Abstract
In this study, the charge selectivity of staphylococcal α-hemolysin (αHL), a bacterial pore-forming toxin, is manipulated by using cyclodextrins as noncovalent molecular adapters. Anion-selective versions of αHL, including the wild-type pore and various mutants, become more anion selective when β-cyclodextrin (βCD) is lodged within the channel lumen. By contrast, the negatively charged adapter, hepta-6-sulfato-β-cyclodextrin (s7βCD), produces cation selectivity. The cyclodextrin adapters have similar effects when placed in cation-selective mutant αHL pores. Most probably, hydrated Cl− ions partition into the central cavity of βCD more readily than K+ ions, whereas s7βCD introduces a charged ring near the midpoint of the channel lumen and confers cation selectivity through electrostatic interactions. The molecular adapters generate permeability ratios (PK+/PCl−) over a 200-fold range and should be useful in the de novo design of membrane channels both for basic studies of ion permeation and for applications in biotechnology.
In keeping with their roles in the cell, natural transmembrane channel proteins are ion selective (1–3). For example, voltage-gated K channels do not handle anions, and they transport K+ ions with a more than 100-fold preference over Na+ ions (1, 3, 4). Other channels are less selective, but their preferences are also of physiological importance. For example, the nicotinic acetylcholine receptor, a ligand-gated channel, is charge selective; it does not transport anions but only weakly discriminates between various cations (5). Poorly selective channels that allow both cations and anions to pass, such as many of the porins (6–8), are not ion channels in their primary physiological roles, yet they are usually weakly charge selective (9–11).
Studies of ion selectivity have been directed toward an understanding of the selectivity of natural channels and, less commonly, the creation of engineered channels with a range of selectivities. The ion selectivity of natural channels has been investigated primarily by site-directed mutagenesis, which has in some cases allowed the identification of one or a few key amino acid residues. More often, the basis of selectivity has been found to be more complex and subtle. For example, in the case of voltage-gated K channels, where structural information is available to temper studies by mutagenesis, selectivity is seen to involve both electrostatic effects and the coordination of transported ions by backbone carbonyls (3, 4). The creation of engineered channels with a range of ion selectivities has been a spin-off from mechanistic studies. For example, the acetylcholine receptor has been converted from a cation-selective to an anion-selective channel by mutations that most likely result in structural changes involving the polypeptide backbone (5). In β-barrel proteins, such as porins, charge selectivity has been altered by manipulating charged residues in the channel lumen (e.g., refs. 9 and 11). In addition, ion selectivity has been achieved in the de novo design of channels and pores (12), primarily by the chemical synthesis of membrane-active peptides and related amphipathic molecules (13–15).
In a different approach for manipulating the properties of transmembrane channels and pores, we demonstrated the remarkable ability of cyclic oligosaccharides comprising glucose units (cyclodextrins) to act as molecular adapters for the pore formed by staphylococcal α-hemolysin (αHL) (16). The αHL pore is a heptamer made up of identical subunits of 293 amino acids (17). Molecules of up to ≈2000 Da can be transported through a wide channel in the pore that is centered on the molecular sevenfold axis (18–20). Polynucleotide strands of much higher mass can move through the pore in extended form (21). Measurements of ionic currents indicate a weak anion selectivity for the unmodified heptamer (22). Cyclodextrins are able to reduce the conductance of the pore by lodging at a point about half way through the channel, where the diameter is at its narrowest (≈14 Å) (16). Further, channel blockers can bind to a cyclodextrin while it is in the channel. For example, β-cyclodextrin (βCD) reduces the conductance of wt (wild-type) αHL from 658 pS to 240 pS in 1 M NaCl (pH 7.5), and a large variety of organic molecules cause transient channel blockades by binding within the wt-αHL⋅βCD complex (16). The results with channel blockers suggest that a substantial fraction of the ionic current flows through the center of the cyclodextrin molecule when it is lodged in the channel lumen. Therefore, we reckoned that cyclodextrins might change the charge selectivity of the αHL pore. Here we show that this is the case.
Materials and Methods
Reagents.
