Abstract
Biochemical studies suggest that positive-strand RNA virus replication involves host as well as viral functions. Brome mosaic virus (BMV) is a member of the alphavirus-like superfamily of animal and plant positive-strand RNA viruses. Yeast expressing the BMV RNA replication proteins 1a and 2a supports BMV RNA replication and mRNA synthesis. Using the ability of BMV to replicate in yeast, we show that efficient BMV RNA replication requires Lsm1p, a yeast protein related to core RNA splicing factors but shown herein to be cytoplasmic. Haploid yeast with an Lsm1p mutation was defective in an early template selection step in BMV RNA replication, involving the helicase-like replication protein 1a and an internal viral RNA element conserved with tRNAs. Lsm1p dependence of this interaction was suppressed by adding 3′ poly(A) to the normally unpolyadenylated BMV RNA. Our results show Lsm1p involvement in a specific step of BMV RNA replication and connections between Lsm1p and poly(A) function, possibly through interaction with factors binding mRNA 5′ ends.
Positive-strand RNA viruses cause numerous human, animal, and plant diseases including encephalitis, hemorrhagic fevers, and hepatitis. Hepatitis C virus, e.g., chronically infects hundreds of millions of people, leading to progressive liver damage and cancer. Such positive-strand RNA viruses encapsidate messenger-sense RNA genomes, replicate through negative-strand RNA intermediates with no natural DNA phase, and share fundamental similarities in RNA replication. At the beginning of infection, the viral genomic RNA is first translated, yielding RNA replication factors that must recruit the genomic RNA out of translation for 3′ to 5′ copying by viral polymerase (1–3). The resulting RNA replication complexes are associated with intracellular membranes, although the nature and functions of this association are poorly understood (4–6).
Genetic and biochemical results suggest that RNA virus replication involves unidentified host factors (7–10). Identifying and characterizing the relevant host factors is thus an important frontier for understanding and controlling viral replication as well as host range and pathology and should also illuminate important host cell pathways. To facilitate such studies, our laboratory identified two higher eukaryotic viruses able to replicate in the genetically tractable yeast Saccharomyces cerevisiae (11, 12). One of these, brome mosaic virus (BMV), is a member of the alphavirus-like superfamily of human-, animal-, and plant-infecting positive-strand RNA viruses (13).
The BMV genome is divided among three 5′ capped RNAs (Fig. 1A). RNA1 and RNA2 encode replication factors 1a and 2a, which share conserved domains with all members of alphavirus-like superfamily. 1a contains domains implicated in RNA helicase and RNA capping functions (14, 15), and 2a contains an RNA-dependent RNA polymerase domain (13). 1a and 2a colocalize in an endoplasmic reticulum-associated replication complex that is the site of BMV-specific RNA synthesis (4, 5). RNA3 encodes cell-to-cell movement and coat proteins that direct systemic infection in the natural plant hosts of BMV but are dispensable for RNA replication. Coat protein is not translated from RNA3 but only from a subgenomic mRNA, RNA4 (11, 16). Expression of the coat gene or genes replacing it thus requires RNA3 replication to produce negative-strand RNA3, followed by subgenomic mRNA synthesis (Fig. 1A). Like RNAs of hepatitis C virus and many other RNA viruses, BMV RNAs lack the 3′ poly(A) of cellular mRNAs. Instead, the BMV RNAs have a tRNA-like structure that interacts with multiple tRNA-specific cellular enzymes (17, 18).
Figure 1.
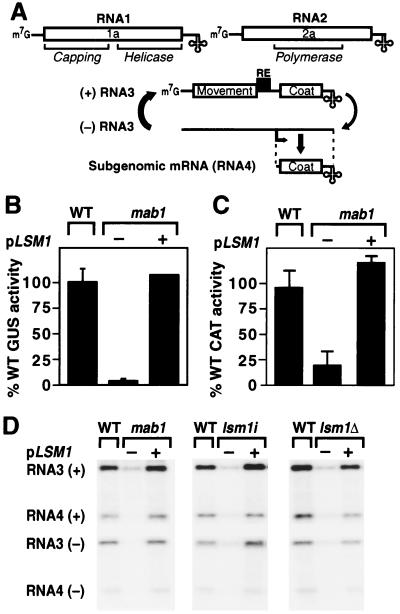
Identification of LSM1. (A) Schematic of the BMV genome, RNA3 replication, and subgenomic mRNA synthesis, showing ORFs (boxed), noncoding regions (single lines), 5′ caps (m7G), intergenic replication enhancer (RE), and tRNA-like 3′ ends (cloverleaf). (B) BMV-directed β-glucuronidase (GUS) expression in 1a- and 2a-expressing wild-type (WT) and mab1 yeast containing a chromosomally integrated B3GUS expression cassette. Total protein was extracted, and GUS activity per milligram of total protein was measured. Averages and SEM from three experiments are shown, except for mab1 + pLSM1, which is the average of two experiments. pLSM1 is a centromeric yeast plasmid containing a 1.5-kilobase (kb) DNA fragment bearing WT LSM1. (C) BMV-directed chloramphenicol acetyl transferase (CAT) expression in mab1 and WT yeast expressing 1a and 2a and electroporated with B3CAT in vitro transcripts. Yeast was incubated in glucose medium and assayed for CAT activity 21 h after electroporation. Averages and SEM from three experiments are shown. (D) Northern blot analysis of positive- and negative-strand BMV RNA3 and subgenomic RNA4 accumulation in WT yeast, the original mab1 mutant strain, the lsm1i isogenic strain, and the lsm1Δ knockout strain expressing 1a, 2a, and WT RNA3. Equal amounts of total yeast RNA were loaded in each lane. The negative-strand PhosphorImager image was printed at higher sensitivity to approximate a 10-fold longer exposure than the positive-strand blot.
