Abstract
Major UV filters have been identified in the lens of the 13 lined ground squirrel (Spermophilus tridecemlineatus). These were found to be N-acetyl-3-hydroxykynurenine and N-acetyl-kynurenine, in addition to a small quantity of 3-hydroxykynurenine. The level of N-acetyl-3-hydroxykynurenine measured in the ground squirrel lens, 8.2 mM, is approximately 11 times the concentration of 3-hyroxykynurenine glucoside reported previously for the human lens. Two additional UV filters of related structure were also present; however, their structures are still under investigation. HPLC elution profiles indicated that the ground squirrel lens cortex and nucleus contained comparable amounts of α-, βH-, βL-, and γ-crystallins. Levels of GSH in the cortex and nucleus were 12.4 and 7.4 mM, respectively. Such high concentrations of GSH may act to inhibit oxidation of the 3-hydroxykynurenine and N-acetyl-3-hydroxykynurenine. N-Acetylated kynurenines are less labile than those with free α-amino groups since N-acetyl-α-amino groups do not undergo spontaneous deamination. This modification thus stabilises the squirrel UV filters. In addition, because deamination is prevented, the decomposition products will not be involved in binding to lens proteins. Because of the similarity of the UV filters present in the ground squirrel to those in man, this species may be a suitable animal model for investigating the effects of UV radiation on cataract, and other ocular diseases, thought to involve exposure to light.
Keywords: UV light, vision, mass spectrometry, cataract, animal model, lens, HPLC, GSH, kynurenine
1. Introduction
The eyes of some diurnal vertebrates contain UV filters. These are most often located in the lens, and a variety of compounds, that typically absorb light in the 300–400 nm band, have been co-opted for this purpose (Van Heyningen, 1973). For example, the lenses of many fish contain mycosporine amino acids (Shick and Dunlap, 2002) that are either derived directly from algal secondary metabolites in the diet, or are elaborated from these precursors. Lenses of the diurnal gecko contain a crystallin that binds 3,4-didehydroretinol, giving a yellow colour (Werten et al., 2000).
Tryptophan (Trp) metabolites have been employed as UV filters by species ranging from primates (Van Heyningen, 1971a,b) and fish, such as the gourami (Truscott et al., 1992) and the deep sea fish Stylephorus chordatus (Thorpe et al., 1992) to the grey squirrel (Van Heyningen, 1971b; Zigman and Paxhia, 1988). Van Heyningen (1971) first reported the identification of the lens pigment in the grey squirrel (Sciurus carolinensis leucotis) as N-acetyl-3-hydroxykynurenine and this was confirmed at a later date using more advanced techniques (Zigman and Paxhia, 1988).
There seems to be two major selective advantages for having ocular UV filters. Firstly, they reduce chromatic aberration and thereby sharpen the retinal image and secondly, they minimise photo-oxidative damage that can result from prolonged exposure of the lens, and retina, to the high energy wavelengths of light.
Collier and Zigman (1987) showed that in the case of the squirrel, the lens can indeed protect the retina from UV light induced damage. They carried out this study by removing one lens from each animal and then exposing the squirrels to UV light. Retinal damage was more noticeable in the aphakic eye.
Many other studies have sought to examine the effects of irradiation by exposing the lenses of experimental animals to UV light in vitro and in vivo (Barron et al., 1987; 1988; Bergbauer et al., 1991; Giblin et al., 2002; Jose and Pitts, 1985; Yu et al., 1985; 1990). Mostly the rationale for these experiments has been to understand the processes involved in human cataract, since there is some evidence that exposure to UV light may increase the risk of cortical (but not nuclear) cataract (Taylor, 1999). Even if the levels of light exposure employed were physiologically relevant, the conclusions may not be generally applicable to the human situation because we primates have lenses that contain a variety of Trp-derived UV filters that effectively absorb the most damaging wavelengths of light that are transmitted through the cornea. Rats and mice are the animals most often used for these investigations, and they appear to be a particularly poor model in this regard because they can see UV light (Gouras and Ekesten, 2005) and their lenses are unlike those of humans in many other respects (Truscott, 2005). Guinea pig lenses are unusual in that they contain very high levels of the UVA chromophore NADPH (Giblin et al., 2002; Rao and Zigler, 1990).
There is therefore a need for an animal whose lenses contain ‘primate UV filters’ since this could be used as a more appropriate model for studying the effect of UV exposure on ocular conditions, including cataract. Here we report the identification of the major UV filters present in the lens of the 13 lined ground squirrel (Spermophilus tridecemlineatus) and show that they are kynurenine (Kyn) and 3-hydroxykynurenine (3OHKyn) derivatives. Trp metabolites with this same molecular chromophore are also the UV filters found in the human lens.
