Abstract
Signal transducer and activator of transcription-3 (STAT3) activation has been associated with suppressed inflammatory processes in experimental animals, murine myeloid cells and macrophage cell lines. Manipulation of STAT3 activity may therefore be a focus for pharmacological intervention of inflammatory diseases in humans. However, the ability of STAT3 to reduce the production of inflammatory mediators by activated human monocytes and macrophages has been characterized inadequately. To establish this, we used a recently optimized adenoviral approach to study the effect of overexpressed STAT3 or a transcriptionally inactive mutant STAT3 in lipopolysaccharide (LPS)-stimulated human monocytes. STAT3 activated by LPS did not directly regulate inhibitor of kappa B α (IκBα) activation or tumour necrosis factor (TNF)-α production, a process dependent on the transcriptional activity of nuclear factor kappa B (NFκB), although the transcriptional activity of STAT3 contributed to the mechanism by which interleukin (IL)-10 suppressed LPS-induced TNF-α levels. This contrasted with the efficient block in IL-10 induction of suppressor of cytokine signalling-3 (SOCS3) in monocytes infected with an adenovirus expressing mutant STAT3. These results indicate that STAT3 activation cannot directly regulate LPS-signalling in human monocytes and represents only part of the mechanism by which IL-10 suppresses TNF-α production by activated human monocytes. This study concludes that pharmacological manipulation of STAT3 transcriptional activity alone would be insufficient to control NFκB-associated inflammation in humans.
Keywords: adenovirus, IL-10, lipopolysaccharide, NFκB, SOCS3
Introduction
Activation of the transcription factor STAT3 (signal transducer and activator of transcription-3) limits the responsiveness of activated macrophages and other haematopoietic cells to inflammatory signals and tumour antigens [1–3]. In murine models of septic peritonitis, STAT3 activation controls the production of inflammatory cytokines and chemokines by resident peritoneal macrophages [1]. STAT3 activation can reduce murine dendritic cell activation [4]. We, and others, have hypothesized that pharmacological enhancement of STAT3 activity in inflammatory tissues may therefore provide a therapeutic approach to control macrophages and their contribution to destructive inflammatory processes.
How STAT3 activation regulates inflammatory cells, especially in humans, is not known. A major pathway of activation of macrophages involves activation of the transcription factor, nuclear factor kappa B (NFκB). In unstimulated cells NFκB is sequestered in the cytoplasm by IκB, an inhibitor of NFκB. However, upon activation, phosphorylation of IκB by IκB kinases causes the release of NFκB, which translocates to the nucleus where it initiates the transcription of inflammatory cytokine genes. Phosphorylation of the IκBα subunit targets it for proteasomal degradation [5–7]. STAT3 may regulate the NFκB pathway at multiple steps, for example through interaction with NFκB proteins [4,8–10]. Alternatively, in murine dendritic cells, STAT3 does not regulate this conventional pathway involving IκBα, but inhibits the transcriptional activity of NFκB by a dominant negative effect in the nucleus [4]. STAT3 can also inhibit activation of cell lines by sequestration of STAT1 and prevention of STAT1-dependent gene activation [11]. In a further mechanism, STAT3 activation may regulate transcriptionally the production of IL-10, a pleiotropic molecule that inhibits proinflammatory cytokine production by activated monocytes, including tumour necrosis factor (TNF)-α [12]. STAT3 can itself be activated by interleukin (IL)-10. It is possible that IL-10 is responsible for many of the repressor effects attributed to STAT3 in activated macrophages [13–17]. However, STAT3 can suppress lipopolysaccharide (LPS)-induced TNF-α production in IL-10-deficient murine macrophages [18].
The novelty of our study is that it focused on primary human monocytes, the cells implicated in driving the human inflammatory process. The involvement of STAT3 in cell activation by LPS, an activator of macrophages that binds to Toll-like receptor-4 (TLR4) and signals via activation of the transcription factor, NFκB, was examined with an emphasis on the production of TNF-α, arguably the most important cytokine in the initiation and progression of inflammatory disease. Either STAT3 or a dominant negative form of STAT3 (STAT3-DN) were overexpressed in primary human monocytes using adenoviral vectors. STAT3-DN contains a mutation in the DNA binding domain, which prevents DNA binding but does not affect tyrosine phosphorylation [19]. To investigate the potential binding to components of the NFκB pathway, the effect of overexpression of STAT3 was examined. To investigate the involvement of the transcriptional activity of STAT3, the STAT3-DN was expressed. This dual approach of forced expression of large amounts of exogenous STAT3, or of blocking the action of endogenous STAT3 by an excess of a transcriptionally inactive STAT3, has not been reported previously. STAT3 activation in human monocytes did not directly control TNF-α production. Further, STAT3 activation contributed only partially to the mechanism by which IL-10 suppressed LPS-induced TNF-α production by human monocytes. These data indicate that, while STAT3 activation may contribute substantially to suppressed inflammatory responses in murine cells, it is less effective in human monocytes. Further, they highlight the need to study primary monocytes for definitive analysis of the actions of signalling intermediates in the control of the human inflammatory response.