In yeast expressing 1a and 2a, RNA3 or RNA3 derivatives introduced by transfection or in vivo transcription are replicated and synthesize subgenomic RNA4 (Fig. 1A). This yeast system reproduces all known features of BMV RNA replication in natural plant hosts, including localization to the endoplasmic reticulum; dependence on 1a, 2a, and the same cis-acting RNA signals; similar ratios of positive to negative-strand RNA; and other features (4, 5, 11, 19, 20).
All eukaryotes encode a set of seven Sm or core small nuclear ribonucleoprotein particle (snRNP) proteins that bind to U1, U2, U4, and U5 snRNAs. These Sm proteins interact through similar Sm motifs to form a ring-shaped complex that binds snRNAs in the cytoplasm and mediates hypermethylation of snRNA 5′ caps (21, 22). Transport of this complex to the nucleus results in predominantly nuclear localization of these Sm proteins (23). A related set of seven Sm motif proteins interacts with U6 snRNA in yeast and humans (24, 25). Yeast encodes another Sm motif protein, Lsm1p, which also has homologs in most or all higher eukaryotes but whose function has been more obscure. Lsm1p interacts with some other Sm-like proteins but not with snRNAs (24, 25). Lsm1p mutation increases the accumulation of capped, poly(A)-deficient mRNAs, indicating a role for Lsm1p in mRNA decapping, which follows mRNA deadenylation and precedes mRNA degradation (26).
Herein, we show that mutation of Lsm1p suppresses BMV RNA replication and subgenomic mRNA synthesis in yeast, reducing the selection of RNA3 templates for replication.
Materials and Methods
Yeast Strains and Plasmids.
Yeast strains and growth were as described (10). BMV 1a and 2a were expressed from pB1CT19 and pB2CT15 by using the ADH1 promoter (11). Where indicated in Fig. 3, 2a was expressed from pB2YT5, a centromeric plasmid with 2a linked to the GAL1 promoter (Y. Tomita, M.I., and S. Naito, unpublished results). BMV RNA3 was expressed to similar levels from the GAL1 promoter induced with 2% (wt/vol) galactose by using pB3RQ39 (see Figs. 4B and 5; ref. 16) or from the CUP1 promoter induced with 0.5 mM CuSO4 (Fig. 4A) by using pJDSAL1. pJDSAL1 was constructed by inserting the 2.2-kb SnaBI–SalI fragment from pB3RQ39 between the XhoI–SphI sites of pSAL1 (27). p1162 was constructed by inserting a 1.8-kb HindIII–BglII fragment encompassing WT LSM1 between the HindIII–BamHI sites of centromeric plasmid Ycplac22 (28). pLSM1 was constructed by subcloning the 1.5-kb HindIII–NsiI fragment of p1162, also encompassing WT LSM1, between the HindIII–PstI sites of Ycplac22.
Figure 3.
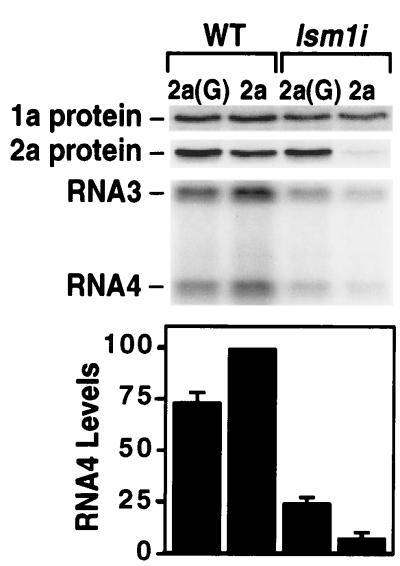
Accumulation of BMV 1a and 2a proteins and WT RNA3 replication products (positive-strand RNA3 and RNA4) in WT and lsm1i yeast. 1a was expressed from the ADH1 promoter, and 2a was expressed from the ADH1 promoter (2a) or GAL1 promoter [2a(G)] as indicated. Equal amounts of total protein or RNA were electrophoresed in each lane for Western and Northern blot analysis. Averages and SEM from three experiments are shown.
Figure 4.
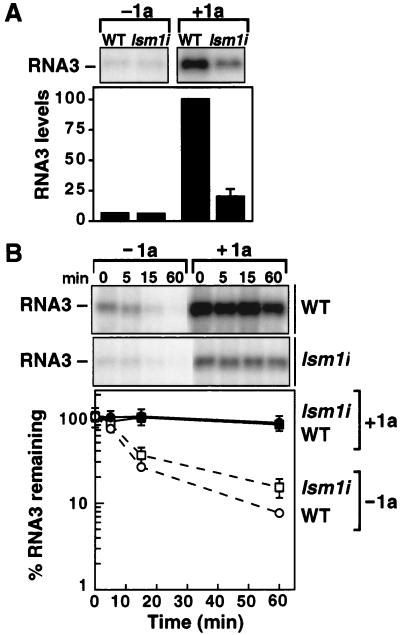
LSM1 is required for efficient 1a-induced RNA3 stabilization. (A) Northern blot analysis of 1a stimulation of RNA3 accumulation in WT and lsm1i yeast in the absence of 2a. Equal amounts of total yeast RNA were loaded in each lane. Averages and SEM from three or more experiments are shown for each sample. Wt RNA3 was expressed from the yeast CUP1 promoter. (B) RNA3 stability analysis in WT and lsm1i yeast in the presence and absence of 1a. Wt RNA3 was expressed from the GAL1 promoter. The indicated yeasts were grown in galactose medium and transferred to glucose medium, which rapidly represses GAL1 transcription (19), and total RNA was extracted at the indicated times. Equal amounts of total yeast RNA were loaded in each lane for Northern analysis. Averages and SEM from three experiments are plotted.