2. Materials and methods
2.1. Isolation and separation of UV filters
Adult 13 lined ground squirrels, weighing approximately 150 g, were obtained from TLS Research (Bloomington, IL, USA) with ethical approval from the Oakland University Institutional Animal Care and Use Committee, protocol #04081. The gender of the animals was not determined. Since the animals were wild-trapped, their exact ages are not known. The supplier has estimated an age of 1–3 years. Following euthanasia of the animals, lenses (27 mg wet wt. each) and retinas were removed from the eyes (the retinas were used in a separate study by another investigator). UV filters were extracted by homogenising each lens in 0.3 mL of 100% (v/v) ethanol, placed at room temperature for 1 h, centrifuged for 10 min at 12 000xg and re-extracted two times in 0.3 mL 80% (v/v) ethanol and treated as above. The supernatant was observed to be a pale yellow in colour, and the precipitated protein was white. The supernatants were pooled and dried in a speed-vac. The dried extracts were then couriered to Australia. UV filters were separated by HPLC using a Varian Microsorb-MV C18 (250×4.6 mm, 100 Å) column connected to a Shimadzu HPLC system controlled by Shimadzu Class VP software. The column was equilibrated in 0.1% (v/v) TFA, at a flow rate of 0.5 mL/min. Samples were resuspended in 100 μL 0.1% (v/v) TFA, centrifuged for 10 min at 12 000xg and loaded onto the column. UV filters were eluted with the following gradient; 0–5 min 0% buffer B (0.08% [v/v] TFA, 80% [v/v] acetonitrile, water), 5–50 min 0–50% B, 50–60 min 50–0% B. The eluant was monitored at 360 nm.
2.2. Synthesis of α-N-acetyl kynurenine and α-N-acetyl-3-hydroxykynurenine
Acetic anhydride was added in a two-fold molar excess over 3OHKyn, or Kyn, in 400 mM sodium phosphate buffer, pH 7.5 and the solutions incubated for 30 min at 37 °C. The reaction products were separated by HPLC as described above, and the major components analysed by mass spectrometry. In each case, both mono- and di-acetylated products were obtained. The di-acetyl compound was always in smaller yields than the mono-. The structures of the N-acetylated compounds, α-N-acetyl kynurenine (N-acetyl-Kyn) and α-N-acetyl-3-hydroxy-kynurenine (N-acetyl-3OHKyn) were confirmed by the masses of the acetylated adducts and by the presence of diagnostic ions at 88, 130 and 158 m/z (Fig. 4(A)).
Fig. 4.
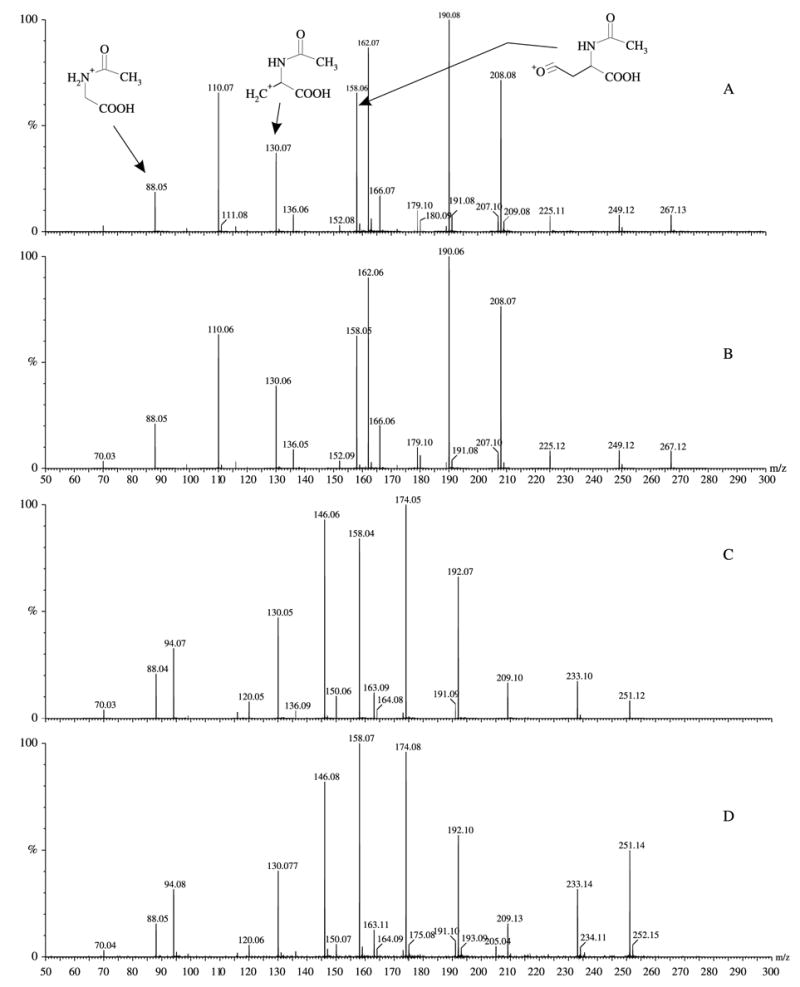
ESI-MS/MS of standards and the compounds purified from the ground squirrel lens. (A) N-acetyl-3OHKyn; (B) peak 4 from Fig. 2; (C) N-acetyl-Kyn; (D) peak 5 from Fig. 2. The proposed structures of the diagnostic fragment ions, containing the N-acetyl moiety, are indicated.
2.3. Quantitation
N-Acetyl-Kyn and N-acetyl-3OHKyn were quantified using standard curves constructed with Kyn or 3OHKyn, respectively. The compounds were analysed by HPLC as described above. The peak areas at 360 nm were determined using the Class-VP software.
