Abstract
PTEN is a tumor suppressor frequently inactivated in brain, prostate, and uterine cancers that acts as a phosphatase on phosphatidylinositol-3,4,5-trisphosphate, antagonizing the activity of the phosphatidylinositol 3′-OH kinase. PTEN manifests its tumor suppressor function in most tumor cells by inducing G1-phase cell cycle arrest. To study the mechanism of cell cycle arrest, we established a tetracycline-inducible expression system for PTEN in cell lines lacking this gene. Expression of wild-type PTEN but not of mutant forms unable to dephosphorylate phosphoinositides reduced the expression of cyclin D1. Cyclin D1 reduction was accompanied by a marked decrease in endogenous retinoblastoma (Rb) protein phosphorylation on cyclin D/CDK4-specific sites, showing an early negative effect of PTEN on Rb inactivation. PTEN expression also prevented cyclin D1 from localizing to the nucleus during the G1- to S-phase cell cycle transition. The PTEN-induced localization defect and the cell growth arrest could be rescued by the expression of a nucleus-persistent mutant form of cyclin D1, indicating that an important effect of PTEN is at the level of nuclear availability of cyclin D1. Constitutively active Akt/PKB kinase counteracted the effect of PTEN on cyclin D1 translocation. The data are consistent with an oncogenesis model in which a lack of PTEN fuels the cell cycle by increasing the nuclear availability of cyclin D1 through the Akt/PKB pathway.
Human cancers are generally characterized by several genetic alterations that converge to the establishment of the fully transformed phenotype (9). The most important advantage over normal cells that tumor cells acquire is probably uncontrolled progression through the cell cycle. The periodic movement of normal cells through the cell cycle is orchestrated by programmed oscillations in the activity of a family of serine/threonine protein kinases called cyclin-dependent kinases (CDKs) (reviewed in reference 28). The activation of CDKs is dependent on the association with cyclin regulatory subunits, and conversely, their inactivation is dependent on the association with CDK inhibitors (CKI). In response to mitogenic signals, normal cells are induced to exit the first gap (G1) phase and enter the DNA synthesis (S) phase of the cell cycle by assembling D-type cyclins with CDK4 and -6. These complexes phosphorylate the inhibitory retinoblastoma (Rb) protein and release it from the E2F transcription factors that trigger progression into the S phase. A fine control of cyclins and CKIs is set in place by both transcriptional and degradation mechanisms for regulation of the succession of distinct events governing progression through the cell cycle.
One of the most frequently inactivated genes in tumors is PTEN (20, 32). It encodes a 403-amino-acid phosphatase that antagonizes the activity of phosphatidylinositol-3′-OH kinase (PI-3 kinase) on phosphoinositide substrates (21). PI-3 kinase has multiple effects, including activation of Akt/PKB, promoting survival signals, of p70S6-kinase, involved in the G1 cell cycle transition, and of the small G protein Rac, mediating cytoskeletal rearrangements (reviewed in reference 36).
In PTEN-deficient cell lines derived from tumors, expression of PTEN induces a marked decrease in proliferation due to cell cycle arrest in the G1 phase (10, 19). This arrest has been attributed to an increase in the CKI p27Kip1 detectable in cell lysates (19) or in cyclin E-cdk2 complexes (6). The increase in the CKI p27Kip1 has been explained by two mechanisms. A first mechanism is based on activation of p27Kip1 gene transcription by Forkhead transcription factors (FKHR) (23, 25). These factors are phosphorylated and inactivated by Akt/PKB (5, 16) and rendered active by inhibition of the PI-3 kinase-Akt/PKB pathway with PI-3 kinase inhibitors (23). A second mechanism consists of reduced degradation of p27Kip1 (22). An effect of PTEN on cyclin D1 expression was also observed (26, 27, 35), but its significance for the cell cycle was not investigated. Other groups have reported that PTEN does not modify cyclin D1 levels (19, 33).
Here, we analyzed the contribution of cyclin D1 to the cell cycle arrest determined by PTEN with an inducible system that we established for PTEN expression in two PTEN-deficient cell lines. We unambiguously found reduction of cyclin D1 expression levels after PTEN induction. We also found that PTEN prevents the increase in nuclear localization of cyclin D1 during cell cycle progression from the G1 to the S phase. In correlation with the decreased nuclear availability of cyclin D1, we found a lack of phosphorylation of endogenous Rb on cyclin D/CDK4-specific sites. As the expression of a nucleus-persistent mutant form of cyclin D1 reestablished the proliferation of PTEN-arrested cells, the subcellular distribution of cyclin D1 appears to be an important mechanism responsible for the effects of PTEN on the cell cycle.
MATERIALS AND METHODS
Plasmid construction.
To establish the retroviral tetracycline-inducible system, wild-type PTEN and the phosphatase-inactive mutant forms PTEN-C124S and PTEN-G129E were inserted in the antisense orientation after the tetracycline operator from the pCXbR(TO) (blasticidin resistance) retroviral vector (1). The pCXn/TR2 (neomycin resistance) retroviral vector encoding the tetracycline-dependent repressor of the tetracycline operator was previously described (1). Human wild-type cyclin D1 cDNA was isolated from human placenta RNA by reverse transcription and PCR (Stratagene). The PCR product was cloned into the vector included in the Topo TA cloning kit (Invitrogen) and completely sequenced. The cDNA was then cloned into the pCXp (puromycin resistance) retroviral vector in frame with an N-terminal Myc tag (pCXp-Myc-CycD1). Myc-tagged wild-type PTEN and the phosphatase-inactive mutant form PTEN-H93A in the pCXb retroviral vector were previously described (11, 18). The cDNAs for mouse wild-type and T286A mutant cyclin D1 (gift of C. Sherr) were cloned into the pCXp retroviral vector. The hemagglutinin (HA)-tagged wild-type and myristoylated PKB/Akt sequences (gift of T. Chan) were cloned into the pCXn retroviral vector.
Cell growth assays.
The U-87 MG (American Type Culture Collection) and U-251 MG (gift of T. J. Liu) glioblastoma cell lines, the MCF7 breast cancer cell line (gift of A. Monteiro), and Bosc23 cells were grown in Dulbecco modified Eagle's medium with 10% fetal calf serum (FCS). Both glioblastoma cell lines are PTEN deficient but differ in p53 gene status (15).
The protocols used for transfection, retroviral infection with amphotropic defective retroviruses, stable expression of PTEN proteins, cell proliferation, and soft agar colony assay have been described in detail elsewhere (11). For stable coexpression of two proteins, cells were simultaneously infected with a mixture of supernatants from Bosc23 cells transfected separately with constructs encoding the respective proteins.
To obtain nonleaky inducible expression of PTEN, U-87 cells dually infected with CXn/TR2 and CXbR(TO)-PTEN were diluted to 102 cells/ml and distributed in 96-well plates in 100 μl of selection medium containing 1 mg of neomycin per ml and 10 μg of blasticidin per ml. Surviving cell colonies were amplified and tested for nonleaky induced expression of PTEN by treatment with 1 μg of doxycycline per ml, followed by cell lysis and Western blot analysis. Single-cell colonies of dually infected U-251 cells were isolated, expanded, and tested as described above for nonleaky induced expression of PTEN.
Protein analysis.