Figure 5.
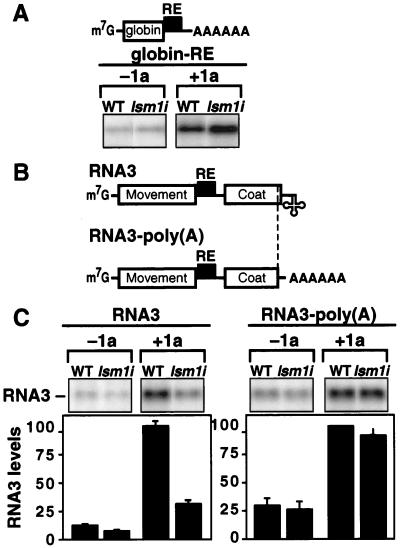
3′ poly(A) suppresses the LSM1 requirement of 1a-induced RNA3 stabilization. (A) Northern blot analysis of 1a stimulation of globin-RE mRNA accumulation in WT and lsm1i yeast. Equal amounts of total yeast RNA were loaded in each lane. (B) Schematic of WT RNA3 and RNA3-poly(A), in which the tRNA-like 3′ end (3′ from a unique StuI site) was replaced by a poly(A) tail generated by the yeast ADH1 polyadenylation site. (C) Northern blot analysis of 1a stimulation of RNA3 and RNA3-poly(A) accumulation in WT and lsm1i yeast. RNA3 and RNA3-poly(A) were expressed from the GAL1 promoter and equal amounts of total yeast RNA were loaded in each lane. Averages and SEM from three or more experiments are shown for each sample.
Linkage Analysis.
URA3 was integrated next to LSM1 by transforming WT mating type a strain YMI08 (10) with a derivative of pRS306, a yeast URA3 integrating plasmid, containing and linearized within a 0.7-kb EcoRV–ClaI fragment of LSM1. The resulting Ura+ strain was crossed with the original mating-type-α mab1 mutant; the diploids were sporulated; and meiotic products were tested for BMV-directed GUS expression and growth at 36°C or on medium lacking uracil.
Gap Repair and Isogenic Strain Construction.
PstI–EcoNI fragments containing the LSM1 ORF plus flanking sequences were cloned from the mab1 mutant strain and WT YPH500 yeast by gap repair (29) by using p1162 and were sequenced in both orientations. To construct strain lsm1Δ, YPH500 yeast was transformed with a DNA fragment containing the LSM1 ORF with its 0.25-kb BsaBI–ClaI fragment replaced by the transcriptionally active URA3 gene; Ura+ colonies were selected, and integrative substitution was verified by Southern blotting. To construct lsm1i, the Ura+ lsm1Δ yeast was transformed with a 0.7-kb HindIII–KpnI mutant lsm1 ORF fragment obtained by gap repair as above, and Ura− cells were selected on 0.1% 5-fluorootic acid.
RNA and Protein Analysis.
RNA transfections, RNA isolation, Northern blots, RNA stability assays, protein extraction, and reporter gene assays were performed as described (10, 11, 19). N-terminal hemagglutinin (HA)-tagged Lsm1p was generated by site-directed mutagenesis to create a NotI site after the LSM1 initiation codon in pLSM1 and inserting a NotI-flanked triple HA tag. Internally tagged LSM1-HA2 and LSM1-HA3 were generated by using a shuttle mutagenesis system (30). Confocal immunofluorescence was performed as described (5) by using a mouse monoclonal antibody against HA (Boehringer Mannheim) and tyramide signal amplification (NEN).
Results
LSM1 Mutation Inhibits BMV RNA Replication.
By screening UV-mutagenized yeast for reduced BMV-directed expression of marker genes, we have shown that yeast mutations that inhibit BMV RNA replication can be isolated (10). Such screens use a yeast strain that expresses BMV replication proteins 1a and 2a from plasmids and contains a chromosomally integrated expression cassette for B3GUS, a BMV RNA3 derivative with the coat gene replaced by the GUS reporter gene. GUS expression from this cassette requires not only DNA-directed transcription of B3GUS RNA but also 1a- and 2a-directed BMV RNA replication and subgenomic mRNA synthesis (Fig. 1A and see above). To test WT RNA3 replication, this yeast can be transformed with a plasmid expressing WT RNA3 (Fig. 1D). RNA replication in WT yeast amplifies positive-strand RNA3 50-fold above the level of DNA-derived RNA3 transcripts (16).
A BMV-inhibiting yeast mutant strain isolated in this way, designated mab1 for maintenance of BMV, has a single, recessive, chromosomal mutation that suppresses BMV-directed RNA3 replication and subgenomic mRNA synthesis by 80–90% (Fig. 1 B–D) and makes yeast growth temperature-sensitive at 36°C. To identify the MAB1 gene, we transformed mab1 yeast with a yeast genomic DNA library (31) and recovered four transformants that grew at 36°C. All four complementing plasmids contained overlapping fragments of yeast chromosome X. Deletion mapping showed that a 1.5-kb fragment containing a single complete yeast ORF, LSM1/YJL124c, complemented the mab1 defects in growth at 36°C and BMV-directed GUS expression from in vivo transcribed B3GUS (Fig. 1B). The same 1.5-kb DNA fragment containing LSM1 restored WT accumulation of RNA3 positive- and negative-strand RNA replication products and subgenomic mRNA in mab1 yeast (Fig. 1D).