2.4. Mass spectrometry
Peaks collected from RP-HPLC were lyophilised, resuspended in 50% (v/v) acetonitrile, 0.5% (v/v) formic acid and analysed in positive ion mode using a Micromass Q-TOF2 equipped with a nanospray source. MS settings were as follows; cone 25 V, LM/HM 12/12, MCP 2300 V, mass range 50–800 m/z. For MS/MS analysis, ions were subjected to a range of collisions energy settings (typically between 10–25 eV).
High resolution mass spectrometry (HR MS) was performed on a micromass Q-TOF Ultima with lock spray. Leucine-encephalin (555.2692 Da) was used as the reference compound.
2.5. HPLC fractionation of squirrel lens proteins
For HPLC analysis, lenses were frozen rapidly in dry ice and dissected into equatorial cortex and nucleus using a 2.7 mm diameter cork borer to produce a cylinder and a ‘doughnut-shaped’ segment. The ends of the cylinder (10% of the total length on each end) were removed with a razor blade and included as cortex. The remaining cylinder, defined as the nucleus, amounted to 40% of the total weight, and the cortex, 60%. The tissues were homogenised (100 mg wet weight of lens per mL buffer) in a nitrogen atmosphere, at 4 °C in 5 mM Tris-HCl buffer, pH 7.5, containing 2 mM EDTA and 50 mM NaCl. The homogenate was separated by centrifugation into water-soluble (WS) protein and a small pellet of water-insoluble (WI) protein, which was discarded. Protein concentration was determined with the Pierce BCA protein assay, using bovine serum albumin as the standard.
WS protein was separated by size exclusion chromatography on a Shimadzu HPLC using a Phenomenex 300×7.8 mm BioSep-SEC-S2000 column. The fractionation buffer was the same as the homogenisation buffer described above. The column was calibrated with the following protein standards: bovine serum albumin (66 kDa), carbonic anhydrase (29 kDa) and cytochrome C (12.4 kDa) and non-proteins: glutathione and Kyn sulfate. The column was equilibrated with 5 mM Tris-HCl, 2 mM EDTA, 50 mM NaCl, pH 7.5 with 5 times the column volume or until the 280 nm reading attained a zero baseline. One milligram of WS protein per run was injected for lens nuclear and cortical proteins. The column had a separation range of 1–300 kDa. SDS-PAGE and Western blot analysis were conducted on isolated HPLC fractions as described (Simpanya et al., (in press)). Lens crystallin antibodies produced in rabbits against calf α-, β- and γ-crystallins were gifts from Dr J.S. Zigler, Jr. (Laboratory of Vision Research, National Eye Institute).
2.6. Determination of reduced glutathione (GSH)
GSH levels were determined using a 5,5′-dithio-bis-(2-nitrobenzoic acid) (DTNB) assay (Sedlak and Lindsay, 1968). Frozen lenses were divided into nucleus and cortex as described above. Each sample was homogenised in 600 μL ice-cold 50 mM EDTA followed by addition of 70 μL of 50% (w/v) trichloroacetic acid and centrifugation.
3. Results
When the lenses of ground squirrels were dissected from the eyes, they were found to be distinctly yellow in colour (Fig. 1). A typical HPLC profile of the UV filters present in the lens of the ground squirrel is shown in Fig. 2. Several major peaks were observed that absorbed at 360 nm. Each peak was collected and analysed by mass spectrometry. The major peak (peak 4) was found to correspond to N-acetyl-3OHKyn, since it had the same HPLC retention time and mass spectrum as a sample of the authentic compound synthesised by acetylation of 3OHKyn.
Fig. 1.
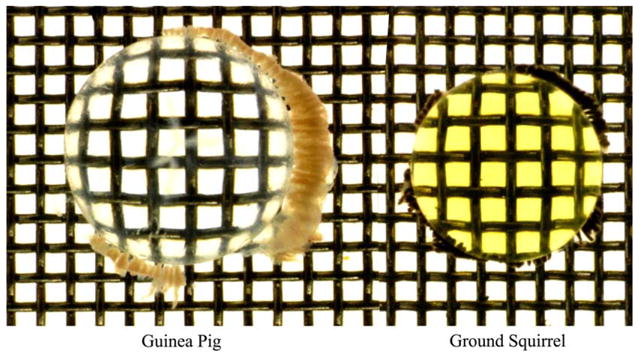
Photograph of the lens of an adult thirteen lined ground squirrel (right) compared with that of a 2-month-old guinea pig.
Fig. 2.
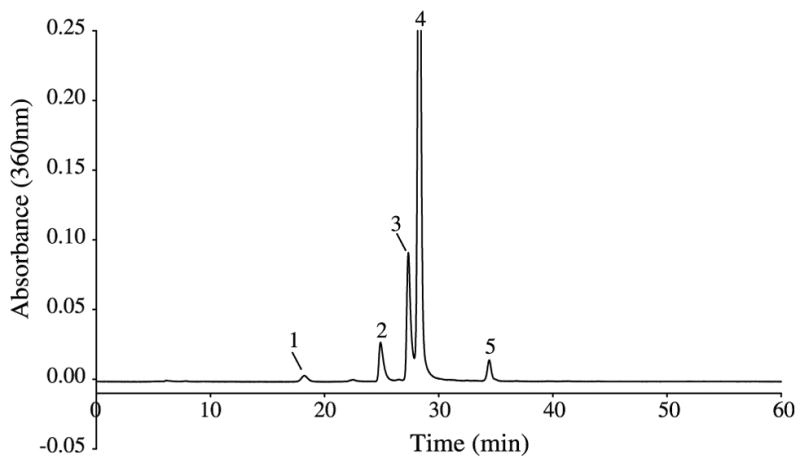
HPLC purification of UV-absorbing compounds from a ground squirrel lens. Peak identity is as follows: (1) 3OHKyn; (2) unknown A; (3) unknown A (major component) and unknown B; (4) N-acetyl-3OHKyn; (5) N-acetyl-Kyn.