Cell lysis in buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 10 mM EDTA, 10% glycerol, and 1% Triton X-100 and Western blot analysis were previously described (11). For cytoplasmic and nuclear extracts, a nuclear and cytoplasm extraction kit (Pierce) was used in accordance with the manufacturer's instructions. All lysis buffers were supplemented with 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 21 μg of aprotinin per ml, 1 mM sodium orthovanadate, and 0.1 mM sodium molybdate.
Antibodies were obtained for PTEN (A2B1), Myc tag (9E10), cyclin D1 (M-20), cyclin A (H432), Rb (C15), PARP (H-250), HA tag (Y-11; Santa Cruz Biotechnology), p27Kip1 (Transduction Laboratories), β-actin (AC74; Sigma), phospho-Rb-S807/S811, phospho-Akt-S473, and Akt/PKB (Cell Signaling).
Immunofluorescence assay.
Serum-starved U-87 cells (2 × 104) were plated on poly-d-lysine-coated glass coverslips (Becton Dickinson) in growth medium containing 10 or 20% FCS. When necessary, the inhibitors LY 294002 and rapamycin were added simultaneously with FCS at concentrations of 10 μM and 20 nM, respectively. After various periods of time, the cells were fixed and stained as described previously (12). The secondary antibodies used were fluorescein isothiocyanate-conjugated anti-mouse and tetramethyl rhodamine isothiocyanate-coupled anti-rabbit (Jackson ImmunoResearch Laboratories).
Cytofluorimetric analysis of cells.
Cell cycle analysis was performed by monitoring the DNA content at various time points by propidium iodide staining. Cells synchronized in G1 by serum starvation for 24 h were fixed in 70% cold ethanol, washed once in phosphate-buffered saline containing 0.1% glucose, and incubated for 30 min at 4°C in phosphate-buffered saline containing 0.1% glucose, 1 mg of RNase A per ml, and 2 μg of propidium iodide per ml. Detection of intracellular antigens was performed with the Fix&Perm cell permeabilization kit (Caltag) in accordance with the directions of the manufacturer. The primary monospecific anti-PTEN and anti-HA tag (Y-11) antibodies were used at a 2-μg/ml concentration, and the secondary antibodies were the same as for immunofluorescence. Cells (10,000 to 20,000) were acquired by a FACScan flow cytometer (Becton Dickinson) and analyzed with the Cell-Quest software.
RESULTS
Establishment of a nonleaky PTEN-inducible system in PTEN-deficient cell lines.
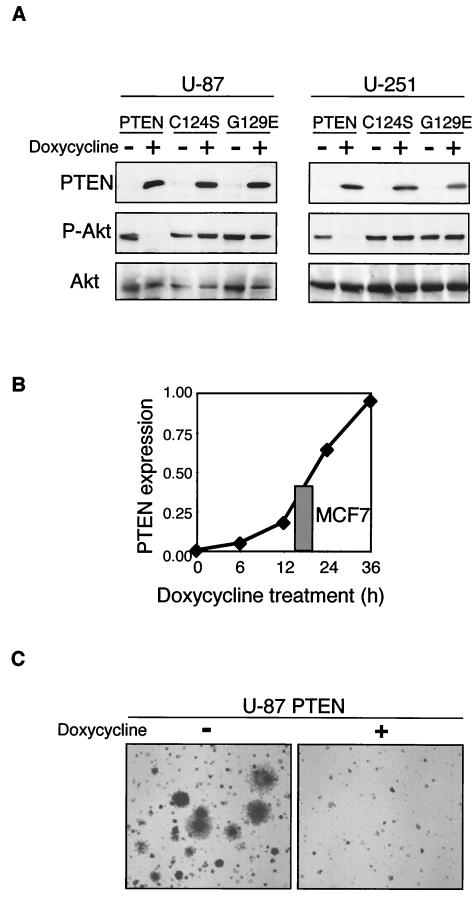
PTEN expression in PTEN-deficient cells induces cell cycle arrest (10, 19). To examine the basis of this arrest, we have engineered a tetracycline-inducible system for retroviral expression of PTEN in cultured mammalian cells (Fig. 1). In this system, two retroviral constructs, pCXn/TR2 and pCXbR(TO)-PTEN, were simultaneously introduced into mammalian cells by infection. The first construct encodes the repressor (TR) of the tetracycline operator (TO) that is present in the second construct within the inducible promoter that controls the transcription of the gene of interest (PTEN). In the absence of tetracycline, the repressor prevents PTEN expression. Upon addition of doxycycline, a derivative of tetracycline that inhibits the repressor, PTEN is expressed. Wild-type PTEN and the catalytically inactive mutant forms PTEN-C124S and PTEN-G129E were inducibly expressed in two PTEN-deficient glioblastoma cell lines, U-87 and U-251 (Fig. 1A). Expression of wild-type PTEN, but not that of the mutant forms of PTEN, efficiently led to the dephosphorylation of Akt/PKB kinase, a downstream target of the PI-3 kinase-PTEN pathway that is dephosphorylated and inactivated by PTEN (31).
FIG. 1.
Retroviral inducible system for PTEN expression. (A) U-87 and U-251 glioblastoma cells were coinfected with pCXn/TR2 retroviruses containing the tetracycline-dependent repressor of the tetracycline operator and pCXbR(TO) retroviruses containing wild-type or enzyme-inactive mutant forms of PTEN (C124S and G129E) inserted after the repressible operator. The cells were grown in medium with 10% FCS and treated with doxycycline for 24 h to induce PTEN expression. Western blot analysis of proteins (50 μg) from total cell lysates was performed with anti-PTEN, anti-phospho-Akt (P-Akt), and anti-total Akt (Akt) antibodies. (B) Graphic representation of the time course of PTEN expression levels normalized to actin levels in PTEN-inducible U-87 cells following doxycycline treatment. The grey bar represents the actin-normalized endogenous PTEN level from MCF7 breast cancer cells. Total U-87 and MCF7 cell lysates were loaded onto the same gel and analyzed simultaneously with PTEN and actin antibodies. (C) Suppression of anchorage-independent growth by PTEN expression in U-87 PTEN-inducible cells. Nontreated cells were evenly distributed in soft agar plates whether containing (+) or not containing (−) doxycycline. Images of plates incubated for 3 weeks were taken with an Axiovert 200 microscope (Zeiss) at an original magnification of ×2.5.
Expression of induced PTEN in U-87 cells was readily detectable by Western blot analysis (Fig. 1A) and increased in a time-dependent manner, reaching levels comparable to that of endogenously expressed PTEN in MCF7 breast cancer cells after more than 12 h of induction (Fig. 1B). To confirm that the expression level of PTEN obtained in the inducible system is functional in reverting the transformation phenotype of the cancer cells, we tested the anchorage-independent growth of U-87 PTEN-inducible cells equally divided in agar plates containing doxycycline or not containing doxycycline (Fig. 1C). No colonies grew in the doxycycline-containing plates, where the expression of PTEN was induced, attesting to the efficiency of PTEN suppression at expression levels comparable to those achieved by endogenous expression.
Effects of PTEN induction on G1- to S-phase cell cycle progression.