DNA-based expression of RNA3 and its derivatives in yeast involves ribozyme self-cleavage to generate the nonpolyadenylated, tRNA-like viral 3′ end (16). mab1 mutation had no effect on the efficiency of ribozyme self-cleavage to release the WT RNA3 3′ end from the longer, easily resolved uncleaved initial transcript. To confirm further whether mab1 inhibition of BMV RNA replication and LSM1 complementation of this defect were independent of in vivo DNA-directed transcription or nucleocytoplasmic transport of BMV RNA3 transcripts, we transfected WT and mab1 yeast with in vitro transcripts of B3CAT, an RNA3 derivative with the coat gene replaced by the CAT gene. CAT expression in mab1 yeast was inhibited 80% relative to WT yeast but was complemented to WT levels by the 1.5-kb fragment containing LSM1 (Fig. 1C).
To determine whether LSM1 corresponds to the WT locus of the causal mab1 mutation or to an extragenic suppressor, we conducted linkage analysis. In WT yeast, URA3 was integrated adjacent to LSM1, and the resulting Ura+ strain was crossed with the mab1 mutant strain. Diploid yeast from this cross was sporulated, and the tetrads were tested for BMV-directed GUS expression, temperature-sensitive growth, and uracil dependence. All 24 tetrads analyzed were parental ditypes with two GUS+ temperature-sensitive Ura+ spores and two GUS− non-temperature-sensitive Ura− spores, where GUS− reflected BMV-directed GUS expression averaging 10-fold lower than GUS+ spores. This close linkage of WT phenotypes to the URA3 marker and the isogenic mutant results below confirmed that LSM1 was the originally mutated MAB1 gene and not an extragenic suppressor.
Because the original mab1 strain was generated by UV mutagenesis, we constructed an isogenic lsm1 mutant strain to ensure the absence of extraneous mutations. The mutant lsm1 gene (see below) from the original mab1 mutant strain was cloned by gap repair (29) and used to replace the chromosomal LSM1 gene in WT yeast by integrative transformation. The resulting lsm1i isogenic strain reproduced the original mab1 phenotypes including temperature-sensitive growth, inhibition of BMV-directed GUS expression (original mab1 = 4% of WT; lsm1i = 6% of WT), and suppressed accumulation of BMV RNA replication products (Fig. 1D, second panel). To test the effect of LSM1 disruption on cell viability, we constructed lsm1Δ, a yeast strain with codons 33–117 of the chromosomal LSM1 ORF replaced by URA3. LSM1 was essential for yeast growth at 36°C but not 30°C. The effects of LSM1 disruption on BMV RNA replication were indistinguishable from the original lsm1i frameshift allele (Fig. 1D, third panel).
Lsm1p Similarities to Core snRNP Proteins.
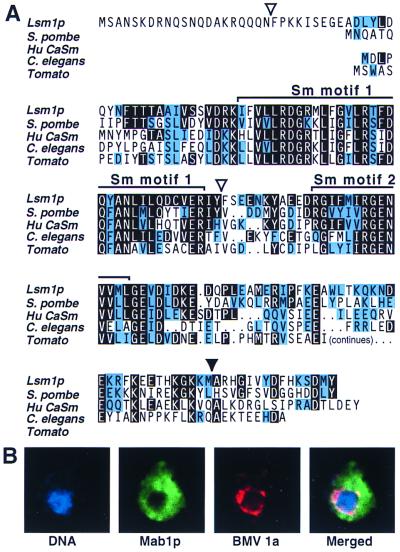
LSM1 encodes a 172-amino acid protein, Lsm1p (Fig. 2A). Fusing an influenza HA epitope tag to the N terminus of the LSM1 ORF in the complementing plasmid and Western blotting with anti-HA antibodies confirmed expression of a protein of the predicted size. Sequencing the lsm1 ORF from the original mab1 strain (see above) revealed a single mutation deleting one adenine from a run of seven, frameshifting the LSM1 ORF after amino acid 156. Lsm1p (Fig. 2A) bears the Sm protein–protein interaction motifs conserved among the core snRNP proteins involved in RNA splicing and other nuclear RNA processing events (25, 32). Lsm1p shares even more extensive similarity (43–45% identity, 68% similarity) with an S. pombe protein and a human protein, CaSm, implicated in pancreatic cancer cell transformation and overexpressed in tumor cell lines from many tissues (33). Phylogenetic comparisons across Sm proteins in multiple organisms further support the close relationship of LSM1 and CaSM, suggesting possible conservation of Lsm1p function from yeast to humans (25), including related proteins in plants (Fig. 2A).
Figure 2.
(A) Alignment of Lsm1p with selected Sm proteins from Schizosaccharomyces pombe (GenBank Z95620), humans (AF000177), Caenorhabditis elegans (Z69302), and a partial sequence from tomato (AI490867). Identical residues are highlighted in black, and conservative substitutions are highlighted in blue. The solid arrowhead indicates the site of the lsm1 frameshift mutation. Open arrowheads indicate sites of functional HA epitope tag insertion in LSM1-HA2 and LSM1-HA3. Dots indicate spaces included to maximize alignment. (B) Intracellular localization of LSM1p. LSM2-HA3 yeast expressing 1a-, 2a-, and RNA3-derivative B3GUS was processed for confocal immunofluorescence by using antibodies against Lsm1p-HA and 1a and stained with propidium iodide for nuclear DNA. Each image is 6 μm per side.
