore tested—in the context of NK-target cell interactions—whether Vav1 trapping by 2DL1/SHP-1(DA) could occur independently of actin polymerization by using 2B4 receptor phosphorylation in the same cells as a reporter for an actin-dependent, KIR-sensitive process. Tyr phosphorylation of 2B4, strongly induced by a 5-min incubation of YTS cells with 221-Cw3 cells, was completely inhibited by pretreatment with cytochalasin D (Fig. 6A), consistent with recent data (57). Inhibition was comparable to that mediated by KIR engagement in YTS-2DL1 cells incubated with 221-Cw15 cells (Fig. 6B). Engagement of the two mutants 2DL1/SHP-1(DA) and 2DL1/SHP-1(RM) lacking catalytic activity did not inhibit 2B4 phosphorylation. Pretreatment with cytochalasin D inhibited 2B4 phosphorylation in all three YTS transfectants incubated with 221-Cw15 cells (Fig. 6B). Vav1 trapping by 2DL1/SHP-1(DA) in the same cells was therefore evaluated to test whether Vav1 trapping could be independent of actin polymerization. No decrease in Vav1 association with 2DL1/SHP-1(DA) after incubation with 221-Cw15 cells was detected in the presence of cytochalasin D, even though phosphorylation of 2B4 was inhibited in the same cells (Fig. 6B). The weaker band detected in the 2DL1/SHP-1(RM) immunoprecipitate may be nonspecific, as it was also present in the immunoprecipitate of the nonchimeric, wild-type KIR2DL1 (Fig. 6B), as shown in Fig. 4. These data establish that Vav1 trapping by 2DL1/SHP-1(DA) occurs prior to, or at least independently of, actin polymerization and 2B4 phosphorylation. Furthermore, Vav1 trapping in the presence of cytochalasin D showed that early activation signals leading to Vav1 phosphorylation were not globally blocked by this inhibitor.
FIG. 6.
Cytochalasin D blocks Tyr phosphorylation of 2B4 but not trapping of Vav1 by 2DL1/SHP-1(DA). YTS-2DL1, 2DL1/SHP1(DA), and 2DL1/SHP1(RM) cells were mixed with 221-Cw3 or 221-Cw15 cells as indicated at the top. Some YTS cells were treated with cytochalasin D (Cyto D) or carrier (DMSO) only. (A) 2B4 immunoprecipitates (IP) with mAb C1.7 were separated by SDS-PAGE, transferred to PVDF membranes, Western blotted with the anti-p-Tyr mAb 4G10, and reprobed with an anti-2B4 rabbit antiserum. For controls, some cells were kept on ice during mixing (time zero). (B) 2B4 immunoprecipitates probed with 4G10 as in panel A (top panels). 2DL1 immunoprecipitates with mAb EB6 from the same cells were probed with anti-Vav1 mAb and reprobed with anti-KT3 mAb (bottom panels).
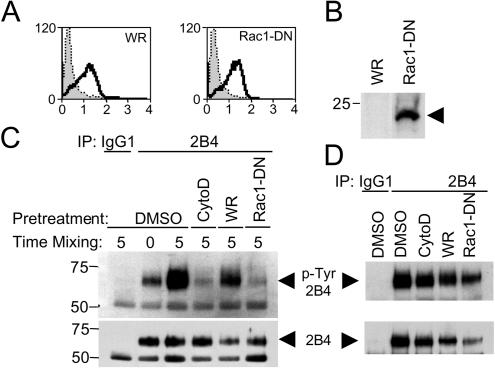
The second prediction was that a KIR-sensitive activation receptor should depend on actin polymerization induced by a Vav1-Rac1 signal (Fig. 5). Activation of the small GTPase Rac1 induces the formation of lamellipodiae (33), and expression of a dominant-negative Rac1 mutant (N17-Rac1) in NK cells blocks natural cytotoxicity (4, 37). A recombinant vaccinia virus was used to express N17-Rac1 in YTS-2DL1 cells (Fig. 7A and B) in order to test its effect on 2B4 phosphorylation. As a control, cells were infected with a wild-type vaccinia virus. Infected cells were mixed with 721.221 cells, and the phosphorylation of 2B4 was monitored by immunoprecipitation and Western blotting (Fig. 7C). For comparison, uninfected cells were treated with cytochalasin D prior to mixing with 721.221 cells. 2B4 phosphorylation was strongly inhibited in cells expressing dominant-negative Rac1 compared to cells infected with a control vaccinia virus (Fig. 7C). The inhibitory effect of dominant-negative Rac1 was specific to the Tyr phosphorylation induced by target cell interaction because it did not inhibit 2B4 phosphorylation induced by pervanadate (Fig. 7D). These data implicate the Vav1 effector Rac1 in the Tyr phosphorylation of 2B4.