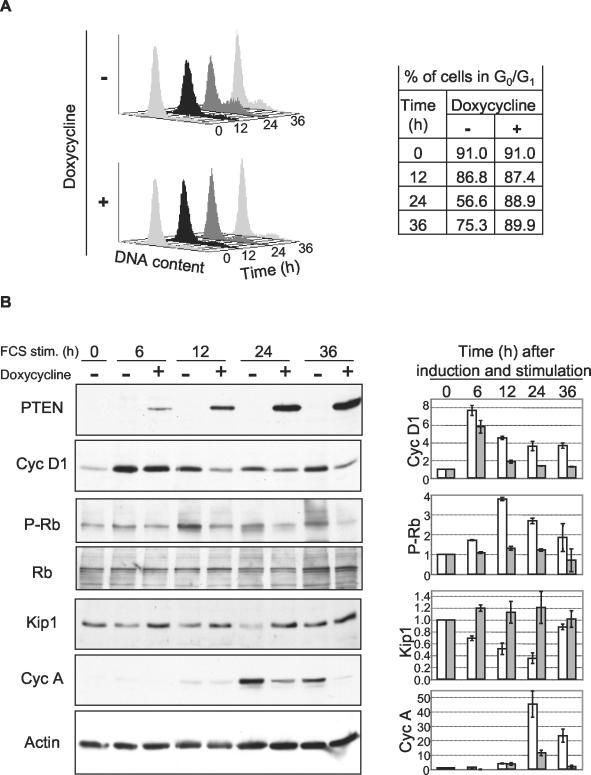
By using the PTEN-inducible system, we next analyzed the protein levels of molecules involved in the transition from the G1 to the S phase of the cell cycle (Fig. 2). PTEN-inducible U-87 cells were arrested in G0 by serum deprivation for 24 to 48 h and subsequently stimulated with serum to synchronously re-enter the cell cycle. Concomitantly with the serum addition, cells were treated with doxycycline to induce PTEN expression or left untreated. The untreated cells progressed through the cell cycle from the G0 phase at 0 h to the late G1 phase at 12 h and the S phase at 24 h and entered a new cell cycle by 36 h after serum stimulation (Fig. 2A). As expected, cells treated with doxycycline for PTEN expression showed no progression to the S phase at 24 h or beyond (Fig. 2A).
FIG. 2.
Time course analysis of G1-S-phase cell cycle regulators following PTEN induction. PTEN-inducible U-87 cells were synchronized in G0 by 24 h of serum deprivation and allowed to re-enter the cell cycle by 10% FCS stimulation (stim.) for the indicated periods of time in the presence (+) or in the absence (−) of doxycycline to induce PTEN expression. (A) Equal numbers of cells were prepared by propidium iodide DNA staining for cell cycle analysis by FACS. The table shows the percentage of cells in G0/G1 calculated with the ModFitLT program for cell cycle analysis. (B) Total cell lysates of equal numbers of cells were subjected to Western blot analysis with antibodies to PTEN, cyclin (Cyc) D1, phospho-Rb (P-Rb), Rb, p27Kip1 (Kip1), cyclin A, and actin. On the right side is shown a densitometric analysis of cells not expressing PTEN (white bars) or expressing PTEN (grey bars) for the expression levels of cyclin D1, p27Kip1, and cyclin A normalized to actin and of phospho-Rb normalized to total Rb. Quantification was performed with the ImageJ software. The data shown are the means and standard deviations of three experiments. Note the decrease in cyclin D1 levels and the increase in p27Kip1 levels in cells expressing PTEN.
The expression levels or activity of cyclins D1 and A and the CKI p27Kip1 were determined at 6 h (early G1 phase) and at the same time points used for the cell cycle analysis (Fig. 2B). In contrast to normal cells, where cyclin D1 expression is very low in G0 and strongly increases in G1 (4), in the transformed cell line tested, cyclin D1 could be detected in the G0 phase and its total expression level increased moderately in the G1 phase. PTEN induction had early effects on the cyclin D1 expression level, which was decreased beginning at 6 h and continued to be decreased throughout the cell cycle. A similar time course of cyclin D1 down-regulation by PTEN expression was also observed in PTEN-inducible U-251 cells (data not shown). The effect of the cyclin D1 decrease on the activity of cyclin D/CDK4 complexes was evaluated by measuring the phosphorylation of Rb by cyclin D/CDK4 with a phospho-Rb-specific antibody recognizing residues phosphorylated only by cyclin D/CDK4 and not by cyclin E or A/CDK2 (38). The phosphorylation of Rb was strongly reduced in the late G1 phase, indicating that the decrease in the cyclin D1 level was accompanied by a strong decrease in cyclin D/CDK4 activity in cells expressing PTEN. As expected, PTEN had the opposite effect on the level of p27Kip1, which was increased starting in G1 phase. No effect on cyclin E levels in the G1 and S phases of the cell cycle was observed (data not shown). Cyclin A, which is the latest cyclin synthesized during the S-phase transition, was decreased in cells expressing PTEN, most likely because of the lack of progression through the cell cycle induced by PTEN.
Phosphatase-inactive mutant forms of PTEN do not reduce cyclin D1 expression.
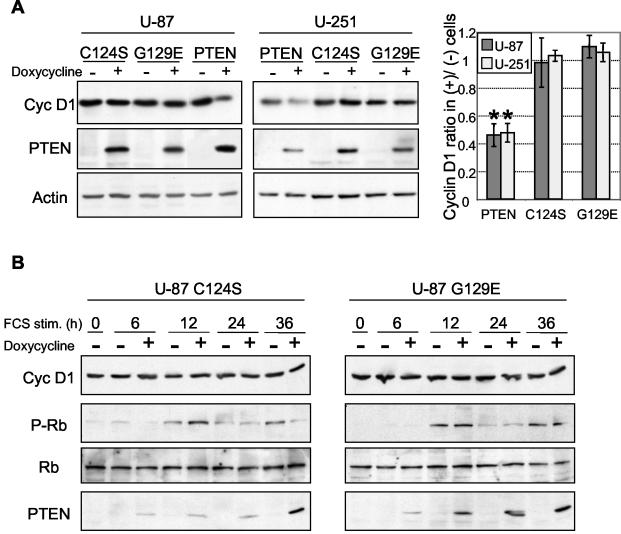
To determine the mechanism by which PTEN causes a reduction in cyclin D1 expression, we first analyzed the requirements of the enzymatic activity of PTEN. Wild-type PTEN and two inactive mutant forms, PTEN-C124S and PTEN-G129E, were inducibly expressed in U-87 and U-251 glioblastoma cells. The G129E mutation has been reported to inactivate PTEN phosphatase activity only on phosphoinositides, and the C124S mutation inactivates the activity on both phospho-polypeptide and phosphoinositide substrates (24). To dissociate the influence of PTEN on cyclin D1 levels from the influence of the cell cycle phase on cyclin D1 levels, and thus to exclude the possibility of an indirect effect of PTEN on cyclin D1 levels through the PTEN-induced modification of the cell cycle, the experiments were carried out with cells deprived of serum for at least 24 h prior to PTEN induction. The cells thus synchronized in the G0 phase were maintained under serum deprivation throughout the course of the experiment. In such experiments carried out with the U-87 and U-251 cell lines, while PTEN decreased the cyclin D1 levels more than twofold, the expression of both mutant forms had no significant influence on cyclin D1 levels (Fig. 3A). To further confirm this finding, which is not in agreement with recently published data (35), time course experiments similar to that presented in Fig. 2 for PTEN were done in parallel for the two phosphatase-inactive mutant forms. The two mutant forms behaved similarly and, in contrast to PTEN, had no effect on cyclin D1 or on Rb phosphorylation (Fig. 3B). These results suggested that the dephosphorylation of phosphoinositides is necessary for PTEN′s effect on cyclin D1 expression levels.