Lsm1p Localizes Predominantly to Cytoplasm.
N-terminally HA-tagged Lsm1p complemented mab1 and lsm1Δ defects in 36°C growth and BMV RNA replication but was not detectable by immunofluorescence with anti-HA antibodies. To facilitate Lsm1p localization, a targeted transposon approach (30) was used to insert a triple HA tag randomly throughout the chromosomal LSM1 gene of WT yeast. From the resulting yeast library, we selected strains LSM1-HA2 and LSM1-HA3, which supported BMV-directed GUS expression to 75% and 85% of that in WT yeast. The HA tags in these strains were inserted after Lsm1p amino acids 21 and 87, respectively (Fig. 2A), and Western blotting confirmed expression of an HA-tagged protein of the expected size.
Confocal immunofluorescence microscopy revealed equivalent localization patterns for both of these functional, tagged Lsm1p derivatives. Unlike core snRNP proteins (23), Lsm1p localized predominantly to the cytoplasm, with only background nuclear signals (Fig. 2B). Lsm1p was distributed through most of the cytoplasm with varying densities. In infected plant cells and yeast, 1a and 2a colocalize on the endoplasmic reticulum at sites of BMV RNA synthesis (4, 5). In yeast expressing 1a, 2a, and replicating RNA3, Lsm1p adjoined these replication sites as detected by 1a immunofluorescence (Fig. 2B). Similar Lsm1p distribution was observed in LSM1-HA2 and LSM1-HA3 yeast with and without BMV components.
lsm1 and 2a Accumulation.
For most experiments in WT and lsm1i yeast, the BMV 1a and 2a RNA replication proteins were expressed from separate plasmids with the same ADH1 promoter and termination signals. The resulting 1a and 2a mRNAs cannot serve as replication templates, because they lack essential RNA replication signals from the 5′ and 3′ ends of RNA1 and RNA2 (Fig. 1A; ref. 11). As for some independent BMV-inhibiting mutants (10), lsm1 mutation allowed normal 1a accumulation but inhibited 2a accumulation (Fig. 3). Because 2a expression can be reduced substantially without inhibiting BMV RNA replication (34), it was unclear whether low 2a accumulation was the sole or primary cause of mab1 defects in RNA replication. To address this issue, WT yeast and lsm1i yeast containing 1a and RNA3 and expressing 2a from the ADH1 or stronger GAL1 promoter were grown, and the levels of 1a, 2a, and BMV RNA replication products were analyzed. GAL1-promoted 2a expression fully restored 2a protein accumulation in lsm1i yeast to WT levels but restored BMV RNA-dependent RNA synthesis only partially (Fig. 3). Under these conditions, positive and negative-strand RNA3 and subgenomic RNA4 accumulation in lsm1i yeast remained only 25–30% of that in WT yeast with either 2a expression plasmid. Thus, BMV RNA replication was inhibited in lsm1i yeast in the presence of normal 1a and 2a levels, showing that lsm1 mutation inhibits one or more additional steps of BMV RNA replication.
lsm1 Mutation Inhibits 1a-Induced RNA3 Stabilization.
In vivo, in the absence of 2a, BMV 1a acts through a cis-acting, intergenic RE (Fig. 1A) to stabilize RNA3 selectively, dramatically increasing the accumulation but not the translation of RNA3 transcripts (Fig. 4; refs. 2 and 19). Multiple findings indicate that these events reflect 1a recruitment of RNA3 into RNA replication and away from translation and degradation. Exchanges between BMV and a related bromovirus show that 1a and the RE are the trans and cis determinants of template selectivity in RNA3 replication (13, 35). The RE is a crucial, 100-fold enhancer of RNA3 negative-strand synthesis (36) and thus of RNA3 replication (19, 37). RE mutations have parallel inhibitory or, in a few cases, stimulatory effects on 1a-induced RNA3 stabilization and (1a+2a)-dependent RNA3 replication (17). The effects of RE mutations on RNA3 replication and the mapped boundaries of the RE are identical in yeast and plant cells (19, 37). Within the RE, an 11-base “box B” sequence conserved with BMV RNA1, RNA2, and the TΨC loop of host tRNAs is crucial for 1a-dependent stabilization and RNA3 replication, suggesting possible host factor involvement (19, 37, 38).
To determine whether these 1a-mediated events were affected in lsm1i mutant yeast, we examined the accumulation of DNA-derived RNA3 transcripts in the absence and presence of 1a (Fig. 4A). 2a was absent in all cases. In the absence of 1a, RNA3 accumulated to similar levels in WT and lsm1i yeast. In WT yeast, 1a increased RNA3 accumulation 10-fold (Fig. 4A). However, in lsm1i yeast, 1a stimulation of RNA3 accumulation was inhibited 70–80% relative to WT yeast, and this inhibition was complemented by a plasmid bearing WT LSM1.
The 1a-induced increase in RNA3 transcript accumulation results from increased RNA3 stability, from a half life of 7 min without 1a to over 3 h in the presence of 1a (2, 19). Reduced 1a stimulation of RNA3 accumulation in lsm1i yeast might result from reducing the number of RNA3 molecules stabilized by 1a, reducing their final stability, or both. To compare RNA3 stability in lsm1i and WT yeast, RNA3 was expressed from the galactose-inducible, glucose-repressible GAL1 promoter. After glucose repression, surviving RNA3 levels were monitored by Northern blotting (Fig. 4B). Without 1a, RNA3 decayed rapidly in both WT and lsm1i yeast. In the presence of 1a, as shown in Fig. 4A, the starting RNA3 level at the time of glucose addition was 4- to 5-fold lower in lsm1i yeast than in WT yeast (Fig. 4B). However, in keeping with the reduced but detectable RNA3 replication in lsm1i yeast (Fig. 1D), the reduced pool of RNA3 in 1a-expressing lsm1i yeast had a stability profile indistinguishable from 1a-stabilized RNA3 in WT yeast (Fig. 4B). Thus, in lsm1i yeast, 1a stabilized a greatly reduced number of RNA3 transcripts, showing that efficient recruitment of RNA3 into the 1a-stabilized state requires LSM1.