βCD was from Aldrich and γ-cyclodextrin from ACROS (Geel, Belgium). Hepta-6-sulfato-β-cyclodextrin (s7βCD) was prepared as described (23) and is available from J & W Scientific (Folsom, CA). Buffers for planar bilayer recording contained various concentrations of KCl or NaCl and 10 mM K2HPO4 or Na2HPO4 (Sigma) in deionized water (Millipore) and were titrated to pH 7.5 with 1 M HCl (EM Science). Experiments with the mutant αHL-CH1 were done in KCl containing 10 mM potassium phosphate buffer (pH 7.4) and 5 μM EDTA.
Proteins.
The mutant αHL genes M113N, M113N/L135N, and E111N/K147N were prepared by cassette mutagenesis in the plasmid αHL-RL2 (24). These constructs contain the following additional changes over wt-αHL: Lys-8 → Ala, Val-124 → Leu, Gly-130 → Ser, Asn-139 → Gln, and Ile-142 → Leu. αHL polypeptides with these mutations behave similarly to wt-αHL in hemolysis assays and in planar bilayer recordings, at the salt concentrations used here (24). The chimeric protein αHL-CH1 features a transmembrane domain derived from the protective antigen of anthrax toxin fused to the cap domain of αHL (laboratories of R. J. Collier and H.B., unpublished data). Residues 119–139 inclusive of αHL (21 residues) were replaced with 22 residues, 302–323, from protective antigen. The register of the β strands in the transmembrane domain is that given by Petosa and colleagues (25).
Heptameric wt-αHL was formed by treating monomeric αHL, purified from Staphylococcus aureus, with deoxycholate (26, 27) and isolated from SDS/polyacrylamide gels as described (28). Mutant αHL polypeptides were prepared by coupled in vitro transcription and translation, with an S30 extract from Escherichia coli (no. L114A, Promega) (24). Heptamers were prepared from the mutants by assembly on rabbit red cell membranes, followed by preparative SDS/PAGE (24).
Bilayer Recordings.
A 25-μm-thick Teflon film (Goodfellow, Malvern, MA) with a 100- to 150-μm diameter orifice was used as the partition between the two chambers (2 ml each) of a Teflon bilayer apparatus. The orifice was pretreated with 1:10 hexadecane (Aldrich)/pentane (Burdick and Jackson). A solvent-free planar lipid bilayer of 1,2-diphytanoyl-sn-glycerophosphatidylcholine (Avanti Polar Lipids) was formed over the orifice (29). A potential was applied across the bilayer with Ag/AgCl electrodes with 1.5% agarose (Ultra Pure DNA Grade, Bio-Rad) bridges containing 3 M KCl. Protein was added to the cis chamber, which was at ground. A positive potential indicates a higher potential in the trans chamber, and a positive current is one in which cations flow from trans to cis. Single-channel currents were recorded with an Axopatch 200B patch-clamp amplifier (Axon Instruments, Foster City, CA) in the whole cell (β = 1) mode with a CV-203BU headstage and filtered at 5 kHz with a built-in 4-pole low-pass Bessel Filter. Data including short events (≈1 msec) were acquired directly by computer by using a Digidata 1200 A/D converter (Axon). The other data were recorded on DAT tape and subsequently transferred to the computer after filtering at 1 kHz through a low-pass 8-pole Bessel filter (model 900, Frequency Devices, Haverhill, MA). The data were acquired by using Clampex 7.0 software (Axon) after sampling at a rate of 20 kHz and analyzed with pClamp 6.03 (Axon) and Origin (Microcal Software, Northampton, MA) software.
Experiments were initiated by the addition of heptameric αHL to the cis compartment with stirring until a single channel inserted into the bilayer. For the wt heptamer, oligomerized with deoxycholate, the final concentration was 3–30 ng/ml. For the mutant heptamers, oligomerized on red cell membranes, the final concentration was ≈0.2 ng/ml. βCD or s7βCD was added to the trans chamber to 40 μM. Experiments were at 22 ± 2°C.
Data Analysis.