FIG. 7.
Expression of dominant-negative Rac1 blocks tyrosine phosphorylation of 2B4. A total of 5 × 106 YTS-2DL1 cells were infected with the WR strain of vaccinia virus (WR) or recombinant vaccinia virus expressing a dominant-negative Rac1 mutant (Rac1-DN) for 3 h at 10 PFU/cell. Infection was monitored by staining with the mAb VV1-IG10 (A) and expression of the FLAG-tagged Rac1-DN by anti-FLAG Western blotting (B). (C) YTS cells were treated with 10 μM cytochalasin D (CytoD) or carrier (DMSO) only or were infected with the indicated vaccinia viruses and mixed with an equal number of 721.221 cells for 0 or 5 min. Cells were lysed and immunoprecipitated with a control antibody (IgG1), followed by anti-2B4 immunoprecipitation. Samples were analyzed by anti-p-Tyr Western blotting (upper panel) and reprobed using an anti-2B4 antibody (lower panel) to show equal loading. (D) The same cells as described for panel C were treated with pervanadate; 2B4 was immunoprecipitated and analyzed as described for panel C.
DISCUSSION
Identification of the molecules dephosphorylated by SHP-1 during inhibition of natural cytotoxicity by an ITIM-containing receptor had the potential to not only illuminate the inhibitory mechanism but to also reveal important steps in the NK cell activation pathway. Furthermore, elucidating the mechanism by which KIR inhibits NK cells may provide relevant information about the many other ITIM-containing receptors that regulate various cell types.
Expression of a trapping mutant of SHP-1 in NK cells made it possible to study the interaction of SHP-1 with substrates encountered during inhibition by KIR when engaged by MHC class I on target cells. As receptors and signaling molecules segregate into discrete domains during the interaction of NK cells with target cells (16, 23, 53, 54), it was necessary to develop a trapping protocol that would be effective during NK-target cell interactions. The data obtained by our approach suggest that the inhibition of NK cell cytotoxicity is achieved by a selective dephosphorylation of Vav1 by the PTPase SHP-1. The trapping 2DL1/SHP-1 chimeric receptor bound specifically to Tyr-phosphorylated Vav1 upon engagement by HLA-Cw15 on target cells. Vav1 trapping was sensitive to vanadate being added either to cell lysates or to intact cells—implying that the SHP-1 catalytic site was essential for Vav1 binding. Vav1 trapping was resistant to cytochalasin D, placing it upstream of actin cytoskeletal rearrangement. The activation of the Vav1 GEF domain by Tyr phosphorylation (1, 14), the established importance of a Vav1-Rac1 signaling pathway in T cells for actin cytoskeleton-dependent membrane reorganization, receptor clustering, phosphatidylinositol 4-phosphate 5-kinase activation (27, 46), and the role of Vav1 in NK cell cytotoxicity (4, 18, 21, 31) all point to the dephosphorylation of Vav1 by SHP-1 as a viable mechanism for the inhibition of NK cell cytotoxicity.
It is possible that the use of a chimeric KIR/SHP-1 receptor in the cell line YTS led to the identification of a substrate that is not normally dephosphorylated during inhibition of NK cells by KIR. However, the inhibitory function of the chimeric KIR/SHP-1 receptor was comparable to that of a wild-type KIR. Dephosphorylation of Vav1 by SHP-1 can also explain how engagement of an ITIM-containing receptor blocked actin polymerization in T cells (26) as well as the actin-dependent Tyr phosphorylation of the NK cell activation receptor 2B4 (57). One of the primary regulatory tyrosines (Tyr174) in Vav1 is within a sequence context (DEIpYEDL) that closely matches two sequence motifs [(D/E)X(L/I/V)XpYXX(L/I/V) and (D/E)XpY, respectively] recently determined to be favored for dephosphorylation by SHP-1 in vitro (56, 61).