3′ Poly(A) Restores 1a-Dependent RNA3 Stabilization in lsm1i Yeast.
The RNA3 RE element (Fig. 1A) is sufficient to direct 1a-induced stabilization and enhanced accumulation of nonviral RNAs such as a globin-RE chimera (19) containing a human β-globin ORF preceded by the yeast GAL1 5′ untranslated region and followed by the RNA3 RE and yeast ADH1 polyadenylation signal (Fig. 5A). Unlike WT RNA3, globin-RE mRNA remained fully 1a-responsive in lsm1i yeast. Coexpressing 1a increased globin-RE mRNA levels 4-fold in WT yeast and 4.5-fold in lsm1i yeast (Fig. 5A). The level of globin-RE mRNA stabilized by 1a in both cases was as great or greater than that for WT RNA3 in WT yeast. The ratio of globin-RE levels in the presence and absence of 1a (4- to 4.5-fold) was lower than for WT RNA3 (8- to 10-fold; Fig. 4A and 5C), only because polyadenylated globin-RE mRNA accumulated to higher levels than unpolyadenylated WT RNA3 in the absence of 1a (see also Fig. 5C).
To determine what feature(s) of globin-RE mRNA allowed it to respond efficiently to 1a in lsm1i yeast, we exchanged selected regions with WT RNA3. Replacing the 3a ORF or WT RNA3 5′ untranslated region with the β-globin ORF or the GAL1 5′ untranslated region, respectively, did not restore 1a-responsiveness to RNA3 in lsm1i yeast. To test whether the remaining unique feature of globin-RE mRNA, the 3′ poly(A), was responsible, we replaced the tRNA-like 3′ end of WT RNA3 with the yeast ADH1 polyadenylation signal (Fig. 5B). In tests with a WT RNA3 control, the behavior of the resulting RNA3-poly(A) derivative paralleled that of globin-RE mRNA both in the presence and the absence of 1a (Fig. 5C). In the absence of 1a, RNA3-poly(A) accumulation was 2- to 3-fold higher than unpolyadenylated RNA3 in WT and lsm1i yeast. In the presence of 1a, RNA3-poly(A) accumulation increased an additional 4-fold in WT yeast. Moreover, unlike WT RNA3, this 1a-dependent increase in accumulation was reproduced in lsm1i yeast (Fig. 5C). Thus, in lsm1i yeast but not WT yeast, a 3′ poly(A) tail facilitates RE-dependent RNA3 responsiveness to 1a.
Discussion
Herein, we have shown that the yeast LSM1 gene is required for efficient BMV RNA replication and linked this LSM1-dependence to the selection of BMV RNA3 templates for replication. Although the Lsm1p protein is related to core snRNP proteins, we found that it localized to the cytoplasm. As discussed below, these results and the ability of 3′ poly(A) to suppress the LSM1-dependence of 1a-RNA3 interaction suggest that LSM1 may have even broader roles in cellular RNA metabolism than a previously identified contribution to mRNA decapping (26).
LSM1 and Poly(A) Effects on 1a–RE Interaction.
BMV 1a is a multifunctional protein with key roles in RNA replication complex assembly and function. One or more activities of its C-proximal DEAD box helicase domain are required for ongoing negative-strand, positive-strand, and subgenomic mRNA synthesis (13). Its N-proximal domain has m7G methyltransferase and m7GMP covalent binding activities implicated in capping viral positive-strand RNAs (14, 15). 1a also targets itself (5) and the polymerase-like 2a protein (39) to the endoplasmic reticulum membrane sites of BMV RNA replication.
Genetic exchanges show that 1a and the intergenic RNA3 RE regulate the selection of RNA3 templates for replication (13, 35). Acting through the RE, 1a strikingly increases the stability and accumulation of RNA3 transcripts (Fig. 4), and these effects are tightly linked to selecting RNA3 templates for replication and simultaneously inhibiting RNA3 translation (2, 19). Loss of the RE and these 1a-mediated events inhibits RNA3 negative-strand synthesis and thus RNA replication approximately 100-fold (19, 36, 37). Similar mechanisms linking selection of viral RNAs for replication to translation inhibition are found in poliovirus and bacteriophage Qβ (3) and may be common or universal among positive-strand viruses to avoid ribosome-polymerase collisions.
LSM1 mutation inhibited the RE-dependent, 2a-independent action of 1a on WT RNA3 templates by 70–80% (Fig. 4A). Equivalent inhibition of 1a-induced RNA3 stabilization was obtained in lsm1i yeast when WT RNA3 templates were expressed by the copper-inducible CUP1 promoter (Fig. 4A) or the galactose-inducible GAL1 promoter (Figs. 4B and 5). After accounting for lesser, ADH1 promoter-specific effects of lsm1 on 2a protein accumulation, the level of this inhibition correlates well with the reduction in RNA3 replication and subgenomic mRNA synthesis (Fig. 3). Thus, the primary effect of lsm1 mutation on BMV RNA replication is inhibition of an early, 1a-mediated step in selecting RNA3 templates for replication.