FIG. 3.
Effects of wild-type PTEN and enzyme-inactive mutant forms of PTEN on cyclin D1 protein levels. (A) Inducible U-87 and U-251 cells were serum starved for 36 h and subsequently treated with doxycycline in the absence of serum for 12 h to induce the expression of PTEN or the enzyme-inactive G129E and C124S mutant forms. The Western blot analysis was performed with the indicated antibodies. The graph shows the densitometric analysis of cyclin (Cyc) D1 levels normalized to the corresponding actin levels and expressed as ratios in PTEN-induced (+) versus noninduced (−) cells. Error bars indicate standard deviations from three experiments. There are significant differences (*) between cyclin D1 ratios for PTEN versus the C124S or G129E mutant form in U-87 cells (P < 0.02 and P < 0.01, respectively) and in U-251 cells (P < 0.01 in both cases). There is no significant difference between the C124S and G129E mutant forms in either U-87 (P = 0.26) or U-251 (P = 0.27) cells. The P values were obtained by using a paired, one-tailed t test. (B) Cell cycle analysis for the enzyme-inactive G129E and C124S mutant forms, showing no modification of cyclin D1 or phosphorylated Rb levels. This analysis was carried out as described in the legend to Fig. 2. stim., stimulation.
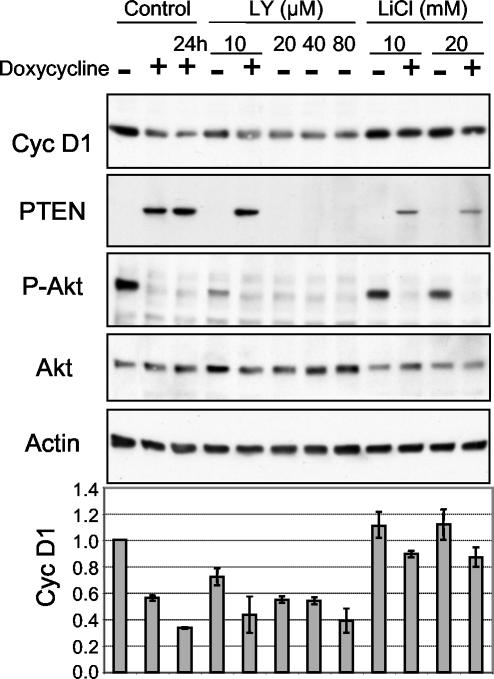
To further confirm that the mechanism responsible for decreased cyclin D1 levels is based on the inhibition of PI-3 kinase activity, we used pharmacological inhibitors of the PI3-kinase pathway. LY 294002 and LiCl specifically inhibit PI-3 kinase and glycogen synthase kinase-3 (GSK-3) (30), respectively. Serum-deprived, PTEN-inducible U-87 cells were simultaneously treated for 12 h with the inhibitors and with doxycycline as indicated (Fig. 4). LY 294002 mimicked the effects of PTEN and reduced cyclin D1 expression in cells, reaching maximum effects at concentrations greater than 10 μM (Fig. 4). Inhibition of GSK-3 by LiCl partially restored cyclin D1 levels in cells expressing PTEN (Fig. 4). GSK-3 is a kinase phosphorylated and inactivated by Akt/PKB (7) that phosphorylates nuclear cyclin D1, enhancing its degradation (8). These data suggest that the decreased levels of cyclin D1 in PTEN-expressing cells result from inhibition of the PI-3 kinase pathway.
FIG. 4.
Effects of PI-3 kinase pathway inhibitors on cyclin (Cyc) D1 expression. PTEN-inducible U-87 cells, serum deprived for 36 h, were induced with doxycycline for 12 h, if not marked otherwise, and simultaneously treated with the inhibitors LY 294002 (LY) and LiCl at the indicated concentrations. Western blot analysis was performed with the indicated antibodies. The graph at the bottom shows the actin-normalized levels of cyclin D1. Note the decrease in cyclin D1 levels following inhibition of the PI-3 kinase by LY 294002 and partial restoration of the decreased cyclin D1 levels in PTEN-expressing cells following inhibition of GSK-3 by LiCl.
PTEN prevents nuclear localization of cyclin D1.
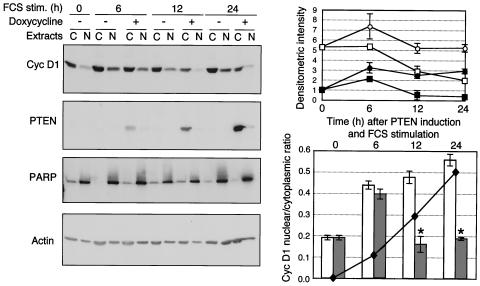
In normal cells that re-enter the cell cycle by serum stimulation from the G0 phase, cyclin D1 localizes to the nucleus in the G1 phase and exits the nucleus as cells progress through the S phase (4). To determine the effect of PTEN on the nuclear translocation of cyclin D1, we investigated the intracellular localization of cyclin D1 by Western blot analysis of cytoplasmic and nuclear extracts from PTEN-inducible U-87 cells progressing through the cell cycle (Fig. 5). In cells not expressing PTEN, as cells entered the G1 phase at 6 h after serum stimulation, cyclin D1 expression increased greatly in the nucleus and only slightly in the cytoplasm (Fig. 5, upper graph), resulting in a marked increase in the nuclear/cytoplasmic ratio (Fig. 5, lower graph). In contrast to normal cells, in these transformed cells, cyclin D1 remained in the nucleus during the S phase (Fig. 5). In serum-stimulated cells that were treated simultaneously with doxycycline to induce PTEN expression, both cytoplasmic and nuclear levels were reduced compared to those in the nontreated controls (Fig. 5, upper graph). In the early G1 phase, however, an initial increase in nuclear cyclin D1 and a corresponding nuclear/cytoplasmic ratio increase were observed in PTEN-expressing cells, similar to the increase that occurred in cells not expressing PTEN (Fig. 5, graphs). This initial increase did not appear to influence the ability of PTEN to prevent the phosphorylation of Rb at 12 h (Fig. 2B) and was likely due to a low level of PTEN expression at 6 h that may be insufficient to prevent the nuclear accumulation of cyclin D1. Starting with the late G1 phase (12 h) in PTEN-expressing cells, the nuclear/cytoplasmic cyclin D1 ratio was significantly reduced (P < 0.01) and remained at low values similar to G0 values throughout the cell cycle (Fig. 5, lower graph). These data show that, in addition to decreasing the cyclin D1 expression levels in both the cytoplasm and the nucleus, PTEN also prevented the localization of cyclin D1 to the nucleus during the G1- to S-phase transition.
FIG. 5.