No change in overall tyrosine phosphorylation of Vav1 was detected in NK cells that were inhibited by KIR2DL1 or by the chimeric 2DL1/SHP-1 receptor when engaged by HLA-C on target cells (data not shown). Several reasons can account for this result. Target cells contribute a good amount of their own phosphorylated Vav1, which is not affected by SHP-1 in the NK cells. Furthermore, SHP-1 may dephosphorylate only a fraction of total cellular Vav1 during inhibition and only one out of the several phosphorylated tyrosines in each active Vav1. Full activation of Vav1 is known to require phosphorylation on multiple tyrosines (14).
The intact SHP-1 inserted in place of the KIR cytoplasmic tail is probably still autoinhibited by its own SH2 domains, as occurs in wild-type SHP-1 (49). A constitutively “open” SHP-1 carrying the trapping mutation would likely be blocked by constitutive occupancy of the catalytic site. Indeed, a chimeric KIR/SHP-1(DA) receptor that lacked the SH2 domains (and thereby lacked autoinhibitory regulation), which was expressed in YTS cells, failed to inducibly trap tyrosine-phosphorylated proteins upon binding to HLA-C on target cells under the same conditions that led to Vav1 trapping by 2DL1/SHP-1(DA), even though the KIR/SHP-1(wt) without SH2 domains was inhibitory (unpublished data). A possible mechanism for the activation of full-length SHP-1 in the 2DL1/SHP-1 chimeric receptor is through intramolecular binding of the N-terminal SH2 domain to a C-terminal tyrosine that is phosphorylated in SHP-1 (63).
Our model of inhibition through Vav1 dephosphorylation differs from previous models of inhibition on the part of ITIM-containing receptors. Rather than promiscuous dephosphorylation of multiple components of signals from activation receptors, it is the selective dephosphorylation of a primary molecule not associated with any given activation receptor that blocks target cell killing. The reduced Tyr phosphorylation of several different proteins upon coligation of inhibitory receptors with activation receptors using antibodies had suggested that SHP-1 dephosphorylated multiple substrates. In the more physiological setting of NK cells or T cells incubated with target cells, Syk and ZAP70, respectively, showed reduced Tyr phosphorylation during inhibition (11, 15). Phosphorylation of LAT in NK cells was probably also reduced by KIR engagement, as it no longer bound Grb2 (52). These data were consistent with an inhibition by KIR-associated SHP-1 that centered on early components of the ITAM activation pathway. Our data support a new model that can account for these earlier findings.
The selective dephosphorylation of Vav1 by SHP-1 during inhibition by KIR leads to some predictions about the mechanism of NK cell activation as well (Fig. 5). Upon initial contact with a target cell, early signals lead to Tyr phosphorylation and activation of Vav1. The GEF activity of Vav1 promotes a Rac1-dependent rearrangement of the actin cytoskeleton. Engagement of KIR by MHC class I on target cells leads to early ITIM phosphorylation and SHP-1 recruitment (29). Vav1 and KIR have both been reported to be phosphorylated by the Src-family kinase p56-Lck (8, 34). Only after formation of a tight actin-dependent NK-target cell interface do receptors cluster, thereby leading to amplification of early signals and binding of additional receptors to their ligand on target cells. Vav1 dephosphorylation by SHP-1 is independent of actin polymerization, as its trapping by the 2DL1/SHP-1(DA) receptor was not sensitive to cytochalasin D. In contrast, Tyr phosphorylation of the activation receptor 2B4 was inhibited by cytochalasin D and by a dominant-negative Rac1 to the same extent as it was by KIR binding to HLA-C on target cells. Therefore, the block of 2B4 phosphorylation by KIR (58) occurs upstream of receptor phosphorylation and not by direct dephosphorylation of 2B4 by SHP-1. Consistent with this interpretation, we have recently shown that engagement of inhibitory KIR by HLA-C on target cells prevents the movement of 2B4 to lipid rafts, thereby blocking 2B4 phosphorylation (57).