Materials and methods
Materials
Recombinant human macrophage-specific colony-stimulating factor (M-CSF) was obtained from CytoLab/PeproTech (Rehovot, Israel) and IL-10 was purchased from ProSpec-Tany TechnoGene (Rehovot, Israel). RPMI-1640, gentamicin and l-glutamine were obtained from Invitrogen Life Technologies (Mount Waverley, VIC, Australia). Dulbecco's modified Eagle's medium (DMEM) was purchased from Sigma (St Louis, MO, USA). Hanks's balanced salts (HBSS) and fetal calf serum (FCS) were obtained from ThermoTrace (Melbourne, VIC, Australia). Anti-phospho-STAT3 (Tyr705) and anti-phospho-IκBα (Ser32) antibodies were purchased from Cell Signalling Technology Inc. (Beverly, MA, USA). Anti-STAT3 (H-190), anti-suppressor of cytokine signalling-3 (SOCS3) (M-20) and anti-IκBα (C-21) antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-β-tubulin antibody was obtained from Abcam (Cambridge, UK). Horseradish peroxidase (HRP)-conjugated rabbit-anti-mouse and donkey-anti-goat secondary antibodies were obtained from Rockland Immunochemicals (Gilbertsville, PA, USA).
Generation of adenoviral vectors expressing STAT3 and dominant negative STAT3
Constructs containing N-terminal haemagglutinin (HA)-tagged STAT3 and dominant negative STAT3 (STAT3-DN) were kindly donated by Masahiko Hibi, Osaka University Graduate School of Medicine, Japan [19]. The STAT3-DN contains a mutation in the DNA binding domain at residues 344 and 345 where the glutamic acid residues are substituted for alanine, preventing DNA binding. The HA-STAT3 and HA-STAT3 DN cDNAs were subcloned into a green fluorescent pattern (GFP)-expressing adenoviral transfer vector, pAdtrack-cytomegalovirus promoter (CMV) (Qbiogene Inc., Carlsbad, CA, USA) using NotI and SalI restriction sites. Positive clones were selected using kanamycin selective media and confirmed by restriction digest and sequence analysis. pAdtrack-CMV constructs containing no insert (GFP alone), STAT3 and STAT3-DN were linearized by digesting with PacI and transformed into BJ5183 AD-1 cells (Stratagene, La Jolla, CA, USA) containing pAdEasy-1 (Qbiogene Inc.). Recombinants containing GFP, STAT3 and STAT3-DN were selected on the basis of colony size and confirmed by restriction digest and sequence analysis. pAdEasy-1 constructs containing GFP, STAT3 and STAT3-DN were transfected into complementing HEK 293 cells using Lipofectamine 2000 (Invitrogen Life Technologies). Human embryonic kidney (HEK) 293 cells were purchased from BD Clontech (Palo Alto, CA, USA) and were maintained in DMEM supplemented with 5% FCS, 5 µg/ml gentamicin, 2 mM l-glutamine and 1 mM sodium pyruvate and incubated at 37°C in 5% CO2. Recombinant viruses were purified from both the HEK 293 cell lysate and culture supernatant using a modified chromatography-based purification system, based on Clontech's BD Adeno-X virus purification kit (BD Clontech).
Isolation of primary human monocytes and adenoviral infection
Human monocytes were purified to ∼ 85% by centrifugal elutriation (Beckman JE-6B, Beckman, Palo Alto, CA, USA) of mononuclear cells isolated from buffy coats on density gradients (Lymphoprep, Axis-Shield, Oslo, Norway) [20,21]. Buffy coats were kindly provided by the Australian RedCross Blood Service, Perth. Monocytes were cultured in RPMI-1640 medium containing 2 mM glutamine, 50 µM 2-mercaptoethanol, 5 µg/ml gentamicin and 2 mM 3-(N-morpholino)propanesulphonic acid. After overnight incubation in Teflon pots (Savillex, Minnetonka, MN, USA) containing RPMI-1640 supplemented with 10% FCS and 25 ng/ml recombinant human M-CSF (to induce αvβ5 expression necessary for AdV infection [22,23]), the cells were harvested and plated at a density of 0·5 × 106 cells/100 µl in polypropylene culture tubes (Nunc Minisorb, Nunc, Roskilde, Denmark). AdV-GFP, AdV-STAT3 or AdV-STAT3-DN were added at a multiplicity of infection (MOI) of 50 (unless stated otherwise) and centrifuged at 1000 g for 60 min at 37°C as described previously [21]. An additional 400 µl of culture medium were added to each tube before incubation for 24 h at 37°C prior to stimulation with LPS (500 ng/ml) or LPS with IL-10 (10 ng/ml).
Determination of infection efficiency by flow cytometry
Cells incubated with no AdV, or with AdV-GFP, AdV-STAT3 or AdV-STAT3-DN were harvested by centrifugation after 24 h and washed once in phosphate-buffered saline (PBS) containing 0·2% bovine serum albumin (BSA) and 0·02% sodium azide. Cells were resuspended in fluorescence activated cell sorter (FACS) fixative (1% formaldehyde in PBS) and stored at 4°C until analysis. CD14 expression confirmed that all larger cells defined by forward and side scatter were monocytes. Infection efficiency was determined as the percentage of GFP-positive cells after 24 h. The amount of virus per cell, estimated by GFP expression, was assessed as mean fluorescence intensity (MFI) by flow cytometry (FACSCalibur, BD Biosciences, San Jose, CA, USA) and expression levels analysed using FlowJo software (version 4·6.2).