The compound in peak 5 was found to correspond to N-acetyl-Kyn, since it had the same HPLC retention time and mass spectrum as a sample of the authentic compound synthesised by acetylation of Kyn. The structures of these acetylated compounds are depicted in Fig. 3. The compound present in peak 1 was found to be 3OHKyn.
Fig. 3.
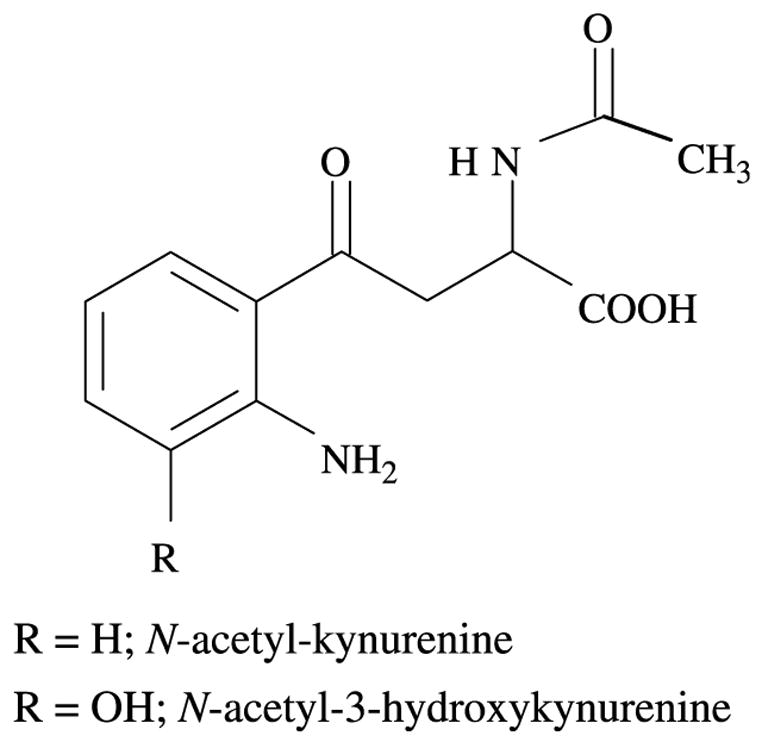
Structures of N-acetyl-3OHKyn and N-acetyl-Kyn.
The identities of the compounds were confirmed using MS/MS analysis. Fig. 4 shows the MS/MS spectra of authentic N-acetyl-Kyn and N-acetyl-3OHKyn, together with those of the compounds present in peaks 4 and 5. In order to provide additional confirmation of the identities of the components in peaks 4 and 5 as N-acetyl-3OHKyn and N-acetyl-Kyn, respectively, HR MS was undertaken. The HR MS for the peak 4 component was determined to be 266.0899 Da (expected mass for N-acetyl-3OHKyn, 266.0903 Da). This represents an error of 1.5 ppm. The HR MS for the peak 5 component was determined to be 250.0976 Da (expected mass for N-acetyl-Kyn 250.0954 Da). This represents an error of 8.8 ppm.
The concentrations of both N-acetyl-3OHKyn and N-acetyl-Kyn in squirrel lens were quantified by HPLC. From the average of three determinations, the amount of N-acetyl-3OHKyn present in the ground squirrel lens was 1.46 μg/mg lens (8.2 mM) and for N-acetyl-Kyn, 0.026 μg/mg lens (0.16 mM).
Although peaks 1, 4 and 5 were identified, two significant peaks in the HPLC traces contained UV filters whose structures were unknown. The identity of the compounds in peak 2 (unknown A, major ion at 346.18 [M+H]1+) and peak 3 (unknown A together with a minor component, unknown B, ion at 364.20 [M+H]1+) have not yet been elucidated. The structures of the 346.18 [M+H]1+ ions present in peaks 2 and 3 are identical by MS/MS. Unknown A may be related to Kyn, based on MS/MS fragmentation data and its appearance in two separate HPLC peaks is consistent with unknown A being a diastereoisomer. The minor component of peak 3 (unknown B) appears to be closely related to N-acetyl-3OHKyn based on MS/MS fragmentation data.