The early clustering and phosphorylation of KIR predicted by our model are consistent with recent data. The striking propensity of KIR to cluster at the site of contact with target cells expressing a KIR ligand (23, 29, 54), which occurs independently of actin polymerization (28), may contribute to an early phosphorylation of the ITIMs. Indeed, we have recently shown that ITIM phosphorylation induced by the binding of KIR to HLA-C on target cells occurs independently of actin polymerization (29). Specific localization of KIR within the “NK immune synapse” and its proximity to other molecules may contribute to the selectivity in Vav1 dephosphorylation by KIR-associated SHP-1.
Upon contact of T cells with antigen-presenting cells, early signals from the T-cell receptor precede adhesion through LFA-1, Vav1-dependent actin polymerization, and formation of the immune synapse (10, 60). Recruitment to the membrane and phosphorylation of Vav1 constitute a very proximal event in T-cell activation, as Vav1-dependent actin polymerization precedes Ca2+ flux induced by contact with an antigen-presenting cell (24). Upon T-cell receptor stimulation, Vav1 is recruited to a signaling complex through a LAT-Gad-SLP76-Vav1 set of interactions (9, 20). However, as LAT and SLP76 are dispensable for NK cytotoxicity (47, 62), and as overexpression of a PH domain-deleted Vav1 still resulted in enhanced lysis of K562 cells by NK cells (6), what signals and what molecular interactions recruit Vav1 during NK cell activation are unclear. The early activation signal(s) in NK cells that leads to Vav1 phosphorylation may be delivered by specialized receptors or, alternatively, by the same activation receptors that induce cytotoxicity once signal amplification through Vav1-dependent receptor clustering has taken place.
A test of our model would be to express a Tyr phosphorylation-independent form of Vav1 in NK cells and to show that it can overcome inhibition by KIR. Such a regulated, Tyr phosphorylation-independent Vav1 has not been developed yet. Constitutively active forms of Vav1 and Rac1 are no substitute, because actin polymerization is under stringent temporal and spatial regulation (50). The model suggests that ITIM-based inhibitory signals in NK cells could be bypassed by a Vav1-independent activation of actin polymerization. It will be interesting to see if the lysis of MHC class I-positive tumor cell lines by NK cells expressing the NKG2D/DAP10 receptor, which is apparently resistant to inhibition by ITIM-containing receptors (3, 17, 25), could be explained by a DAP10/phosphatidylinositol 3-kinase-dependent (59) but Vav1-independent signal.
The main conclusion from our work is that an early, actin polymerization-independent dephosphorylation of Vav1 by SHP-1 during engagement of KIR by MHC class I on target cells may block activation signals that depend on actin cytoskeleton rearrangements. Such a mechanism is possible because of the unique ability of KIR to cluster and become Tyr phosphorylated in the absence of actin polymerization and of cell adhesion (29). Whether Vav1 dephosphorylation alone can account for the full inhibitory effect of KIR and if this proposed mechanism accounts for inhibition of normal NK cells by a wild-type KIR remains to be seen. A selective dephosphorylation of Vav1 by SHP-1 is consistent with the known inhibition of early NK activation signals upon KIR binding to its ligand on target cells and suggests that other ITIM-based inhibitory pathways may also rely on a selective dephosphorylation of substrates by SHP-1.
Acknowledgments
We thank T. Yi, J. Gumperz, P. Parham, and G. Cohen for the kind gift of reagents, and we thank M. Sandusky for DNA sequencing.