PTEN prevents the nuclear localization of cyclin (Cyc) D1. Western blot analysis of cyclin D1 subcellular distribution in PTEN-inducible U-87 cells. Equal volumes of nuclear (N) and cytoplasmic (C) extracts from cells processed as described in the legend to Fig. 2 were analyzed with the indicated antibodies. Because the protein concentrations in cytoplasmic extracts were twice as high as those in nuclear extracts after loading of the same volume of extract, the data presented here underestimate the nuclear cyclin D1 amounts by twofold. The intensity of cyclin D1 expression was measured in the cytoplasmic extracts and normalized to the cytoplasmic actin levels, and that measured in the nuclear extracts was normalized to the nuclear PARP levels. The normalized cyclin D1 levels are plotted in the upper graph (○ and □ represent cytoplasmic values for noninduced and PTEN-induced cells, respectively, and • and ▪ represent nuclear values for noninduced and PTEN-induced cells, respectively). The lower graph shows the nuclear/cytoplasmic ratio of the normalized levels of cyclin D1 for noninduced cells (empty columns) and PTEN-induced cells (filled columns). The means and standard deviations from three experiments are shown. Asterisks represent significant differences (P < 0.01) between the nuclear/cytoplasmic ratios of noninduced and PTEN-induced cells. The superimposed curve shows the normalized values of PTEN levels in PTEN-induced cells (black diamonds) during the time course. stim., stimulation.
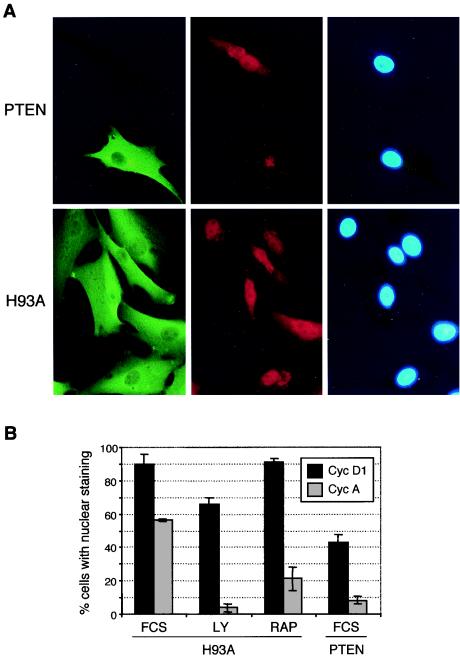
Because the levels of PTEN in the inducible system are too low for detection by immunofluorescence assay, we stably expressed PTEN and the phosphatase-inactive mutant form PTEN-H93A in U-87 cells. At 10 to 12 h after serum stimulation of G0-synchronized cells, in cells expressing PTEN-H93A or in cells not expressing PTEN, cyclin D1 was detected by immunofluorescence assay in both the cytoplasm and the nucleus (Fig. 6A) and nuclear staining was present in 90% of the cells (Fig. 6B). In contrast, in PTEN-expressing cells, the percentage of cells with nuclear cyclin D1 decreased to approximately 40% (Fig. 6). LY 294002 at the subliminal concentration of 10 μM had a less pronounced effect on cyclin D1 nuclear distribution than did PTEN, and no effect was observed with rapamycin, which indirectly inhibits p70 S6 kinase, a downstream kinase that is activated by PI-3 kinase and is implicated in protein synthesis (Fig. 6B). Both drugs were efficient at inducing cell cycle arrest, as determined by the reduced nuclear expression of cyclin A determined in the S phase in control experiments.
FIG. 6.
PTEN prevents the nuclear localization of cyclin (Cyc) D1 in stable PTEN-expressing cells. (A) Immunofluorescence of formaldehyde-fixed cells with anti-PTEN monoclonal (green fluorescence) and anti-anti-D1 polyclonal (red fluorescence) antibodies and nuclear 4′,6′-diamidino-2-phenylindole (DAPI) staining for U-87 cells stably expressing wild-type PTEN or the H93A enzyme-inactive mutant form of PTEN. Images were taken with a 40× objective and a Nikon fluorescence microscope. (B) Effects of LY 294002, rapamycin (RAP), and PTEN on the nuclear localization of cyclins D1 and A. Serum-starved cells stably expressing PTEN or the H93A mutant form of PTEN were stimulated with 10% FCS and treated with the indicated inhibitors for cyclin D1 or cyclin A detection, respectively. Cyclins D1 and A were detected by immunofluorescence as for panel A. A rabbit polyclonal antibody was used for cyclin A. The percentage of cells that presented nuclear localization of cyclins among the total number of cells expressing wild-type PTEN or the H93A mutant form of PTEN was averaged from three different fields for more than 150 cells in each category.
Nucleus-persistent mutant cyclin D1 rescues PTEN-induced proliferation arrest.
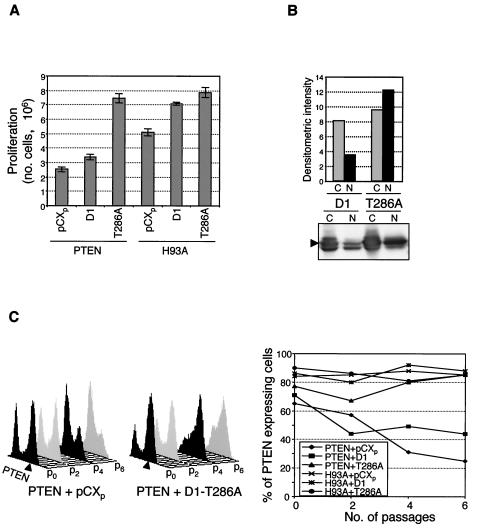
To assess the role of the nuclear localization of cyclin D1 in PTEN-induced growth suppression, we analyzed whether the growth defect induced by PTEN can be rescued by forced nuclear localization of cyclin D1. The export of cyclin D1 from the nucleus to the cytoplasm, followed by its degradation by the proteasome, depends on phosphorylation of Thr 286 by GSK-3β (8). Accordingly, the T286A mutant form of cyclin D1, which is defective in phosphorylation by GSK-3β, has been reported to persist in the nucleus and accumulate in cells because of a lower degradation rate (8). We stably coexpressed PTEN with wild-type cyclin D1 or the T286A mutant form and assessed the proliferation of the cells. The T286A mutant form of cyclin D1 completely rescued the proliferation defect of PTEN-expressing cells while it had a moderate effect on the proliferation of control PTEN-H93A-expressing cells (Fig. 7A). As expected, T286A mutant cyclin D1 accumulated in the nuclei of PTEN-expressing cells significantly more than did overexpressed wild-type cyclin D1 (Fig. 7B), suggesting that increased nuclear expression of this mutant form represents the main mechanism of the proliferation rescue observed.
FIG. 7.