Western blot analysis
Following isolation and overnight culture with M-CSF, monocytes were infected with AdV-GFP, AdV-STAT3, AdV-STAT3-DN or left uninfected (no virus) for 24 h. They were then stimulated with 500 ng/ml LPS, without or with 10 ng/ml IL-10, for 0–120 min. Cells were harvested by centrifugation and monocytes lysed in protein lysis buffer [10 mM Tris, 50 mM NaCl, 5 mM ethylenediamine tetraacetic acid (EDTA), 1% Triton X-100, pH 7·6] supplemented with 5 mM sodium fluoride, 10 mM sodium molybdate, 1 mM sodium pyrophosphate, 2 mM sodium orthovanadate and 1× protease inhibitors (complete mini, protease inhibitor cocktail tablets; Roche, Penzberg, Germany). For the analysis of STAT3 and STAT3-(Tyr705) phosphorylation by Western blot, approximately 7·5 µg protein lysate was resolved per lane of either a 10% or 12% sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) gel. Alternatively, for the detection of endogenous SOCS3, IκBα and phosphorylated IκBα (Ser32) approximately 25 µg protein lysate was loaded per lane of a 12% SDS-PAGE gel and transferred onto nitrocellulose membrane (Pall Scientific, MI, USA). Membranes were blocked for at least 1 h in 5% skimmed milk in Tris-buffered saline (TBS)/0·05% Tween-20 (block buffer) followed by incubation with primary antibodies diluted in block buffer or 5% BSA in TBS/0·05% Tween-20 according to the manufacturer's guidelines. Following four sequential 5-min washes in TBS/0·05% Tween-20 (TBS/T), membranes were incubated with HRP-conjugated anti-rabbit IgG or anti-goat IgG secondary antibodies diluted in block buffer. Bound secondary antibody was detected using chemiluminescence (Roche Diagnostics), visualized using CL-XPosure film (Pierce, Rockford, IL, USA) and evaluated using Image Gauge, version 3·4 (Fujifilm, Japan).
Assay of TNF-α
TNF-α was assayed in culture supernatants using a human TNF-α enzyme-linked immunosorbent assay (ELISA) assay kit (BD OptEIA, BD Biosciences, San Diego, CA, USA) and the dissociated enhanced lantanide fluoroimmuno assay (DELFIA) system (Perkin Elmer). For each experiment samples were assayed in triplicate. The assays were sensitive to TNF-α levels of > 10 pg/ml. Levels have been presented as box-and-whisker plots showing the median, upper and lower quartiles and range.
Statistical analysis
Significance of the results has been evaluated using one-way analysis of variance (anova). A P-value of < 0·05 was considered significant.
Results
STAT3 and STAT3-DN expression in primary human monocytes
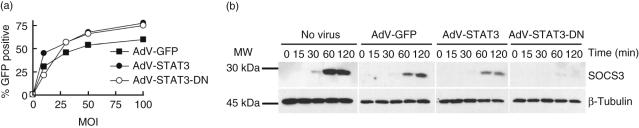
To determine optimal infection efficiency, human monocytes were infected at different MOI for 24 h with an empty adenoviral vector (AdV-GFP) or one encoding either STAT3 (AdV-STAT3) or a dominant negative STAT3 (AdV-STAT3-DN). GFP levels were measured by flow cytometry. For all AdV constructs investigated, maximal infection efficiency was achieved at MOI 50 (Fig. 1a), and was the MOI used in all subsequent experiments. After 24 h at MOI 50, mean infection efficiencies were 62 ± 5% (± s.e.m., n = 7 experiments), 68 ± 4% and 65 ± 5% for monocytes infected with AdV-GFP, AdV-STAT3 and AdV-STAT3-DN, respectively.
Fig. 1.
AdV-GFP-, AdV-STAT3- and AdV-STAT3-DN-infection of primary human monocytes. (a) AdV titration. Primary human monocytes were infected with AdV-GFP (empty vector control), AdV-STAT3 and AdV-STAT3-DN viruses at different multiplicity of infection (MOI) and the percentage of green fluorescent protein (GFP)-positive cells measured by flow cytometry 24 h following infection. (b) Suppressor of cytokine signalling-3 (SOCS3) expression. Monocytes were uninfected (no virus) or infected with AdV-GFP, AdV-STAT3 or AdV-STAT3-DN at MOI 50 before stimulation with lipopolysaccharide (LPS) (500 ng/ml) + interleukin (IL)-10 (10 ng/ml) for 0–120 min and lysates harvested. SOCS3 expression was determined by Western blot (25 µg/lane). Lysates from monocytes infected with AdV-GFP, AdV-STAT3 and AdV-STAT3-DN (MOI 50) with mean infection efficiencies of 79, 79 and 77%, respectively, are shown. In this experiment the mean fluorescence intensity for GFP was 2995, 3325 and 2810 for AdV-GFP- and AdV-STAT3- and AdV-STAT3-DN-infected monocytes, respectively; these values provide a measure of the extent of infection per monocyte. Protein loading was visualized using an anti-β-tubulin antibody. The data presented are from one of two independent experiments showing similar results using monocytes harvested from different donors.