REFERENCES
- 1.Aghazadeh, B., W. E. Lowry, X. Y. Huang, and M. K. Rosen. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell 102:625-633. [DOI] [PubMed] [Google Scholar]
- 2.Barford, D., and B. G. Neel. 1998. Revealing mechanisms for SH2 domain mediated regulation of the protein tyrosine phosphatase SHP-2. Structure 6:249-254. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, S., V. Groh, J. Wu, A. Steinle, J. H. Phillips, L. L. Lanier, and T. Spies. 1999. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science 285:727-729. [DOI] [PubMed] [Google Scholar]
- 4.Billadeau, D. D., K. M. Brumbaugh, C. J. Dick, R. A. Schoon, X. R. Bustelo, and P. J. Leibson. 1998. The Vav-Rac1 pathway in cytotoxic lymphocytes regulates the generation of cell-mediated killing. J. Exp. Med. 188:549-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Billadeau, D. D., and P. J. Leibson. 2002. ITAMs versus ITIMs: striking a balance during cell regulation. J. Clin. Investig. 109:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billadeau, D. D., S. M. Mackie, R. A. Schoon, and P. J. Leibson. 2000. Specific subdomains of Vav differentially affect T cell and NK cell activation. J. Immunol. 164:3971-3981. [DOI] [PubMed] [Google Scholar]
- 7.Binstadt, B. A., D. D. Billadeau, D. Jevremovic, B. L. Williams, N. Fang, T. L. Yi, G. A. Koretzky, R. T. Abraham, and P. J. Leibson. 1998. SLP-76 is a direct substrate of SHP-1 recruited to killer cell inhibitory receptors. J. Biol. Chem. 273:27518-27523. [DOI] [PubMed] [Google Scholar]
- 8.Binstadt, B. A., K. M. Brumbaugh, C. J. Dick, A. M. Scharenberg, B. L. Williams, M. Colonna, L. L. Lanier, J. P. Kinet, R. T. Abraham, and P. J. Leibson. 1996. Sequential involvement of Lck and SHP-1 with MHC-recognizing receptors on NK cells inhibits FcR-initiated tyrosine kinase activation. Immunity 5:629-638. [DOI] [PubMed] [Google Scholar]
- 9.Boerth, N. J., J. J. Sadler, D. E. Bauer, J. L. Clements, S. M. Gheith, and G. A. Koretzky. 2000. Recruitment of SLP-76 to the membrane and glycolipid-enriched membrane microdomains replaces the requirement for linker for activation of T cells in T cell receptor signaling. J. Exp. Med. 192:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromley, S. K., W. R. Burack, K. G. Johnson, K. Somersalo, T. N. Sims, C. Sumen, M. M. Davis, A. S. Shaw, P. M. Allen, and M. L. Dustin. 2001. The immunological synapse. Annu. Rev. Immunol. 19:375-396. [DOI] [PubMed] [Google Scholar]
- 11.Brumbaugh, K. M., B. A. Binstadt, D. D. Billadeau, R. A. Schoon, C. J. Dick, R. M. Ten, and P. J. Leibson. 1997. Functional role for Syk tyrosine kinase in natural killer cell-mediated natural cytotoxicity. J. Exp. Med. 186:1965-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burshtyn, D. N., and E. O. Long. 1997. Regulation through inhibitory receptors: lessons from natural killer cells. Trends Cell Biol. 7:473-479. [DOI] [PubMed] [Google Scholar]
- 13.Burshtyn, D. N., J. Shin, C. Stebbins, and E. O. Long. 2000. Adhesion to target cells is disrupted by the killer cell inhibitory receptor. Curr. Biol. 10:777-780. [DOI] [PubMed] [Google Scholar]