Inhibitory effects of PTEN on cell growth are overcome by coexpression of the nucleus-persistent T286A mutant form of cyclin D1. (A) The PTEN-induced proliferation defect is complemented by the expression of the T286A mutant form of cyclin D1. Equal numbers of U-87 cells doubly infected with either PTEN or the H93A mutant form of PTEN and the pCXp vector, wild-type cyclin D1 (D1), or the T286A mutant form of cyclin D1 (T286A) were subjected to double-drug selection. The efficiency of virus infection was near 100%, and essentially no cells died during drug selection. Proliferation was scored 3 days after the completion of drug selection by counting the cells with a hemacytometer. (B) Increased nuclear localization of the overexpressed T286A mutant form of cyclin D1. Western blot analysis with cyclin D1 antibody of nuclear (N) and cytoplasmic (C) extracts from cells coexpressing PTEN and either wild-type cyclin D1 (D1) or the T286A mutant form of cyclin D1 (T286A) in randomly growing cells in medium supplemented with 10% FCS. Overexpressed (arrowhead) cyclin D1 levels were quantified and normalized to those of actin and PARP as described in the legend to Fig. 5, and the corresponding cytoplasmic and nuclear expression levels are shown graphically. (C) The proportion of PTEN-expressing cells decreased as the passage number increased but was maintained when the cells coexpressed the T286A mutant form of cyclin D1. The cells described in panel A were subjected to serial passages (p), and the number of cells expressing PTEN was assessed by FACS analysis after intracellular staining with anti-PTEN antibody. On the right, PTEN-expressing cells (arrowheads) are indicated for two cell populations, the first coexpressing PTEN and the pCXp vector and the second coexpressing PTEN and the T286A mutant form of cyclin D1. On the left, the graph shows the evolution of PTEN-expressing cells along passages of the six cell populations analyzed, in percentages of PTEN-expressing cells among the total number of cells. These experiments were repeated at least twice with similar results.
To further analyze the importance of cyclin D1 in the growth suppression caused by PTEN, we performed an assay of growth competition between cells expressing PTEN and cells not expressing PTEN that were grown in the same dish. U-87 cells were coinfected, on the one hand, with either PTEN or PTEN-H93A and, on the other hand, with a pCXp vector control, wild-type cyclin D1, or the T286A mutant form (Fig. 7C). For these six combinations, the number of cells expressing PTEN or PTEN-H93A was determined by fluorescence-activated cell sorter (FACS) analysis of intracellular PTEN expression over serial passages. After infection with retroviruses containing wild-type PTEN, approximately 70% of the infected cells expressed PTEN, although all of the cells were resistant to drug selection. After serial passages starting from the initial mixed cell population, the cells not expressing PTEN overgrew the growth-disadvantaged PTEN-expressing cells that decreased to 20% of all cells at passage 6 (Fig. 7C, graph and left FACS plot). When PTEN was coexpressed with wild-type cyclin D1, the number of cells expressing PTEN decreased, although not as dramatically as when PTEN was coexpressed with the control vector (Fig. 7C, graph). In contrast, when T286A mutant cyclin D1 was coexpressed, the number of cells expressing PTEN showed rather a tendency to increase over the cell passages (Fig. 7C, graph and right FACS plot). These results suggest that nuclear localization of cyclin D1 is important for the proliferation control induced by PTEN.
PKB/Akt restores the cyclin localization defect induced by PTEN.
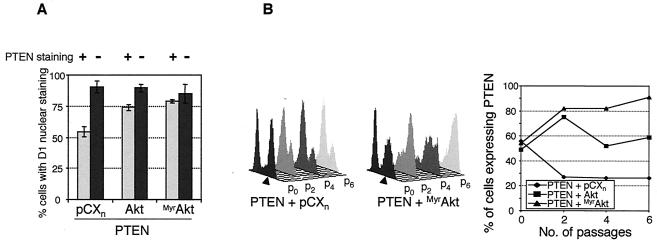
To test whether PTEN relocalizes cyclin D1 by inactivating Akt/PKB, we coexpressed PTEN with either wild-type Akt/PKB or a constitutively active form, MyrAkt, and observed the cellular localization of cyclin D1. Cyclin D1 was localized to the nuclei of a higher percentage of the cells expressing both PTEN and wild-type Akt or PTEN and MyrAkt than to those of cells expressing PTEN and vector control (Fig. 8A).
FIG. 8.
Akt/PKB rescues the cyclin D1 nuclear localization defect induced by PTEN. (A) Nuclear cyclin D1 was analyzed by immunofluorescence assay of fixed cells expressing PTEN in combination with the empty vector (pCXn), wild-type Akt/PKB (Akt), or constitutively active Akt/PKB (MyrAkt). Cells at passage 3 were serum deprived, stimulated with 20% FCS for 13 h, and stained with monoclonal anti-PTEN and polyclonal anti-cyclin D1 antibodies and DAPI. The percentage of cells that presented nuclear cyclin D1 staining was calculated separately for cells expressing PTEN (plus signs and light bars) or not expressing PTEN (minus signs and dark bars). The results represent the average of two or three random fields recorded at a magnification of 20× and containing more than 100 cells. (B) The number of PTEN-expressing cells increased with the passage number when the cells coexpressed activated MyrAkt. Cells in successive passages were monitored for PTEN expression by cytofluorimetry as described in the legend to Fig. 7C. Arrowheads show PTEN-expressing cells.
Despite its effect on cyclin D1, overexpression of MyrAkt in U-87 cells had no effect on proliferation and caused only an increase in cell size (data not shown). Nevertheless, both wild-type Akt and MyrAkt rescued the growth-suppressive effects of PTEN in the cell growth competition assay (Fig. 8B). This is shown by the maintenance of the number of cells expressing PTEN and wild-type Akt or by the increase in the number of cells coexpressing PTEN and MyrAkt. In the same experiment, the number of cells expressing MyrAkt remained constant (data not shown). This indicates that PKB/Akt complements the negative effects induced by PTEN on proliferation.
DISCUSSION
Cell cycle arrest in the G1 phase is the mechanism for the proliferation defect induced by PTEN in most glioblastoma and prostate cancer cells (10, 19; our unpublished data). While information supporting the effect of PTEN on p27Kip1 and its role in cell cycle arrest accumulates, the influence of PTEN on cyclin D1 was marginally addressed and is subject to contradictory results. Some researchers have reported decreased cyclin D1 levels after PTEN overexpression (26, 27, 35), while others have found no modification of cyclin D1 levels (19, 33). Interestingly, one report described decreased levels of cyclin D3 but not of cyclin D1 after PTEN expression in PTEN-deficient endometrial cells (39). We have shown here that PTEN decreased the levels and nuclear localization of cyclin D1, and we established a role for cyclin D1 in the growth arrest induced by PTEN. The difficulty in assessing the contributions of cyclin D1 and p27Kip1 to the cell cycle arrest caused by PTEN most likely came from the lack of a system in which the effects of PTEN on the cell cycle could be accurately examined. Because PTEN influences the cell cycle, both the constitutive expression of PTEN in PTEN-deficient cancer cells (19) and the constitutive deletion of PTEN from embryonic stem cells (33) may determine compensatory modifications of the cell cycle machinery in these cells. These confounding effects most likely explain the apparent lack of an effect of PTEN on cyclin D1 reported by these researchers. In our study, the generation of a nonleaky inducible expression system in which PTEN expression could be pulsed for short periods of time in PTEN-deficient cells allowed dissection of the mechanism pertaining to the down-regulation of cyclin D1 by PTEN.
Cyclin D1 levels were reduced early after the re-entry from G0 into G1, when the levels of induced PTEN protein were comparable to those of endogenous PTEN protein. Cyclin D1 reduction was accompanied by a strong decrease in the phosphorylation of Rb by cyclin D1/CDK4 complexes. In parallel with previous reports showing a reduction by PTEN of cyclin E/CDK2 activity (6, 19), our data support an early suppression by PTEN of cyclin D1/CDK4-mediated Rb phosphorylation.