As a confirmatory STAT3-dependent process [14], induction of SOCS3 by IL-10 was determined. Uninfected AdV-GFP-, AdV-STAT3- and AdV-STAT3-DN-infected monocytes were incubated with IL-10 (10 ng/ml), together with LPS (500 ng/ml), and the level of SOCS3 expression determined. The Western blots shown (Fig. 1b) are from one of two similar experiments using separate donors. Endogenous SOCS3 protein expression was detected after 30 min and increased at 60 and 120 min following exposure to IL-10 with LPS in uninfected and AdV-GFP-infected cells. Enhancing the potential activity of STAT3 by infecting with AdV-STAT3 did not increase the level or kinetics of SOCS3 expression. SOCS3 levels were almost undetectable in AdV-STAT3-DN-infected cells; after 60 min the level of SOCS3 detected was 21% and 15%, respectively, for the two donors, of the level measured for AdV-GFP-infected monocytes. We hypothesize that a population of uninfected cells within the AdV-STAT3-DN lysate would account for the remaining SOCS3. This result validated the use of the dominant negative STAT3 to measure responses dependent on the transcriptional activity of STAT3.
LPS-induced TNF-α production is STAT3-independent
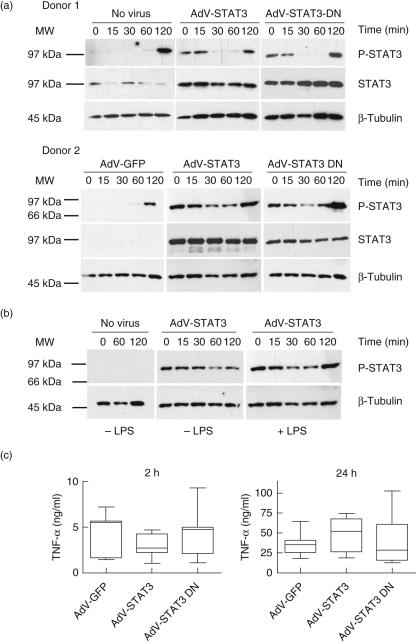
STAT3 phosphorylation following LPS exposure for 2 h was determined in primary human monocytes from each of two donors that had been exposed to no virus, or previously infected for 24 h with AdV-GFP, AdV-STAT3 or AdV-STAT3-DN (Fig. 2). There was enhanced STAT3 phosphorylation at time 0 (prior to LPS exposure) in cells expressing increased STAT3 protein due to infection with AdV-STAT3 or AdV-STAT3-DN. However, the early levels of phosphorylation decreased with longer incubation. Levels of STAT3 phosphorylation in response to LPS were similar in all groups at 120 min (Fig. 2). Enhanced STAT3 phosphorylation at time 0 and 120 min was investigated further (Fig. 2b). STAT3 phosphorylation was compared directly in AdV-STAT3-infected cells incubated without or with LPS. The results confirm that the increased STAT3 phosphorylation at 120 min was due to LPS. Further, the increased levels of STAT3 in the AdV-STAT3- and AdV-STAT3-DN-infected cells indicated that if a cell expresses high levels of STAT3, that protein is sensitive to phosphorylation upon manipulation. STAT3 regulation of LPS-induced TNF-α production was determined by assaying culture supernatants from AdV-STAT3- or AdV-STAT3-DN-infected cells (Fig. 2c). LPS-induced TNF-α levels after 2 and 24 h were not significantly different between treatment groups and suggested that endogenous STAT3 activation did not regulate LPS-induced TNF-α production by human monocytes.
Fig. 2.
Lipopolysaccharide (LPS)-induced tumour necrosis factor (TNF)-α production by human monocytes is independent of signal transducer and activator of transcription-3 (STAT3) activation. Monocytes were left uninfected (no virus) or infected with AdV-GFP, AdV-STAT3 or AdV-STAT3-DN at multiplicity of infection (MOI) 50 for 24 h prior to stimulation with 500 ng/ml LPS. (a) STAT3 phosphorylation by Western blot analysis of cell lysates (showing results from two different donors) prepared at 0–120 min after stimulation with LPS (7·5 µg lysate/lane of a 10% gel). Overexpression of STAT3 by AdV-STAT3- and AdV-STAT3-DN-infected monocytes was confirmed using an anti-STAT3 antibody (in donor 2, the strength of the signal for AdV-STAT3- and AdV-STAT3-DN-infected lysates prevented detection of the low signal for lysates from uninfected and AdV-GFP-infected cells). Protein loading was visualized using anti-β-tubulin. (b) STAT3 phosphorylation of AdV-STAT3-infected monocytes incubated without or with LPS. Uninfected cells (no virus) were a control. (c) LPS-induced TNF-α levels (ng/ml) in culture supernatants after 2 and 24 h. The data presented in box-and-whisker plots are from seven individual donors assayed in triplicate (median, quartiles and range of TNF-α levels).
LPS-activated NFκB signalling is not regulated by STAT3 in a negative feedback loop
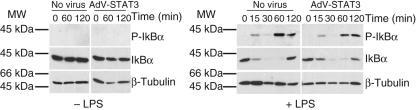
To confirm that high levels of activated STAT3 detected in untreated and LPS-exposed AdV-STAT3-infected cells did not regulate LPS-induced signal transduction, the effect of STAT3 over-expression on LPS-induced NFκB signalling intermediates was determined. Upon LPS activation of cells, phosphorylation of Ser32 and Ser36 of the IκBα subunit targets it for proteasomal degradation [5–7]. As phosphorylation of either one of these residues is critical for NFκB activation, the degree of phosphorylation on Ser32 and the subsequent degradation of IκBα were measured. STAT3 activation in AdV-STAT3-infected cells at time 0 (Fig. 2a,b), or upon culture for 120 min, did not activate IκBα (Fig. 3). Further, the finding that there were no changes in IκBα-activation or LPS-induced, NFκB-driven TNF-α production in uninfected and AdV-STAT3-infected monocytes suggests that STAT3 is unlikely to have any direct regulatory effect on LPS-activated NFκB (Fig. 3).