- 14.Bustelo, X. R. 2000. Regulatory and signaling properties of the Vav family. Mol. Cell. Biol. 20:1461-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carena, I., A. Shamshiev, A. Donda, M. Colonna, and G. D. Libero. 1997. Major histocompatibility complex class I molecules modulate activation threshold and early signaling of T cell antigen receptor-γ/δ stimulated by nonpeptidic ligands. J. Exp. Med. 186:1769-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlin, L. M., K. Eleme, F. E. McCann, and D. M. Davis. 2001. Intercellular transfer and supramolecular organization of human leukocyte antigen C at inhibitory natural killer cell immune synapses. J. Exp. Med. 194:1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cerwenka, A., J. L. Baron, and L. L. Lanier. 2001. Ectopic expression of retinoic acid early inducible-1 gene (RAE-1) permits natural killer cell-mediated rejection of a MHC class I-bearing tumor in vivo. Proc. Natl. Acad. Sci. USA 98:11521-11526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan, G. D., T. Hanke, and K. D. Fischer. 2001. Vav-1 regulates NK T cell development and NK cell cytotoxicity. Eur. J. Immunol. 31:2403-2410. [DOI] [PubMed] [Google Scholar]
- 19.Chiang, G. G., and B. M. Sefton. 2001. Specific dephosphorylation of the Lck tyrosine protein kinase at Tyr 394 by the SHP-1 tyrosine phosphatase. J. Biol. Chem. 276:23173-23178. [DOI] [PubMed] [Google Scholar]
- 20.Clements, J. L., N. J. Boerth, J. R. Lee, and G. A. Koretzky. 1999. Integration of T cell receptor-dependent signaling pathways by adapter proteins. Annu. Rev. Immunol. 17:89-108. [DOI] [PubMed] [Google Scholar]
- 21.Colucci, F., E. Rosmaraki, S. Bregenholt, S. I. Samson, V. Di Bartolo, M. Turner, L. Vanes, V. Tybulewicz, and J. P. Di Santo. 2001. Functional dichotomy in natural killer cell signaling. Vav1-dependent and -independent mechanisms. J. Exp. Med. 193:1413-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colucci, F., E. Schweighoffer, E. Tomasello, M. Turner, J. R. Ortaldo, E. Vivier, V. L. Tybulewicz, and J. P. Di Santo. 2002. Natural cytotoxicity uncoupled from the Syk and ZAP-70 intracellular kinases. Nat. Immunol. 3:288-294. [DOI] [PubMed] [Google Scholar]
- 23.Davis, D. M., I. Chiu, M. Fassett, G. B. Cohen, O. Mandelboim, and J. L. Strominger. 1999. The human natural killer cell immune synapse. Proc. Natl. Acad. Sci. USA 96:15062-15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delon, J., N. Bercovici, R. Liblau, and A. Trautmann. 1998. Imaging antigen recognition by naive CD4+ T cells: compulsory cytoskeletal alterations for the triggering of an intracellular calcium response. Eur. J. Immunol. 28:716-729. [DOI] [PubMed] [Google Scholar]
- 25.Diefenbach, A., E. R. Jensen, A. M. Jamieson, and D. H. Raulet. 2001. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413:165-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dietrich, J., M. Cella, and M. Colonna. 2001. Ig-like transcript 2 (ILT2)/leukocyte Ig-like receptor 1 (LIR1) inhibits TCR signaling and actin cytoskeleton reorganization. J. Immunol. 166:2514-2521. [DOI] [PubMed] [Google Scholar]
- 27.Dustin, M. L., and A. C. Chan. 2000. Signaling takes shape in the immune system. Cell 103:283-294. [DOI] [PubMed] [Google Scholar]
- 28.Fassett, M. S., D. M. Davis, M. M. Valter, G. B. Cohen, and J. L. Strominger. 2001. Signaling at the inhibitory natural killer cell immune synapse regulates lipid raft polarization but not class I MHC clustering. Proc. Natl. Acad. Sci. USA 98:14547-14552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faure, M., D. F. Barber, S. M. Takahashi, T. Jin, and E. O. Long. 2003. Spontaneous clustering and tyrosine phosphorylation of NK cell inhibitory receptor induced by ligand binding. J. Immunol. 170:6107-6114. [DOI] [PubMed]
- 30.Flint, A. J., T. Tiganis, D. Barford, and N. K. Tonks. 1997. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 94:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galandrini, R., G. Palmieri, M. Piccoli, L. Frati, and A. Santoni. 1999. Role for the Rac1 exchange factor Vav in the signaling pathways leading to NK cell cytotoxicity. J. Immunol. 162:3148-3152. [PubMed] [Google Scholar]