We present here a dual mechanism of PTEN-induced down-regulation of cyclin D1 that involves a decrease in both the level and nuclear localization of cyclin D1. Both of these effects could be mimicked by LY 294002, a specific inhibitor of PI-3 kinase, indicating that production of phosphatidylinositol-3,4,5-trisphosphate is necessary for cyclin D1 maintenance. Consistent with these results, we also found that the phosphatase activity of PTEN on phosphoinositide substrates is necessary for its effect on cyclin D1. In disagreement with a previous report (35), we found that a mutation of PTEN disrupting the phosphoinositide phosphatase activity (G129E), or both phosphoinositide and phospho-peptide phosphatase activities (C124S), impaired the ability of PTEN to decrease cyclin D1 levels.
Cyclin D1 turnover is regulated in a complex manner. Its transcription is regulated by Ras and mitogen-activated protein kinases (2, 17) and by β-catenin (34), which is negatively regulated by GSK-3 (37). The degradation of cyclin D1 is less well understood, but distinct degradation pathways have been suggested for the CDK-bound and free pools of cyclin D1 (13). Nuclear CDK4-bound cyclin D1 is phosphorylated by GSK-3β, exported from the nucleus, and subsequently degraded (3, 8). The mechanism of degradation of free cyclin D1 is not known (13). As PTEN inactivates Akt/PKB (31), it activates GSK-3β, which is phosphorylated and inactivated by Akt/PKB (7). Activation of GSK-3β by PTEN may thus down-regulate both the transcription and the degradation of cyclin D1. A previous study has shown a severe decrease of the cyclin D1 mRNA levels in PC3 prostate cancer cells after overexpression of either PTEN or GSK-3, suggesting that PTEN decreases the transcription of cyclin D1 via a mechanism involving GSK-3-mediated inactivation of β-catenin (27). In inducible U-87 cells, we found only a modest reduction in cyclin D1 mRNA after PTEN induction (1.8-fold by Northern analysis; data not shown). However, we also found that the degradation of cyclin D1 contributes to the decrease in cyclin D1 levels (1.7-fold higher turnover following PTEN induction; data not shown). Although the inhibition of GSK-3 by lithium partially restored the levels of cyclin D1 after PTEN expression, this effect was probably due to stabilization of the nuclear pool of cyclin D1 by lithium (3). Indeed, we showed that PTEN prevents the nuclear localization of cyclin D1. The partial effect of GSK-3 activity on cyclin D1 levels suggested that PTEN reduces cyclin D1 levels through GSK-3-independent mechanisms. One possibility could involve translation control by Akt/PKB and p70 S6 kinase through inhibition of the translation repressor 4E-BP1 (14). In our study, rapamycin, which indirectly inhibits p70 S6 kinase, had no effect on cyclin D1 levels (data not shown) or nuclear localization. It is more likely that PTEN influences the degradation of free cyclin D1, which has been reported to be independent of GSK-3 phosphorylation (13). Recently, PTEN has been shown to regulate p27Kip1 degradation by modulating the expression of the F-box protein from the E3 ligase complex targeting its ubiquitination (22). It would be interesting to identify the E3 ligase complex targeting cyclin D1 for degradation and to analyze whether PTEN also controls the degradation of cyclin D1 at this level.
The localization of cyclin/CDK complexes in the nucleus is important for the phosphorylation of nuclear proteins involved in cell cycle progression. We have shown that, besides the expression levels, PTEN also decreased the nuclear localization of cyclin D1. We observed this effect in cells progressing through the cell cycle but also, as with the decreased cyclin D1 expression, independently of the cell cycle, in serum-deprived cells, where induction of PTEN expression resulted in a twofold decrease in the nuclear/cytoplasmic ratio (data not shown). The decreased nuclear localization was restored by a constitutively active mutant form of Akt/PKB in the presence of PTEN, indicating that the pathway linking PTEN to cyclin D1 involves the inactivation of Akt/PKB by PTEN. The nuclear localization defect of cyclin D1 played a role in the growth arrest induced by PTEN, as overexpression of a mutant form of cyclin D1 that is resistant to GSK-3β phosphorylation and persists in the nucleus (8) completely overcame the inhibitory effects of PTEN on proliferation. The availability of nuclear cyclin D1/CDK4 complexes may promote the G1-to-S transition by phosphorylating Rb but also by sequestering p27Kip1 from cyclinE/CDK2 complexes (29). In agreement with this alternate mechanism, we also found significantly increased levels of p27Kip1 in cells overexpressing the nucleus-persistent mutant form of cyclin D1 (data not shown). Thus, in addition to the direct effects on p27Kip1 transcription (23, 25) and degradation (22), PTEN may also have an indirect effect on the distribution of p27Kip1 in the various cyclin/CDK complexes. The decreases in cyclin D1 expression and nuclear localization by PTEN may thus inhibit the cyclin E/CDK2 complexes by preventing the distribution of p27Kip1 from cyclin E/CDK2 to cyclin D1/CDK4 complexes. In conclusion, our data show that PTEN negatively regulates the expression and nuclear localization of cyclin D1. Together with the effects of PTEN on p27Kip1, these mechanisms may constitute the basis of the suppression of cell proliferation by PTEN.
Acknowledgments
We thank C. Sherr, N. Ahmed, T. Chen, and P. Tsichlis for generously providing reagents and F. Cross for critical reading of the manuscript.
In the initial stages of this work, which were performed at The Rockefeller University, New York, N.Y., M.M.G. was supported by fellowships from the Medical Research Council of Canada and the National Cancer Institute (CA09673). A.R. was supported in part by National Institutes of Health grant RO1 GM57569.