Fig. 3.
Lipopolysaccharide (LPS)-activated nuclear factor kappa B (NFκB) signalling is not regulated by signal transducer and activator of transcription-3 (STAT3). IκBα phosphorylation and degradation was determined by Western blot. Cells were incubated without (– LPS, left) or with 500 ng/ml LPS (+ LPS, right). Lysates were isolated from AdV-STAT3-infected monocytes with a mean infection efficiency of 64% and mean fluorescence intensity for glomerular filtered phosphate (GFP) of 2045. Protein loading was visualized using anti-β-tubulin.
IL-10 regulation of LPS-induced TNF-α production is dependent on the transcriptional properties of STAT3
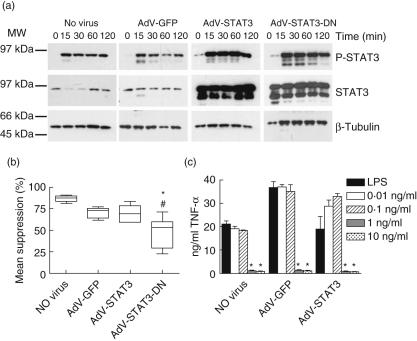
Having established that STAT3 did not control LPS-activated TNF-α production, we then investigated STAT3 regulation of IL-10 function in human monocytes. STAT3 phosphorylation following simultaneous exposure to IL-10 and LPS was determined in uninfected, AdV-GFP-, AdV-STAT3- and AdV-STAT3-DN-infected monocytes. There was again a high basal level of STAT3 phosphorylation at time 0 in AdV-STAT3- and AdV-STAT3-DN-infected monocytes (Fig. 4a). STAT3 phosphorylation was induced 15 min following exposure to IL-10 with LPS, and high levels of STAT3 phosphorylation were maintained for 120 min. Unlike the levels observed for LPS-treated monocytes, STAT3 phosphorylation was greater in cells infected with AdV-STAT3 and AdV-STAT3-DN and paralleled their increased STAT3 protein levels. Similar kinetics of STAT3 phosphorylation were observed in uninfected cells and monocytes infected with AdV-GFP, AdV-STAT3 and AdV-STAT3-DN (Fig. 4a).
Fig. 4.
The suppressive effects of interleukin (IL)-10 on lipopolysaccharide (LPS)-induced tumour necrosis factor (TNF)-α production require signal transducer and activator of transcription-3 (STAT3). Monocytes were left uninfected (no virus) or infected with AdV-GFP, AdV-STAT3 or AdV-STAT3-DN at multiplicity of infection (MOI) 50 for 24 h prior to stimulation with IL-10 (10 ng/ml) with LPS (500 ng/ml). (a) STAT3 phosphorylation by Western blot analysis of cell lysates prepared at 0–120 min after stimulation by IL-10 with LPS (7·5 µg lysate/lane of a 12% gel). Overexpression of STAT3 by AdV-STAT3- and AdV-STAT3-DN-infected monocytes was confirmed using an anti-STAT3 antibody. Protein loading was visualized using anti-β-tubulin. The blots shown are representative of two independent experiments using monocytes harvested from different donors. These same lysates were probed for suppressor of cytokine signalling-3 (SOCS3) (see Fig. 1b). (b) TNF-α levels in culture supernatants at 24 h presented as the mean percentage suppression by IL-10 of LPS-induced TNF-α levels. The data presented in box-and-whisker plots are from seven individual donors (median, quartiles and range). *Significantly different to AdV-GFP, #significantly different to AdV-STAT3, P < 0·05. (c) Monocytes were left uninfected or infected with AdV-GFP or AdV-STAT3 at multiplicity of infection (MOI) 50 for 24 h prior to stimulation with LPS (500 ng/ml) alone or together with 0·01, 0·1, 1 or 10 ng/ml IL-10. *Significantly different to LPS-stimulated TNF-α levels for triplicate cultures, mean ± s.e.m., P < 0·05.
When results for monocytes from seven independent donors that were infected with AdV-STAT3-DN (compared with AdV-STAT3) were pooled, a significant reduction in the ability of IL-10 to suppress LPS-induced TNF-α production was observed (Fig. 4b). This result suggests that both STAT3 phosphorylation and its transcriptional activity contribute to optimal suppression of LPS-induced TNF-α production by human monocytes. However, significant suppressive activity of IL-10 remained in AdV-STAT3 and AdV-STAT3-DN-infected cells that could not be explained by IL-10 regulating the remaining AdV-uninfected cells (approximately 35% of cells due to mean infection efficiency of 65% for the seven donors), particularly as AdV-STAT3-DN infection efficiently suppressed IL-10-induced SOCS3 expression (Fig. 1b). For cells from the seven donors, the suppressive effects of IL-10 in AdV-GFP- and AdV-STAT3-infected cells were reversed by a mean of 31·8 ± 11·2% and 33·3 ± 8·2%, respectively, in AdV-STAT3-DN-infected cells. High levels of STAT3 phosphorylation did not enhance the suppressive effects of IL-10 in AdV-STAT3-infected monocytes (Fig. 4b).