In order to obtain a more complete picture of the UV filtering capacity of the lens and determine whether any soluble filters were bound to crystallins, selected lenses were divided into nucleus and cortex and extracted with buffer for HPLC analysis (Section 2). The nuclear and cortical WS proteins were separated by size exclusion HPLC and the eluates monitored at both 280 nm (for protein) and 360 nm (for UVA-absorbing compounds) (Fig. 5(A) and (B)). Kyn, injected separately into the column, was found to exhibit both 280 and 360 nm absorbance peaks (data not shown). Six major 280 nm peaks (1–4, 6 and 7) were present in both the nuclear and cortical chromatograms. Peak 5 was more prominent in the nucleus (Fig. 5(A)) and peak 8 was more pronounced in the cortex (Fig. 5(B)). Elution times for bovine lens crystallins injected separately into the column are shown in the legend for Fig. 5. Based on these results, it appears that peaks 1–4 correspond to α-, βH-, βL- and γ-crystallin, respectively. SDS-PAGE and Western blot data (not shown) confirmed the presence of α-crystallin in peak 1, β-crystallin in peaks 2 and 3 and γ-crystallin in peak 4. The identity of peak 5 (Fig. 5(A)), is not known, whilst peak 6 (Fig. 5(A) and (B)) appears to contain the majority of unknown A. Analysis of peaks 5–8 for protein revealed detectable protein present only in peak 5.
Fig. 5.
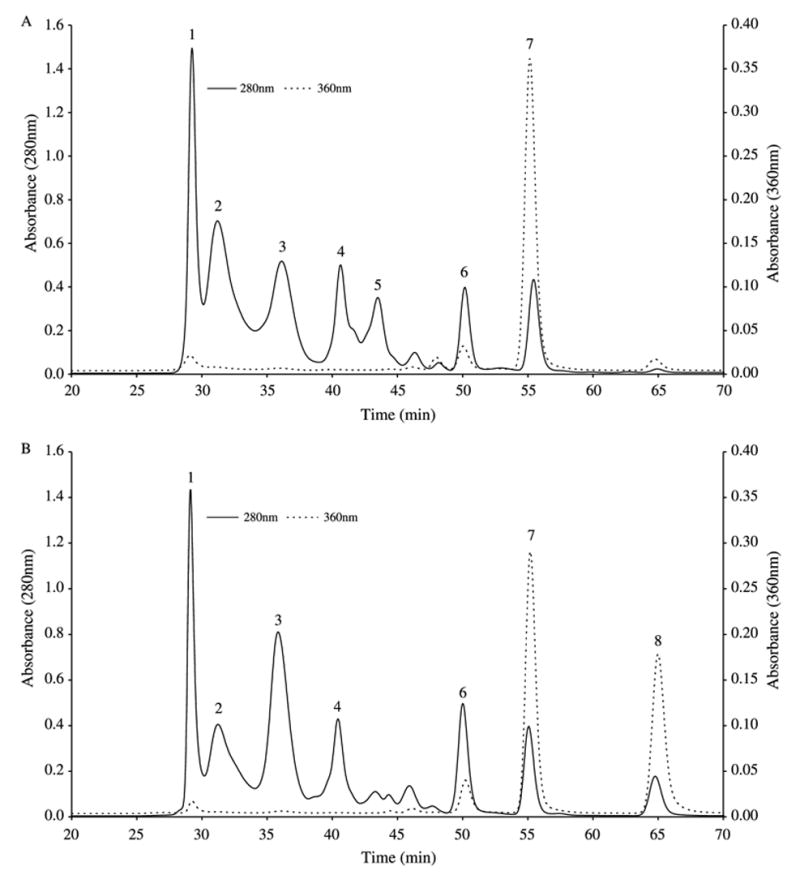
Size-exclusion HPLC elution profiles of ground squirrel lens water-soluble (WS) components. The WS proteins (1 mg protein of each sample was applied to the column) were separated by size exclusion at a flow rate of 200 μL/min on a BioSep-SEC-S2000 column using 5 mM Tris-HCI buffer, pH 7.5, containing 2 mM EDTA and 50 mM NaCl. A. Lens nuclear WS protein; B. Lens cortical WS protein. The eluate was monitored at both 280 nm (–) and 360 nm (- - -). Bovine lens crystallins, injected separately as standards, eluted as follows: α-crystallin (28.5 min), βH-crystallin (30.6 min), βL-crystallin (36.0 min), γ-crystallin (42.3 min). Injected GSH and Kyn sulfate eluted at 54 and 66 min, respectively.
Peak 7 (Fig. 5(A) and (B)) was present in both the nuclear and cortical extracts, however, the 360 nm absorbance was approximately 25% higher in the nucleus. GSH, injected by itself into the column, eluted at 54 min (280 nm peak), corresponding to peak 7 (55 min elution). Similarly, ‘spiking’ the protein sample with GSH increased the 280 nm absorbance at peak 7. Peak 8 was present largely in the cortex (Fig. 5(B)). Kyn sulfate, injected separately into the column, eluted at 66 min, close to peak 8 (65 min elution). When a mixture of GSH and Kyn sulfate was injected, 280 nm peaks were seen at 56 min (close to peak 7) and 66 min (close to peak 8), and a 360 nm peak was observed only at 66 min (close to peak 8, data not shown). The total amount of 360 nm absorbing compounds in the cortex (peaks 7 and 8, Fig. 5(B)) was slightly higher than that present in the nucleus (peak 7, Fig. 5(A)).
GSH was also measured in the two regions of the squirrel lens. The values obtained were 12.4±0.5 mM (n=4) for the cortex and 7.4±0.5 mM (n=4) for the nucleus.
4. Discussion
Major UV filters in the lens of the thirteen lined ground squirrel (S. tridecemlineatus) have been identified as N-acetyl-3OHKyn and N-acetyl-Kyn. These compounds were identified from their elution times, ESI-MS/MS fragmentation pattern and HR MS by comparison to standard compounds. Minor levels of 3OHKyn were also detected. If Kyn was present in the lenses, it was below the detection limit.