REFERENCES
- 1.Akagi, T., T. Shishido, K. Murata, and H. Hanafusa. 2000. v-Crk activates the phosphoinositide 3-kinase/AKT pathway in transformation. Proc. Natl. Acad. Sci. USA 97:7290-7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese, C., J. Johnson, G. Watanabe, N. Eklund, D. Vu, A. Arnold, and R. G. Pestell. 1995. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem. 270:23589-23597. [DOI] [PubMed] [Google Scholar]
- 3.Alt, J. R., J. L. Cleveland, M. Hannink, and J. A. Diehl. 2000. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 14:3102-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldin, V., J. Lukas, M. J. Marcote, M. Pagano, and G. Draetta. 1993. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 7:812-821. [DOI] [PubMed] [Google Scholar]
- 5.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 6.Cheney, I. W., S. T. Neuteboom, M. T. Vaillancourt, M. Ramachandra, and R. Bookstein. 1999. Adenovirus-mediated gene transfer of MMAC1/PTEN to glioblastoma cells inhibits S phase entry by the recruitment of p27Kip1 into cyclin E/CDK2 complexes. Cancer Res. 59:2318-2323. [PubMed] [Google Scholar]
- 7.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 8.Diehl, J. A., M. Cheng, M. F. Roussel, and C. J. Sherr. 1998. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12:3499-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fearon, E. R., and B. Vogelstein. 1990. A genetic model for colorectal tumorigenesis. Cell 61:759-767. [DOI] [PubMed] [Google Scholar]
- 10.Furnari, F. B., H. J. Huang, and W. K. Cavenee. 1998. The phosphoinositol phosphatase activity of PTEN mediates a serum-sensitive G1 growth arrest in glioma cells. Cancer Res. 58:5002-5008. [PubMed] [Google Scholar]
- 11.Georgescu, M. M., K. H. Kirsch, T. Akagi, T. Shishido, and H. Hanafusa. 1999. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc. Natl. Acad. Sci. USA 96:10182-10187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Georgescu, M. M., K. H. Kirsch, P. Kaloudis, H. Yang, N. P. Pavletich, and H. Hanafusa. 2000. Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res. 60:7033-7038. [PubMed] [Google Scholar]
- 13.Germain, D., A. Russell, A. Thompson, and J. Hendley. 2000. Ubiquitination of free cyclin D1 is independent of phosphorylation on threonine 286. J. Biol. Chem. 275:12074-12079. [DOI] [PubMed] [Google Scholar]
- 14.Gingras, A. C., S. G. Kennedy, M. A. O'Leary, N. Sonenberg, and N. Hay. 1998. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 12:502-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishii, N., D. Maier, A. Merlo, M. Tada, Y. Sawamura, A. C. Diserens, and E. G. Van Meir. 1999. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 9:469-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kops, G. J., N. D. de Ruiter, A. M. De Vries-Smits, D. R. Powell, J. L. Bos, and B. M. Burgering. 1999. Direct control of the Forkhead transcription factor AFX by protein kinase B. Nature 398:630-634. [DOI] [PubMed] [Google Scholar]
- 17.Lavoie, J. N., G. L'Allemain, A. Brunet, R. Muller, and J. Pouyssegur. 1996. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J. Biol. Chem. 271:20608-20616. [DOI] [PubMed] [Google Scholar]
- 18.Lee, J. O., H. Yang, M. M. Georgescu, A. Di Cristofano, T. Maehama, Y. Shi, J. E. Dixon, P. Pandolfi, and N. P. Pavletich. 1999. Crystal structure of the PTEN tumor suppressor: implications for its phosphoinositide phosphatase activity and membrane association. Cell 99:323-334. [DOI] [PubMed] [Google Scholar]
- 19.Li, D. M., and H. Sun. 1998. PTEN/MMAC1/TEP1 suppresses the tumorigenicity and induces G1 cell cycle arrest in human glioblastoma cells. Proc. Natl. Acad. Sci. USA 95:15406-15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, J., C. Yen, D. Liaw, K. Podsypanina, S. Bose, S. I. Wang, J. Puc, C. Miliaresis, L. Rodgers, R. McCombie, S. H. Bigner, B. C. Giovanella, M. Ittmann, B. Tycko, H. Hibshoosh, M. H. Wigler, and R. Parsons. 1997. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275:1943-1947. [DOI] [PubMed] [Google Scholar]
- 21.Maehama, T., and J. E. Dixon. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol-3,4,5-trisphosphate. J. Biol. Chem. 273:13375-13378. [DOI] [PubMed] [Google Scholar]
- 22.Mamillapalli, R., N. Gavrilova, V. T. Mihaylova, L. M. Tsvetkov, H. Wu, H. Zhang, and H. Sun. 2001. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2). Curr. Biol. 11:263-267. [DOI] [PubMed] [Google Scholar]
- 23.Medema, R. H., G. J. Kops, J. L. Bos, and B. M. Burgering. 2000. AFX-like Forkhead transcription factors mediate cell cycle regulation by Ras and PKB through p27kip1. Nature 404:782-787. [DOI] [PubMed] [Google Scholar]
- 24.Myers, M. P., I. Pass, I. H. Batty, J. Van der Kaay, J. P. Stolarov, B. A. Hemmings, M. H. Wigler, C. P. Downes, and N. K. Tonks. 1998. The lipid phosphatase activity of PTEN is critical for its tumor suppressor function. Proc. Natl. Acad. Sci. USA 95:13513-13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura, N., S. Ramaswamy, F. Vazquez, S. Signoretti, M. Loda, and W. R. Sellers. 2000. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol. Cell. Biol. 20:8969-8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paramio, J. M., M. Navarro, C. Segrelles, E. Gomez-Casero, and J. L. Jorcano. 1999. PTEN tumour suppressor is linked to the cell cycle control through the retinoblastoma protein. Oncogene 18:7462-7468. [DOI] [PubMed] [Google Scholar]
- 27.Persad, S., A. A. Troussard, T. R. McPhee, D. J. Mulholland, and S. Dedhar. 2001. Tumor suppressor PTEN inhibits nuclear accumulation of β-catenin and T cell/lymphoid enhancer factor 1-mediated transcriptional activation. J. Cell Biol. 153:1161-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sherr, C. J. 2000. The Pezcoller lecture: cancer cell cycles revisited. Cancer Res. 60:3689-3695. [PubMed] [Google Scholar]
- 29.Sherr, C. J., and J. M. Roberts. 1999. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13:1501-1512. [DOI] [PubMed] [Google Scholar]
- 30.Stambolic, V., L. Ruel, and J. R. Woodgett. 1996. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6:1664-1668. [DOI] [PubMed] [Google Scholar]
- 31.Stambolic, V., A. Suzuki, J. L. de la Pompa, G. M. Brothers, C. Mirtsos, T. Sasaki, J. Ruland, J. M. Penninger, D. P. Siderovski, and T. W. Mak. 1998. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell 95:29-39. [DOI] [PubMed] [Google Scholar]
- 32.Steck, P. A., M. A. Pershouse, S. A. Jasser, W. K. Yung, H. Lin, A. H. Ligon, L. A. Langford, M. L. Baumgard, T. Hattier, T. Davis, C. Frye, R. Hu, B. Swedlund, D. H. Teng, and S. V. Tavtigian. 1997. Identification of a candidate tumour suppressor gene, MMAC1, at chromosome 10q23.3 that is mutated in multiple advanced cancers. Nat. Genet. 15:356-362. [DOI] [PubMed] [Google Scholar]
- 33.Sun, H., R. Lesche, D. M. Li, J. Liliental, H. Zhang, J. Gao, N. Gavrilova, B. Mueller, X. Liu, and H. Wu. 1999. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol-3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc. Natl. Acad. Sci. USA 96:6199-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tetsu, O., and F. McCormick. 1999. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398:422-426. [DOI] [PubMed] [Google Scholar]
- 35.Weng, L. P., J. L. Brown, and C. Eng. 2001. PTEN coordinates G1 arrest by down-regulating cyclin D1 via its protein phosphatase activity and up-regulating p27 via its lipid phosphatase activity in a breast cancer model. Hum. Mol. Genet. 10:599-604. [DOI] [PubMed] [Google Scholar]
- 36.Wymann, M. P., and L. Pirola. 1998. Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta. 1436:127-150. [DOI] [PubMed] [Google Scholar]
- 37.Yost, C., M. Torres, J. R. Miller, E. Huang, D. Kimelman, and R. T. Moon. 1996. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 10:1443-1454. [DOI] [PubMed] [Google Scholar]
- 38.Zarkowska, T., and S. Mittnacht. 1997. Differential phosphorylation of the retinoblastoma protein by G1/S cyclin-dependent kinases. J. Biol. Chem. 272:12738-12746. [DOI] [PubMed] [Google Scholar]
- 39.Zhu, X., C. H. Kwon, P. W. Schlosshauer, L. H. Ellenson, and S. J. Baker. 2001. PTEN induces G1 cell cycle arrest and decreases cyclin D3 levels in endometrial carcinoma cells. Cancer Res. 61:4569-4575. [PubMed] [Google Scholar]