The higher levels of STAT3 phosphorylation in AdV-STAT3-infected monocytes did not enhance the suppressive effect of 10 ng/ml IL-10 (Fig. 4a). As the suppressive effects of IL-10 on TNF-α production by LPS-activated human monocytes were dose-dependent (Fig. 4c), enhancement of the regulatory properties of lower concentrations of IL-10 were investigated in AdV-STAT3-infected monocytes. Increased STAT3 expression and STAT3 activation did not enhance the regulatory properties of 0·1 or 0·01 ng/ml IL-10 (Fig. 4c).
Discussion
This study demonstrated that only STAT3 activated by IL-10 was functionally important in regulating TNF-α production by human monocytes. Further, suppression of LPS-induced TNF-α levels by IL-10 was only partially dependent on the transcriptional activity of STAT3, a finding at variance with that accepted for murine macrophages [13,14,16,17,24,25]. IL-10 was one of three stimuli of phosphorylation of STAT3. Exposure of human monocytes to LPS and to IL-10 both stimulated STAT3 phosphorylation, each with different kinetics. The third stimulus was physical manipulation (washing by centrifugation, resuspension in new medium) of monocytes expressing high levels of STAT3 protein due to infection with adenoviral vectors encoding its production.
This study suggests therefore that STAT3 phosphorylation is not sufficient for regulation of TNF-α production by activated human monocytes, and that pharmacological enhancement of STAT3 activation would not directly regulate human monocyte TNF-α production. STAT3 phosphorylation following LPS stimulation did not negatively regulate LPS-induced NFκB activation or limit TNF-α production. STAT3 phosphorylation in cells overexpressing STAT3 did not limit LPS stimulation of TNF-α, nor was it cumulative with STAT3 activated by IL-10 for control of LPS-induced TNF-α levels. The results of this study suggest that a functionally relevant interaction of STAT3 with NFκB in human monocytes is unlikely.
Upon infection of monocytes with an adenoviral vector coding a transcriptionally inactive STAT3, significant regulation by IL-10 of LPS-induced TNF-α production remained. This result suggested both a STAT3-dependent and a STAT3-independent mechanism of action of IL-10 in its regulation of TNF-α production. Attempts were made to measure TNF-α levels by flow cytometry in AdV-infected individual cells. This would provide a direct correlation between transgene expression and extent of regulation of TNF-α production. However, we have been unable to retain AdV-associated GFP expression after staining for intracellular TNF-α. An early (within the first hour of LPS exposure) STAT3-independent and a late (subsequent to the first hour of LPS exposure) STAT3-dependent mechanism of action of IL-10 on human monocytes have also been proposed [14]; our study suggests maintenance for up to 24 h of two parallel mechanisms of action of IL-10. This is the first study using a viral vector expressing transcriptionally inactive mutant STAT3 which has confirmed that the transcriptional activity of STAT3 is required for optimal suppression of TNF-α production by IL-10 in human monocytes.
There have been few studies of protein overexpression in human monocytes and macrophages. Previous studies have been limited by an inability to transfect them to high efficiency. In our study, an adenoviral transfection system optimized previously in this laboratory [21] was used to overexpress in primary human monocytes full length STAT3 (STAT3α) [24], and a transcriptionally inactive form of STAT3 which could block the activity of endogenous STAT3 (STAT3-DN). Using this approach, infection efficiencies of greater than 60% were achieved. Importantly, in our studies, adenoviral infection of monocytes per se (no LPS stimulus) did not induce TNF-α mRNA or protein production [21,26]. Both the α and β isoforms of STAT3 are expressed by human monocytes [24]. This is demonstrated by use of a 12% SDS-PAGE gel in the results of Fig. 4a (a 10% SDS-PAGE gel was used in Fig. 2a). STAT3α has been attributed with modulation of murine macrophage responses to IL-10 while STAT3β, the C-terminal truncated isoform, can induce the expression of specific STAT3 target genes [24].
In the absence of an exogenous stimulator, STAT3 phosphorylation was detected in cells expressing high levels of STAT3 protein. Viral proteins were considered a potential underlying cause of this STAT3 activation because adenoviral infection was initiated 24 h before preparation of lysates and persisted throughout the 2-h time course. However, STAT3 phosphorylation in the absence of LPS exposure was not seen in cells infected with AdV-GFP, and levels of activated STAT3 in AdV-STAT3- and AdV-STAT3-DN-infected cells were significantly lower 60 min after initiation of cultures. A previous study of murine bone marrow macrophages also showed STAT3 phosphorylation in untreated cells that diminished with incubation [27], but activation by physical manipulation of the cells was not addressed.
It could be argued that the activation of STAT3 by LPS after 60–120 min was too late to control the transcriptional effects of NFκB. However, early phosphorylation of STAT3 in AdV-STAT3- and AdV-STAT3-DN-infected monocytes was without effect on activation of IκBα, or TNF-α levels. It remains possible that endogenous IL-10 was responsible for the LPS-induced phosphorylation of STAT3 at 120 min. The time of first induction of IL-10 mRNA by LPS varies with the cell type studied and can vary from after 2 h (murine bone marrow macrophages) to after 8 h (RAW 264·7 cells) [27]. In our studies of LPS-stimulated human monocytes, IL-10 mRNA levels as detected by quantitative reverse transcription–polymerase chain reaction (RT–PCR) were barely perceptible after 2 h and thus our studies do not allow us to link LPS-induced phosphorylation of STAT3 with the induction of endogenous IL-10.