In common with the grey squirrel, the lens of the ground squirrel contains N-acetyl-3OHKyn as the major UV filter; however, N-acetyl-Kyn appears unique to the ground squirrel as do the other, as yet unidentified, 360 nm-absorbing species. The crystal structure of N-acetyl-Kyn has been determined previously (Kennard et al., 1979). Previous studies have not quantified N-acetyl-3OHKyn in grey squirrel lenses, so it is not possible to compare levels (Van Heyningen, 1971b; Zigman and Paxhia, 1988). A concentration of 8.2 mM for N-acetyl-3OHKyn in the ground squirrel lens is approximately 11 times the level of 3OHKyn glucoside present in the lens of a teenage human (Bova et al., 2001). Ground squirrels are active during daylight hours only and hibernate during the colder months, as such, they will be exposed to high levels of UV radiation. Chou and Cullen (1984) showed the lens of S. tridecemlineatus absorbs all wavelengths below 410 nm, effectively protecting the retina from UV damage.
As noted above, N-acetyl-3OHKyn is the major UV filter in the ground squirrel lens. 3OHKyn is also present, but in greatly reduced amounts, suggesting an efficient lenticular mechanism for acetylation of the α-amino group. The identification of N-acetyl-Kyn indicates the α-amino groups of both Kyn and 3OHKyn can act as an acceptor for the acetyl group and that the same enzyme may be involved. The lenses of many species contain acetylating enzymes since the N-terminal amino groups of α- and β-crystallins are acetylated (David et al., 1996; de Jong et al., 1975). It is not known if the same enzyme is involved.
The reason for acetylation of 3OHKyn and Kyn may at first seem odd, until one recognises that deamination of Kyn UV filters is a spontaneous reaction (Taylor et al., 2002b) that leads to the generation of highly reactive α,β-unsaturated ketones (Garner et al., 1999). These can readily modify proteins (Aquilina and Truscott, 2002) and the levels of protein-bound Kyn and 3OHKyn-glucoside increase in the human lens after middle age (Hood et al., 1999; Vazquez et al., 2002). This reaction can be prevented by GSH via interception of the reactive ketone. Protein bound UV filters play a role in the age-dependent yellowing of the human lens (Hood et al., 1999; Vazquez et al., 2002). The soluble yellow UV filters in the ground squirrel lens appear to be unbound. It is not known whether these filters are present in the lenses of very young animals (the age of the animals used in this study was estimated at 1–3 years).
Acetylation of the α-amino group will prevent this deamination and therefore, this represents an efficient means for stabilising UV filters in the squirrel lens. Although the side chain is stabilised in this way, the aminophenol moiety of N-acetyl-3OHKyn remains sensitive to oxidation (Aquilina et al., 1997; Taylor et al., 2002b). Oxidation of 3OHKyn produces reactive o-quinonimines that can rapidly bind to protein (Aquilina et al., 1997). This post-translational modification can also be prevented by GSH (Stutchbury and Truscott, 1993).
The concentration of GSH found in the ground squirrel lens cortex, 12.4 mM, is twice that present in a young human lens cortex (Lou and Dickerson, 1992), but only one-half that in the lens cortex of two other diurnal species, the rabbit and guinea pig (Garadi et al., 1987; Giblin et al., 1995). The concentration of GSH found in the ground squirrel lens nucleus (7.4 mM) is slightly higher than that present in the central region of the lens for the rabbit, guinea pig and young human. Seven millimolar GSH would prevent oxidation of 3OHKyn, as well as any possible binding of reactive UV filters to lens proteins (Bova et al., 2001; Dickerson and Lou, 1997; Taylor et al., 2002a). Such a process can take place in the lens nucleus under low GSH conditions in the presence of oxygen, which is known to exist in the lens centre at a detectable level (McNulty et al., 2004).
There are two other major UV absorbing species present in the lens. As judged by absorbance at 360 nm, one of these compounds is present in greater amounts than N-acetyl-Kyn (Fig. 2). The identity of these compounds is still under investigation; however, unknown A, with a mass of 345.1322 Da, may be related to Kyn, as judged by similar fragments when subjected to ESI-MS/MS. Unknown B, with a mass of 363.1428 Da, appears to be related to N-acetyl-3OHKyn.
Based on HPLC elution profiles obtained in this study for lens WS proteins, as well as crystallin standards (Fig. 5(A) and (B)), the ground squirrel lens nucleus and cortex contained comparable levels of α-, βH-, βL- and γ-crystallins. The protein-containing peak 5, observed in the nucleus (Fig. 5(A)) has not yet been identified. Peak 6 appears to contain the majority of unknown A present in the lens. Major UVA-absorbing materials were present in peaks 7 and 8, however, these apparently degraded prior to RP-HPLC analysis.
In young humans free UV filters predominate in the lens and the levels decrease with age (Bova et al., 2001). After middle age, human lenses contain both free and protein-bound UV filters (Hood et al., 1999; Vazquez et al., 2002). The fact that the ground squirrel lens contains UV filters that are of a closely related structure to those present in the human lens, and at comparable concentrations, suggests that this animal may be a good model for investigating the impact of UV light exposure on various ocular conditions, including cataract.