The requirement for LSM1 to support efficient 1a-induced stabilization of RNA3 is related to the state of RNA3, because the need for LSM1 function was suppressed by transferring the 1a-responsive RE element to a polyadenylated globin mRNA or replacing the tRNA-like 3′ end of RNA3 with a poly(A) tail. Unlike WT RNA3, these polyadenylated, RE-containing RNAs were fully 1a-responsive in lsm1i yeast (Fig. 5). Thus, in lsm1i but not WT yeast, a 3′ poly(A) tail facilitates RE-dependent RNA3 responsiveness to 1a. Conversely, the WT LSM1 gene allows WT BMV RNAs to function without a 3′ poly(A) tail for responding to 1a and possibly other factors. Given the importance of poly(A) for cell mRNA function (ref. 40; see also below), it is not surprising that nonpolyadenylated viral RNAs depend on specific host factors to compensate.
LSM1 mutation did not seem to affect 1a directly. In lsm1i yeast, 1a accumulated normally and efficiently stabilized WT levels of a globin mRNA hybrid bearing the RE or the RNA3-poly(A) derivative (Fig. 5). Moreover, although the number of WT RNA3 molecules stabilized by 1a was reduced severely in lsm1i yeast, those RNA3 molecules affected by 1a were as stable as in WT yeast (Fig. 4B). Thus, loss of LSM1 function suppressed the accessibility of WT RNA3 for 1a-mediated recruitment into the stabilized state but did not affect the final state of the few RNA3 molecules recruited.
Possible LSM1 Functions.
The shared ability of poly(A) and LSM1 to support RE-dependent 1a action on RNA3 implies links between their functions. The known effects of poly(A) are mediated by poly(A) binding protein, Pab1p, which enhances mRNA translation and stability by recruiting factors that bind the 5′ m7G caps of cell mRNAs and BMV RNAs (40, 41). The only other LSM1 function identified to date also involves mRNA 5′ ends and poly(A). A screen for mutations allowing yeast to grow without Pab1p identified LSM1 (26). LSM1 was found to be required for efficient 5′ decapping of mRNAs, which normally follows poly(A) shortening (causing loss of Pab1p binding) and triggers mRNA degradation. Like our results, these findings show that Lsm1p and poly(A) are involved in common processes, that Lsm1p facilitates the effective interaction of some factors with nonpolyadenylated RNAs, and that Lsm1p facilitates the transition of mRNA from translation to a new fate.
In keeping with the connections among LSM1, 5′ ends, and 3′ poly(A), the RE-dependent actions of 1a on RNA3 are also linked to viral RNA 5′ ends. Efficient 1a-induced RNA3 stabilization requires a functional, cis-linked viral or cellular 5′ untranslated region (19). Moreover, 1a inhibits RNA3 translation (2). Both results suggest 1a communication with the RNA3 5′ end or factors binding it. Interaction of 1a with PAB1-stabilizable factors binding mRNA 5′ ends would explain the influence of the 5′ untranslated region, 1a inhibition of RNA3 translation, and poly(A) stimulation of 1a action.
The core snRNP proteins, to which LSM1 is related, bind snRNAs and mediate the interaction of a cap methyltransferase with snRNA 5′ ends (22). Thus, a possible similar role for LSM1 consistent with the results above would be to facilitate the interaction of decapping and perhaps other factors with mRNA 5′ ends. If such factors included or interacted with any factors associated with the PAB1-stabilized translation initiation complex, such a role could explain LSM1 involvement in decapping and 1a-RNA3 interaction, and the ability of LSM1 and poly(A) to independently facilitate 1a-RNA3 interaction through 5′-associated factors. A related hypothesis would be that, because lsm1 mutation delays the decay of capped, deadenylated mRNAs (26), their caps might compete for factors involved in 1a-RNA3 recognition. This hypothesis would also imply the involvement of cap binding factors in 1a–RNA3 interaction but seems less likely, because Fig. 5 shows that any cap-binding factors required for 1a-RNA3 interaction must be strongly stabilized by poly(A).
Acknowledgments
We thank Jack Hietpas for excellent technical assistance, Michael Janda for assistance with 2a expression, and Bill Sugden, Marv Wickens, and John Young for comments on the manuscript. This research was supported by National Institutes of Health Grant GM35072. P.A. is an investigator of the Howard Hughes Medical Institute.