Single-channel conductances were determined by fitting the peaks in amplitude histograms to Gaussian functions. The permeability ratios (PK+/PCl−) were calculated from reversal potentials by using the Goldman-Hodgkin-Katz (GHK) equation (1):
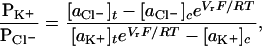 |
where Vr is the reversal potential (i.e., the electrical potential giving zero current), aX is the activity of ion X (30), subscripts c and t represent the cis and trans compartments, and the other symbols have their usual meanings. Vr was obtained by a polynomial fit of the current-voltage (I–V) data near zero current. For asymmetrical conditions, one chamber (cis or trans) contained 1000 mM KCl, whereas the other chamber contained 200 mM KCl, except for the αHL-CH1 pore where cis contained 1000 mM KCl and trans, 100 mM KCl. After the measurements, the membrane was broken to determine the contribution of electrode junction potentials (normally smaller than 0.5 mV). Permeability ratios for αHL depend on several variables including pH and bilayer composition (31). The results obtained here are valid only for the conditions stated.
Results
Cyclodextrins Act as Molecular Adapters To Enhance the Anion Selectivity of αHL Pores.
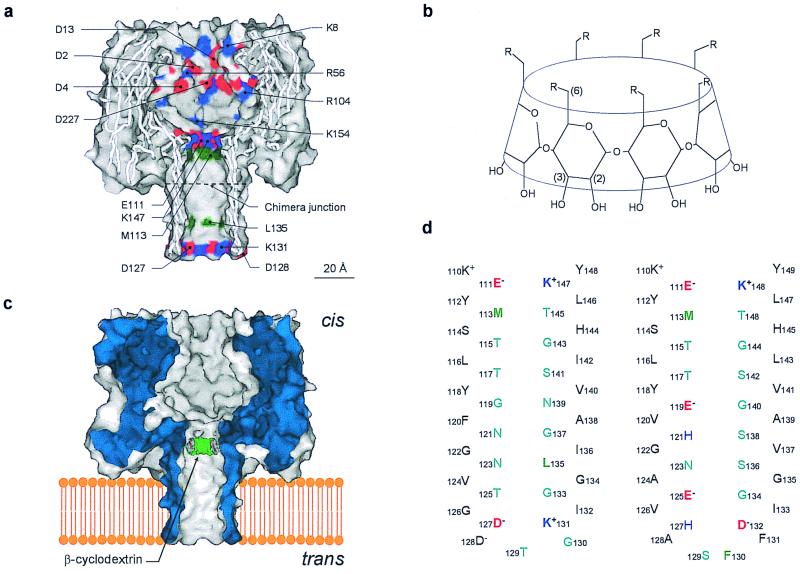
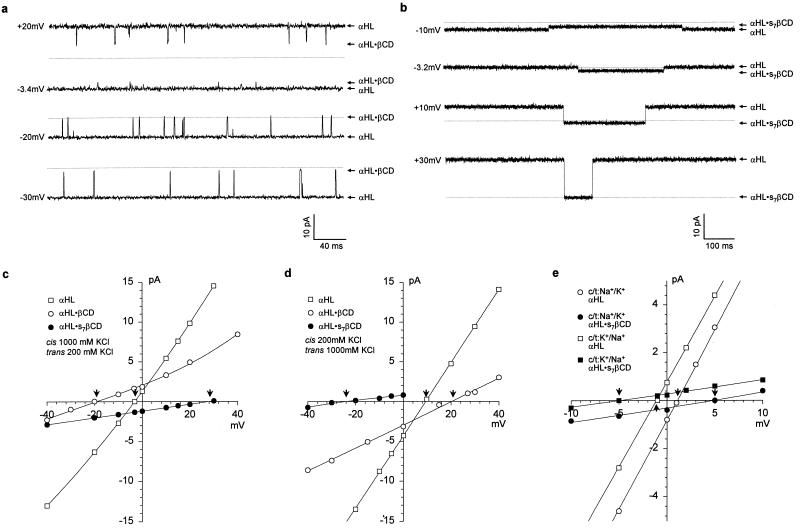
Various α-hemolysins and cyclodextrins were used in this study (Fig. 1). We showed previously that βCD lodges transiently in the lumen of the wt-αHL pore, where it acts as a noncovalent molecular adapter reducing the unitary conductance (Table 1) (16). The homoheptameric wt-αHL pore is weakly anion selective (22). To determine whether the selectivity is altered while βCD is in the channel lumen, single-channel currents were recorded under asymmetric conditions: 1000 mM KCl cis, 200 mM KCl trans (pH 7.5) (Fig. 2a). I–V curves were plotted for the contributions arising from the unmodified wt-αHL pore and the wt-αHL·βCD complex (Fig. 2c). Experiments were also performed with the opposite KCl asymmetry: 200 mM KCl cis, 1000 mM KCl trans (pH 7.5) (Fig. 2d). The charge selectivities under the various conditions were then calculated from Vr and the GHK equation. The wt-αHL·βCD complex (PK+/PCl− = 0.23–0.25) is significantly more anion selective than the unmodified wt-αHL pore (PK+/PCl− = 0.55–0.79) (Table 1).