Acknowledgments
This study was funded in part by grants from the National Health and Medical Research Council of Australia (NH&MRC) #162707 and NIH: EY13570, EY02027 and EY014803. The authors appreciate the technical assistance of Victor Leverenz and the generosity of Dr Barry Winkler (Oakland University) in donating lenses from his experimental animals for this study.
References
- Aquilina JA, Truscott RJW. Identifying sites of attachment of UV filters to proteins in older human lenses. Biochim Biophys Acta. 2002;1596:6–15. doi: 10.1016/s0167-4838(01)00313-2. [DOI] [PubMed] [Google Scholar]
- Aquilina JA, Carver JA, Truscott RJ. Oxidation products of 3-hydroxykynurenine bind to lens proteins: relevance for nuclear cataract. Exp Eye Res. 1997;64:727–735. doi: 10.1006/exer.1996.0258. [DOI] [PubMed] [Google Scholar]
- Barron BC, Yu NT, Kuck JF., Jr Tryptophan Raman/457.9-nm-excited fluorescence of intact guinea pig lenses in ageing and ultraviolet light. Invest Ophthalmol Vis Sci. 1987;28:815–821. [PubMed] [Google Scholar]
- Barron BC, Yu NT, Kuck JF., Jr Raman spectroscopic evaluation of ageing and long-wave UV exposure in the guinea pig lens: a possible model for human ageing. Exp Eye Res. 1988;46:249–258. doi: 10.1016/s0014-4835(88)80082-4. [DOI] [PubMed] [Google Scholar]
- Bergbauer KL, Kuck JFR, Jr, Su KC, Yu NT. Use of a UV-blocking contact lens in evaluation of UV-induced damage to the guinea pig lens. Int Contact Lens Clin. 1991;18:182–187. [Google Scholar]
- Bova LM, Sweeney MHJ, Jamie JF, Truscott RJW. Major changes in human ocular UV protection with age. Invest Ophthalmol Vis Sci. 2001;42:200–205. [PubMed] [Google Scholar]
- Chou BR, Cullen AP. Spectral transmittance of the ocular media of the thirteen-lined ground squirrel Spermophilus tridecemlineatus. Can J Zool. 1984;62:825–830. [Google Scholar]
- Collier R, Zigman S. The grey squirrel lens protects the retina from near-UV radiation damage. Prog Clin Biol Res Barron. 1987;247:571–585. [PubMed] [Google Scholar]
- David LL, Lampi KJ, Lund AL, Smith JB. The sequence of human βB1-crystallin cDNA allows mass spectrometric detection of βB1 protein missing portions of its N-terminal extension. J Biol Chem. 1996;271:4273–4279. doi: 10.1074/jbc.271.8.4273. [DOI] [PubMed] [Google Scholar]
- de Jong WW, Terwindt EC, Bloemendal H. The amino acid sequence of the A chain of human α-crystallin. FEBS Lett. 1975;58:310–313. doi: 10.1016/0014-5793(75)80286-9. [DOI] [PubMed] [Google Scholar]
- Dickerson JE, Jr, Lou MF. Free cysteine levels in normal human lenses. Exp Eye Res. 1997;65:451–454. doi: 10.1006/exer.1997.0343. [DOI] [PubMed] [Google Scholar]
- Garadi R, Giblin FJ, Reddy VN. Disulfide-linked membrane proteins in X-ray-induced cataract. Opthalmol Res. 1987;19:141–149. doi: 10.1159/000265486. [DOI] [PubMed] [Google Scholar]
- Garner B, Vazquez S, Griffith R, Linders RA, Carver JA, Truscott RJW. Identification of glutathionyl-3-hydroxykynurenine glucoside as a novel fluorophore associated with ageing of the human lens. J Biol Chem. 1999;274:20847–20854. doi: 10.1074/jbc.274.30.20847. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, Padgaonkar VA, Leverenz VR, Lin LR, Lou MF, Unakar NJ, Dang L, Dickerson JE, Jr, Reddy VN. Nuclear light scattering, disulfide formation and membrane damage in lenses of older guinea pigs treated with hyperbaric oxygen. Exp Eye Res. 1995;60:219–235. doi: 10.1016/s0014-4835(05)80105-8. [DOI] [PubMed] [Google Scholar]
- Giblin FJ, Leverenz VR, Padgaonkar VA, Unakar NJ, Dang L, Lin LR, Lou MF, Reddy VN, Borchman D, Dillon JP. UVA light in vivo reaches the nucleus of the guinea pig lens and produces deleterious, oxidative effects. Exp Eye Res. 2002;75:445–458. [PMC free article] [PubMed] [Google Scholar]
- Gouras P, Ekesten B. Why do mice have ultra-violet vision? Exp Eye Res. 2005;79:887–892. doi: 10.1016/j.exer.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Hood BD, Garner B, Truscott RJW. Human lens colouration and ageing. Evidence for crystallin modification by the major ultraviolet filter, 3-hydroxy-kynurenine O-β-D-glucoside. J Biol Chem. 1999;274:32547–32550. doi: 10.1074/jbc.274.46.32547. [DOI] [PubMed] [Google Scholar]
- Jose JG, Pitts DG. Wavelength dependency of cataracts in albino mice following chronic exposure. Exp Eye Res. 1985;41:545–563. doi: 10.1016/s0014-4835(85)80011-7. [DOI] [PubMed] [Google Scholar]