Although AdV-STAT3- and AdV-STAT3-DN-infected monocytes expressed significantly increased STAT3 protein levels, LPS-induced STAT3 phosphorylation was quantitatively similar in all cell groups and suggested a tight regulatory control of the phosphorylation process. In contrast, in response to IL-10, STAT3 phosphorylation was significantly greater in AdV-STAT3- and AdV-STAT3-DN-infected monocytes and was proportional to the increased levels of STAT3 protein in these cells. Measurements of TNF-α production suggested that the biological activity induced by STAT3 phosphorylation was maximal in AdV-STAT3- and AdV-STAT3-DN-infected cells, and perhaps limited the synthesis of IL-10-induced new proteins [28,29]. One new protein induced by IL-10 in a STAT3-dependent manner in both human monocytes and murine macrophages is SOCS3 [13,14,30–32]. However, a role for SOCS3 in regulating the anti-inflammatory effects of IL-10 has been the subject of much debate [26,31–35]. There was no increase in IL-10-induced SOCS3 protein in AdV-STAT3-infected cells when compared with that induced in AdV-GFP-infected monocytes. In contrast, in a recent study of THP-1 cells, different levels of STAT3 expression were created using a lentiviral system for overexpressing STAT3 and short hairpin RNAs to STAT3 [11]. In turn, in response to stimulation with IFN-α, different levels of SOCS3 expression were created, including those higher and lower than the level detected in non-transfected cells. We hypothesize that the tight control of overexpressed STAT3 for SOCS3 expression in AdV-STAT3-infected monocytes reflects a difference between primary cells and a transformed cell line and highlights an important control mechanism in monocytes.
For treatment of human inflammatory disease, pathways of activation and de-activation of key regulators in primary human cells must be better understood. STAT3 activation pathways were the subject of this investigation. No direct effects of STAT3 phosphorylation on LPS-induced TNF-α production were identified. STAT3 may be involved only indirectly in control of inflammation via mediators such as IL-10 induced as part of a negative feedback response to cell activation. However, regulation of TNF-α production by IL-10 was only partially dependent on STAT3 in human monocytes. To understand more clearly the balance of STAT3 activity in stimulatory and inhibitory processes associated with inflammation, further experimentation with human tissue, and cells isolated from it, is required. This study could not support pharmacological manipulation of STAT3 activity as a means of effective control of NFκB-associated human inflammation.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (no. 275546 to PHH).
References
- 1.Matsukawa A, Kudo S, Maeda T, et al. Stat3 in resident macrophages as a repressor protein of inflammatory response. J Immunol. 2005;175:3354–9. doi: 10.4049/jimmunol.175.5.3354. [DOI] [PubMed] [Google Scholar]
- 2.Matsukawa A, Takeda K, Kudo S, Maeda T, Kagayama M, Akira S. Aberrant inflammation and lethality to septic peritonitis in mice lacking STAT3 in macrophages and neutrophils. J Immunol. 2003;171:6198–205. doi: 10.4049/jimmunol.171.11.6198. [DOI] [PubMed] [Google Scholar]
- 3.Kortylewski M, Kujawski M, Wang T, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–21. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 4.Nefedova Y, Cheng P, Gilkes D, et al. Activation of dendritic cells via inhibition of Jak2/STAT3 signaling. J Immunol. 2005;175:4338–46. doi: 10.4049/jimmunol.175.7.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finco TS, Beg AA, Baldwin AS., Jr Inducible phosphorylation of I kappa B alpha is not sufficient for its dissociation from NF-kappa B and is inhibited by protease inhibitors. Proc Natl Acad Sci USA. 1994;91:11884–8. doi: 10.1073/pnas.91.25.11884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DiDonato J, Mercurio F, Rosette C, et al. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–8. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 8.Yu Z, Kone BC. The STAT3 DNA-binding domain mediates interaction with NF-kappaB p65 and inducible nitric oxide synthase transrepression in mesangial cells. J Am Soc Nephrol. 2004;15:585–91. doi: 10.1097/01.asn.0000114556.19556.f9. [DOI] [PubMed] [Google Scholar]
- 9.Welte T, Zhang SS, Wang T, et al. STAT3 deletion during hematopoiesis causes Crohn's disease-like pathogenesis and lethality: a critical role of STAT3 in innate immunity. Proc Natl Acad Sci USA. 2003;100:1879–84. doi: 10.1073/pnas.0237137100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–74. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 11.Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281:14111–8. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- 12.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J Immunol. 2000;165:1612–7. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 13.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–63. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 14.Williams L, Bradley L, Smith A, Foxwell B. Signal transducer and activator of transcription 3 is the dominant mediator of the anti-inflammatory effects of IL-10 in human macrophages. J Immunol. 2004;172:567–76. doi: 10.4049/jimmunol.172.1.567. [DOI] [PubMed] [Google Scholar]