- 32.Gordon, J. A. 1991. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 201:477-482. [DOI] [PubMed] [Google Scholar]
- 33.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 34.Han, J., B. Das, W. Wei, L. Van Aelst, R. D. Mosteller, R. Khosravi-Far, J. K. Westwick, C. J. Der, and D. Broek. 1997. Lck regulates Vav activation of members of the Rho family of GTPases. Mol. Cell. Biol. 17:1346-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huyer, G., S. Liu, J. Kelly, J. Moffat, P. Payette, B. Kennedy, G. Tsaprailis, M. J. Gresser, and C. Ramachandran. 1997. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J. Biol. Chem. 272:843-851. [DOI] [PubMed] [Google Scholar]
- 36.Jia, Z., D. Barford, A. J. Flint, and N. K. Tonks. 1995. Structural basis for phosphotyrosine peptide recognition by protein tyrosine phosphatase 1B. Science 268:1754-1758. [DOI] [PubMed] [Google Scholar]
- 37.Jiang, K., B. Zhong, D. L. Gilvary, B. C. Corliss, E. Hong-Geller, S. Wei, and J. Y. Djeu. 2000. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat. Immunol. 1:419-425. [DOI] [PubMed] [Google Scholar]
- 38.Kaufman, D. S., R. A. Schoon, M. J. Robertson, and P. J. Leibson. 1995. Inhibition of selective signaling events in natural killer cells recognizing major histocompatibility complex class I. Proc. Natl. Acad. Sci. USA 92:6484-6488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kon-Kozlowski, M., G. Pani, T. Pawson, and K. A. Siminovitch. 1996. The tyrosine phosphatase PTP1C associates with Vav, Grb2, and mSos1 in hematopoietic cells. J. Biol. Chem. 271:3856-3862. [DOI] [PubMed] [Google Scholar]
- 40.Leibson, P. J. 1997. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity 6:655-661. [DOI] [PubMed] [Google Scholar]
- 41.Long, E. O. 1999. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 17:875-904. [DOI] [PubMed] [Google Scholar]
- 42.Lou, Z. K., D. D. Billadeau, D. N. Savoy, R. A. Schoon, and P. J. Leibson. 2001. A role for a RhoA/ROCK/LIM-kinase pathway in the regulation of cytotoxic lymphocytes. J. Immunol. 167:5749-5757. [DOI] [PubMed] [Google Scholar]
- 43.Nishimura, H., T. Okazaki, Y. Tanaka, K. Nakatani, M. Hara, A. Matsumori, S. Sasayama, A. Mizoguchi, H. Hiai, N. Minato, and T. Honjo. 2001. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science 291:319-322. [DOI] [PubMed] [Google Scholar]
- 44.Oldenborg, P. A., A. Zheleznyak, Y. F. Fang, C. F. Lagenaur, H. D. Gresham, and F. P. Lindberg. 2000. Role of CD47 as a marker of self on red blood cells. Science 288:2051-2054. [DOI] [PubMed] [Google Scholar]
- 45.Palmieri, G., V. Tullio, A. Zingoni, M. Piccoli, L. Frati, M. López-Botet, and A. Santoni. 1999. CD94/NKG2-A inhibitory complex blocks CD16-triggered Syk and extracellular regulated kinase activation, leading to cytotoxic function of human NK cells. J. Immunol. 162:7181-7188. [PubMed] [Google Scholar]
- 46.Penninger, J. M., and G. R. Crabtree. 1999. The actin cytoskeleton and lymphocyte activation. Cell 96:9-12. [DOI] [PubMed] [Google Scholar]
- 47.Peterson, E. J., J. L. Clements, Z. K. Ballas, and G. A. Koretzky. 1999. NK cytokine secretion and cytotoxicity occur independently of the SLP-76 adaptor protein. Eur. J. Immunol. 29:2223-2232. [DOI] [PubMed] [Google Scholar]
- 48.Ravetch, J. V., and L. L. Lanier. 2000. Immune inhibitory receptors. Science 290:84-89. [DOI] [PubMed] [Google Scholar]
- 49.Siminovitch, K. A., and B. G. Neel. 1998. Regulation of B cell signal transduction by SH2-containing protein-tyrosine phosphatases. Semin. Immunol. 10:329-347. [DOI] [PubMed] [Google Scholar]