Abbreviations
- BMV
brome mosaic virus
- RE
replication enhancer
- kb
kilobase
- CAT
chloramphenicol acetyl transferase
- GUS
β-glucuronidase
- snRNP
small nuclear ribonucleoprotein particle
- WT
wild type
- HA
hemagglutinin
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.080072997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.080072997
References
- 1.Novak J, Kirkegaard K. Genes Dev. 1994;8:1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 2.Janda M, Ahlquist P. Proc Natl Acad Sci USA. 1998;95:2227–2232. doi: 10.1073/pnas.95.5.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamarnik A V, Andino R. Genes Dev. 1998;12:2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Restrepo-Hartwig M, Ahlquist P. J Virol. 1996;70:8908–8916. doi: 10.1128/jvi.70.12.8908-8916.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Restrepo-Hartwig M, Ahlquist P. J Virol. 1999;73:10303–10309. doi: 10.1128/jvi.73.12.10303-10309.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlegel A, Giddings J T, Ladinsky M, Kirkegaard K. J Virol. 1996;70:6576–6588. doi: 10.1128/jvi.70.10.6576-6588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lai M. Virology. 1998;244:1–12. doi: 10.1006/viro.1998.9098. [DOI] [PubMed] [Google Scholar]
- 8.Strauss J H, Strauss E G. Science. 1999;283:802–804. doi: 10.1126/science.283.5403.802. [DOI] [PubMed] [Google Scholar]
- 9.Gamarnik A V, Andino R. EMBO J. 1996;15:5988–5998. [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa M, Diez J, Restrepo-Hartwig M, Ahlquist P. Proc Natl Acad Sci USA. 1997;94:13810–13815. doi: 10.1073/pnas.94.25.13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janda M, Ahlquist P. Cell. 1993;72:961–970. doi: 10.1016/0092-8674(93)90584-d. [DOI] [PubMed] [Google Scholar]
- 12.Price B D, Rueckert R, Ahlquist P. Biochemistry. 1996;93:9465–9470. doi: 10.1073/pnas.93.18.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahlquist P. Curr Opin Genet Dev. 1992;2:71–76. doi: 10.1016/s0959-437x(05)80325-9. [DOI] [PubMed] [Google Scholar]
- 14.Ahola T, Ahlquist P. J Virol. 1999;73:10061–10069. doi: 10.1128/jvi.73.12.10061-10069.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kong F, Sivakumaran K, Kao C. Virology. 1999;259:200–210. doi: 10.1006/viro.1999.9763. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa M, Janda M, Krol M A, Ahlquist P. J Virol. 1997;71:7781–7790. doi: 10.1128/jvi.71.10.7781-7790.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan M, Ahlquist P. Semin Virol. 1997;8:221–230. [Google Scholar]
- 18.Rao A L N, Dreher T W, Marsh L E, Hall T C. Proc Natl Acad Sci USA. 1989;86:5335–5339. doi: 10.1073/pnas.86.14.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sullivan M, Ahlquist P. J Virol. 1999;73:2622–2632. doi: 10.1128/jvi.73.4.2622-2632.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krol M A, Olson N H, Tate J, Johnson J E, Baker T S, Ahlquist P. Proc Natl Acad Sci USA. 1999;96:13650–13655. doi: 10.1073/pnas.96.24.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kambach C, Walke S, Young R, Avis J M, de la Fortelle E, Raker V A, Lührmann R, Li J, Nagai K. Cell. 1999;96:375–387. doi: 10.1016/s0092-8674(00)80550-4. [DOI] [PubMed] [Google Scholar]
- 22.Plessel G, Fischer U, Luhrmann R. Mol Cell Biol. 1994;14:4160–4172. doi: 10.1128/mcb.14.6.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pellizzoni L, Kataoka N, Charroux B, Dreyfuss G. Cell. 1998;95:615–624. doi: 10.1016/s0092-8674(00)81632-3. [DOI] [PubMed] [Google Scholar]
- 24.Mayes A E, Verdone L, Legrain P, Beggs J D. EMBO J. 1999;18:4321–4331. doi: 10.1093/emboj/18.15.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salgado-Garrido J, Bragado-Nilsson E, Kandels-Lewis S, Seraphin B. EMBO J. 1999;18:3451–3462. doi: 10.1093/emboj/18.12.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boeck R, Lapeyre B, Brown C E, Sachs A B. Mol Cell Biol. 1998;18:5062–5072. doi: 10.1128/mcb.18.9.5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascorro-Gallardo J O, Covarrubias A A, Gaxiola R. Gene. 1996;172:169–170. doi: 10.1016/0378-1119(96)00059-5. [DOI] [PubMed] [Google Scholar]
- 28.Gietz R D, Sugino A. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 29.Orr-Weaver T L, Szostak J W, Rothstein R J. Proc Natl Acad Sci USA. 1981;78:6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross-Macdonald P, Sheehan A, Roeder G S, Snyder M. Proc Natl Acad Sci USA. 1997;94:190–195. doi: 10.1073/pnas.94.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose M D, Novick P, Thomas J H, Botstein D, Fink G R. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- 32.Hermann H, Fabrizio P, Raker V A, Foulaki K, Hornig H, Brahms H, Luhrmann R. EMBO J. 1995;14:1076–1088. doi: 10.1002/j.1460-2075.1995.tb07199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweinfest C W, Graber M W, Chapman J M, Papas T S, Baron P L, Watson D K. Cancer Res. 1997;57:2961–2965. [PubMed] [Google Scholar]
- 34.Dinant S, Janda M, Kroner P A, Ahlquist P. J Virol. 1993;67:7181–7189. doi: 10.1128/jvi.67.12.7181-7189.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pacha R F, Ahlquist P. J Virol. 1991;65:3693–3703. doi: 10.1128/jvi.65.7.3693-3703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quadt R, Ishikawa M, Janda M, Ahlquist P. Proc Natl Acad Sci USA. 1995;92:4892–4896. doi: 10.1073/pnas.92.11.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.French R, Ahlquist P. J Virol. 1987;61:1457–1465. doi: 10.1128/jvi.61.5.1457-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pogue G P, Marsh L E, Connell J P, Hall T C. Virology. 1992;188:742–753. doi: 10.1016/0042-6822(92)90529-x. [DOI] [PubMed] [Google Scholar]
- 39.Chen, J. & Ahlquist P. (2000) J. Virol., 74, in press. [DOI] [PMC free article] [PubMed]
- 40.Wickens M, Anderson P, Jackson R J. Curr Opin Genet Dev. 1997;7:2320–2323. doi: 10.1016/s0959-437x(97)80132-3. [DOI] [PubMed] [Google Scholar]
- 41.Tarun S Z, Sachs A B. EMBO J. 1996;15:7168–7177. [PMC free article] [PubMed] [Google Scholar]