- Kennard CHL, Matsuura Y, Tanaka N, Kakudo M. Crystal structure of N-acetylkynurenine. Aust J Chem. 1979;32:911–915. [Google Scholar]
- Lou MF, Dickerson JE., Jr Protein-thiol mixed disulfides in human lens. Exp Eye Res. 1992;55:889–896. doi: 10.1016/0014-4835(92)90015-k. [DOI] [PubMed] [Google Scholar]
- McNulty R, Wang H, Mathias RT, Ortwerth BJ, Truscott RJW, Bassnett S. Regulation of tissue oxygen levels in the mammalian lens. J Physiol. 2004;559:883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PV, Zigler JS., Jr Extremely high levels of NADPH in guinea pig lens: correlation with zeta-crystallin concentration. Biochem Biophys Res Commun. 1990;167:1221–1228. doi: 10.1016/0006-291x(90)90654-6. [DOI] [PubMed] [Google Scholar]
- Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- Shick JM, Dunlap WC. Mycosporine-like amino acids and related gadusols: biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annu Rev Physiol. 2002;64:223–262. doi: 10.1146/annurev.physiol.64.081501.155802. [DOI] [PubMed] [Google Scholar]
- Simpanya MF, Ansari RR, Suh KI, Leverenz VR, Giblin FJ. Aggregation of lens crystallins in an in vivo hyperbaric oxygen/guinea pig model for nuclear cataract: dynamic light scattering and HPLC analysis. Invest Ophthal Vis Sci. doi: 10.1167/iovs.05-0843. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stutchbury GM, Truscott RJW. The modification of proteins by 3-hydroxykynurenine. Exp Eye Res. 1993;57:149–155. doi: 10.1006/exer.1993.1110. [DOI] [PubMed] [Google Scholar]
- Taylor HR. Epidemiology of age-related cataract. Eye. 1999;13:445–448. doi: 10.1038/eye.1999.119. [DOI] [PubMed] [Google Scholar]
- Taylor LM, Aquilina JA, Jamie JF, Truscott RJW. Glutathione and NADH, but not ascorbate, protect lens proteins from modification by UV filters. Exp Eye Res. 2002a;74:503–511. doi: 10.1006/exer.2001.1165. [DOI] [PubMed] [Google Scholar]
- Taylor LM, Aquilina JA, Jamie JF, Truscott RJW. UV filter instability: consequences for the human lens. Exp Eye Res. 2002b;75:165–175. doi: 10.1006/exer.2002.2012. [DOI] [PubMed] [Google Scholar]
- Thorpe A, Truscott RJ, Douglas RH. Kynurenine identified as the short-wave absorbing lens pigment in the deep-sea fish Stylephorus chordatus. Exp Eye Res. 1992;55:53–57. doi: 10.1016/0014-4835(92)90091-6. [DOI] [PubMed] [Google Scholar]
- Truscott RJW. Age related nuclear cataract: oxidation is the key. Exp Eye Res. 2005;80:709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Truscott RJW, Carver JA, Thorpe A, Douglas RH. Identification of 3-hydroxykynurenine as the lens pigment in the gourami Trichogaster trichopterus. Exp Eye Res. 1992;54:1015–1017. doi: 10.1016/0014-4835(92)90167-q. [DOI] [PubMed] [Google Scholar]
- Van Heyningen R. Fluorescent glucoside in the human lens. Nature. 1971a;230:393–394. doi: 10.1038/230393a0. [DOI] [PubMed] [Google Scholar]
- Van Heyningen R. Fluorescent derivatives of 3-hydroxy-L-kynurenine in the lens of man, the baboon and the grey squirrel. Biochem J. 1971b;123:30P–31P. doi: 10.1042/bj1230030p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Heyningen R. The glucoside of 3-hydroxykynurenine and other fluorescent compounds in the human lens. Ciba Found Symp. 1973;19:151–171. [Google Scholar]
- Vazquez S, Aquilina JA, Jamie JF, Sheil MM, Truscott RJW. Novel protein modification by kynurenine in human lenses. J Biol Chem. 2002;277:4867–4873. doi: 10.1074/jbc.M107529200. [DOI] [PubMed] [Google Scholar]
- Werten PJ, Roll B, van Aalten DM, de Jong WW. Gecko iota-crystallin: how cellular retinol-binding protein became an eye lens ultraviolet filter. Proc Natl Acad Sci USA. 2000;97:3282–3287. doi: 10.1073/pnas.050500597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu NT, DeNagel DC, Pruett PL, Kuck JF., Jr Disulfide bond formation in the eye lens. Proc Natl Acad Sci USA. 1985;82:7965–7968. doi: 10.1073/pnas.82.23.7965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu NT, Bando M, Kuck JF., Jr Localisation of UV-induced changes in mouse lens. Exp Eye Res. 1990;50:327–329. doi: 10.1016/0014-4835(90)90218-j. [DOI] [PubMed] [Google Scholar]
- Zigman S, Paxhia T. The nature and properties of squirrel lens yellow pigment. Exp Eye Res. 1988;47:819–824. doi: 10.1016/0014-4835(88)90065-6. [DOI] [PubMed] [Google Scholar]