- 50.Srinivasan, S., F. Wang, S. Glavas, A. Ott, F. Hofmann, K. Aktories, D. Kalman, and H. R. Bourne. 2003. Rac and Cdc42 play distinct roles in regulating PI(3,4,5)P3 and polarity during neutrophil chemotaxis. J. Cell Biol. 160:375-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Timms, J. F., K. Carlberg, H. H. Gu, H. Y. Chen, S. Kamatkar, M. J. S. Nadler, L. R. Rohrschneider, and B. G. Neel. 1998. Identification of major binding proteins and substrates for the SH2-containing protein tyrosine phosphatase SHP-1 in macrophages. Mol. Cell. Biol. 18:3838-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Valiante, N. M., J. H. Phillips, L. L. Lanier, and P. Parham. 1996. Killer cell inhibitory receptor recognition of human leukocyte antigen (HLA) class I blocks formation of a pp36/PLC-γ signaling complex in human natural killer (NK) cells. J. Exp. Med. 184:2243-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vyas, Y. M., H. Maniar, and B. Dupont. 2002. Cutting edge: differential segregation of the Src homology 2-containing protein tyrosine phosphatase-1 within the early NK cell immune synapse distinguishes noncytolytic from cytolytic interactions. J. Immunol. 168:3150-3154. [DOI] [PubMed] [Google Scholar]
- 54.Vyas, Y. M., K. M. Mehta, M. Morgan, H. Maniar, L. Butros, S. Jung, J. K. Burkhardt, and B. Dupont. 2001. Spatial organization of signal transduction molecules in the NK cell immune synapses during MHC class I-regulated noncytolytic and cytolytic interactions. J. Immunol. 167:4358-4367. [DOI] [PubMed] [Google Scholar]
- 55.Wagtmann, N., S. Rajagopalan, C. C. Winter, M. Peruzzi, and E. O. Long. 1995. Killer cell inhibitory receptors specific for HLA-C and HLA-B identified by direct binding and by functional transfer. Immunity 3:801-809. [DOI] [PubMed] [Google Scholar]
- 56.Wang, P., H. Fu, D. F. Snavley, M. A. Freitas, and D. Pei. 2002. Screening combinatorial libraries by mass spectrometry. 2. Identification of optimal substrates of protein tyrosine phosphatase SHP-1. Biochemistry 41:6202-6210. [DOI] [PubMed] [Google Scholar]
- 57.Watzl, C., and E. O. Long. 2003. Natural killer cell inhibitory receptors block actin cytoskeleton-dependent recruitment of 2B4 (CD244) to lipid rafts. J. Exp. Med. 197:77-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watzl, C., C. C. Stebbins, and E. O. Long. 2000. Cutting edge: NK cell inhibitory receptors prevent tyrosine phosphorylation of the activation receptor 2B4 (CD244). J. Immunol. 165:3545-3548. [DOI] [PubMed] [Google Scholar]
- 59.Wu, J., H. Cherwinski, T. Spies, J. H. Phillips, and L. L. Lanier. 2000. DAP10 and DAP12 form distinct, but functionally cooperative, receptor complexes in natural killer cells. J. Exp. Med. 192:1059-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wülfing, C., M. D. Sjaastad, and M. M. Davis. 1998. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc. Natl. Acad. Sci. USA 95:6302-6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang, J., Z. L. Cheng, T. Q. Niu, X. S. Liang, Z. Z. J. Zhao, and G. W. Zhou. 2000. Structural basis for substrate specificity of protein-tyrosine phosphatase SHP-1. J. Biol. Chem. 275:4066-4071. [DOI] [PubMed] [Google Scholar]
- 62.Zhang, W., C. L. Sommers, D. N. Burshtyn, C. C. Stebbins, J. B. DeJarnette, R. P. Trible, A. Grinberg, H. C. Tsay, H. M. Jacobs, C. M. Kessler, E. O. Long, P. E. Love, and L. E. Samelson. 1999. Essential role of LAT in T cell development. Immunity 10:323-332. [DOI] [PubMed] [Google Scholar]
- 63.Zhang, Z. S., K. Shen, W. Lu, and P. A. Cole. 2003. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J. Biol. Chem. 278:4668-4674. [DOI] [PubMed] [Google Scholar]