- 15.Takeda K, Clausen BE, Kaisho T, et al. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity. 1999;10:39–49. doi: 10.1016/s1074-7613(00)80005-9. [DOI] [PubMed] [Google Scholar]
- 16.Riley JK, Takeda K, Akira S, Schreiber RD. Interleukin-10 receptor signaling through the JAK-STAT pathway. Requirement for two distinct receptor-derived signals for anti-inflammatory action. J Biol Chem. 1999;274:16513–21. doi: 10.1074/jbc.274.23.16513. [DOI] [PubMed] [Google Scholar]
- 17.Williams LM, Ricchetti G, Sarma U, Smallie T, Foxwell BM. Interleukin-10 suppression of myeloid cell activation − a continuing puzzle. Immunology. 2004;113:281–92. doi: 10.1111/j.1365-2567.2004.01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Butcher BA, Kim L, Panopoulos AD, Watowich SS, Murray PJ, Denkers EY. IL-10-independent STAT3 activation by Toxoplasma gondii mediates suppression of IL-12 and TNF-alpha in host macrophages. J Immunol. 2005;174:3148–52. doi: 10.4049/jimmunol.174.6.3148. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima K, Yamanaka Y, Nakae K, et al. A central role for Stat3 in IL-6-induced regulation of growth and differentiation in M1 leukemia cells. EMBO J. 1996;15:3651–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Hart PH, Vitti GF, Burgess DR, Whitty GA, Piccoli DS, Hamilton JA. Potential antiinflammatory effects of interleukin 4. suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci USA. 1989;86:3803–7. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayne GC, Borowicz RA, Greeneklee KV, Finlay-Jones JJ, Williams KA, Hart PH. Centrifugation facilitates transduction of green fluorescent protein in human monocytes and macrophages by adenovirus at low multiplicity of infection. J Immunol Meth. 2003;278:45–56. doi: 10.1016/s0022-1759(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 22.Foxwell B, Browne K, Bondeson J, et al. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc Natl Acad Sci USA. 1998;95:8211–15. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang S, Endo RI, Nemerow GR. Upregulation of integrins alpha v beta 3 and alpha v beta 5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–63. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maritano D, Sugrue ML, Tininini S, et al. The STAT3 isoforms alpha and beta have unique and specific functions. Nat Immunol. 2004;5:401–9. doi: 10.1038/ni1052. [DOI] [PubMed] [Google Scholar]
- 25.O'Farrell AM, Liu Y, Moore KW, Mui AL. IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms. evidence for Stat3-dependent and -independent pathways. EMBO J. 1998;17:1006–18. doi: 10.1093/emboj/17.4.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prele CM, Keith-Magee AL, Yerkovich ST, Murcha M, Hart PH. Suppressor of cytokine signalling-3 at pathological levels does not regulate lipopolysaccharide or interleukin-10 control of tumour necrosis factor-alpha production by human monocytes. Immunology. 2006;119:8–17. doi: 10.1111/j.1365-2567.2006.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carl VS, Gautam JK, Comeau LD, Smith MF., Jr Role of endogenous IL-10 in LPS-induced STAT3 activation and IL-1 receptor antagonist gene expression. J Leukoc Biol. 2004;76:735–42. doi: 10.1189/jlb.1003526. [DOI] [PubMed] [Google Scholar]
- 28.Aste-Amezaga M, Ma X, Sartori A, Trinchieri G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol. 1998;160:5936–44. [PubMed] [Google Scholar]
- 29.Bogdan C, Paik J, Vodovotz Y, Nathan C. Contrasting mechanisms for suppression of macrophage cytokine release by transforming growth factor-beta and interleukin-10. J Biol Chem. 1992;267:23301–8. [PubMed] [Google Scholar]
- 30.Ding Y, Chen D, Tarcsafalvi A, Su R, Qin L, Bromberg JS. Suppressor of cytokine signaling 1 inhibits IL-10-mediated immune responses. J Immunol. 2003;170:1383–91. doi: 10.4049/jimmunol.170.3.1383. [DOI] [PubMed] [Google Scholar]
- 31.Lang R, Pauleau AL, Parganas E, et al. SOCS3 regulates the plasticity of gp130 signaling. Nat Immunol. 2003;4:546–50. doi: 10.1038/ni932. [DOI] [PubMed] [Google Scholar]
- 32.Niemand C, Nimmesgern A, Haan S, et al. Activation of STAT3 by IL-6 and IL-10 in primary human macrophages is differentially modulated by suppressor of cytokine signaling 3. J Immunol. 2003;170:3263–72. doi: 10.4049/jimmunol.170.6.3263. [DOI] [PubMed] [Google Scholar]
- 33.Berlato C, Cassatella MA, Kinjyo I, Gatto L, Yoshimura A, Bazzoni F. Involvement of suppressor of cytokine signaling-3 as a mediator of the inhibitory effects of IL-10 on lipopolysaccharide-induced macrophage activation. J Immunol. 2002;168:6404–11. doi: 10.4049/jimmunol.168.12.6404. [DOI] [PubMed] [Google Scholar]
- 34.Qasimi P, Ming-Lum A, Ghanipour A, et al. Divergent mechanisms utilized by SOCS3 to mediate interleukin-10 inhibition of tumor necrosis factor alpha and nitric oxide production by macrophages. J Biol Chem. 2006;281:6316–24. doi: 10.1074/jbc.M508608200. [DOI] [PubMed] [Google Scholar]
- 35.Yasukawa H, Ohishi M, Mori H, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nat Immunol. 2003;4:551–6. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]