Figure 1.
Representations of the proteins and cyclodextrins used in this work. (a), Sagittal section through the wt-αHL pore showing the location of all of the charged side chains in the channel lumen (red, negative; blue, positive) and the key hydrophobic residues M113 and L135 (green). The site of the junction in the chimera αHL-CH1 is indicated; (b), Structures of the βCDs used in this work. βCD, R = −OH; s7βCD, R = −OSO3−; (c), Schematic of the wt-αHL pore showing βCD lodged in the lumen of the channel. The location is based on mutagenesis data (16); (d), Sequences of the transmembrane β barrels in wt-αHL (Left) and αHL-CH1 (Right).
Table 1.
Permeability ratios (PK+/PCl−) and conductance values (g) for various α-HLs with and without adapters
| Adapter | Charge, e | Minimum diameter, Å | wt-αHL
|
αHL-M113N
|
αHL-E111N/K147N
|
αHL-CH1
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vr, mV | PK+/PCl− | g, pS* | Vr, mV | PK+/PCl− | g, pS* | Vr, mV | PK+/PCl− | g, pS* | Vr, mV | PK+/PCl− | g, pS† | |||
| None | 14 | +9.1‡ | 0.55 ± 0.02 | 658 ± 11 | +5.96‡ | 0.68 ± 0.03 | 622 ± 9 | +2.71§ | 1.2 ± 0.1 | 634 ± 12 | +27.1¶ | 5.1 ± 0.2 | 541 ± 11 | |
| −3.7§ | 0.79 ± 0.02 | −2.21§ | 0.87 ± 0.04 | |||||||||||
| βCD | 0 | 6.2 | +19.6‡ | 0.25 ± 0.01 | 240 ± 5 | +29.7‡ | 0.079 ± 0.005 | 261 ± 4 | −24.9§ | 0.15 ± 0.02 | 207 ± 7 | −3.9¶ | 0.82 ± 0.01 | 109 ± 9 |
| −20.5§ | 0.23 ± 0.01 | −32.3§ | 0.046 ± 0.006 | |||||||||||
| s7βCD | −7 | <6.2 | −24.1‡ | 6.7 ± 0.4 | 53 ± 6 | −19.3‡ | 4.1 ± 0.02 | 40 ± 1 | ND | ND | ND | ND | ND | ND |
| +28.1§ | 10.0 ± 0.2 | +23.2§ | 6.1 ± 0.1 | |||||||||||
| γCD | 0 | 7.9 | −14.0§ | 0.38 ± 0.04 | 328 ± 4 | ND | ND | 287 ± 5 | ND | ND | 275 ± 4 | ND | ND | ND |
For each entry, three or more separate experiments were performed, and data acquired for at least 1 min were analyzed. The reversal potentials (Vr) are mean values under the conditions stated. Permeability ratios are quoted as the mean ± SD. ND, not determined.
*−40 mV, 1 M NaCl, 10 mM sodium phosphate (pH 7.5).
†−40 mV 1 M KCl, 10 mM potassium phosphate (pH 7.4).
‡pH 7.5, KCl in mM (cis/trans) was (200/1000).
§pH 7.5, KCl in mM (cis/trans) was (1000/200).
¶pH 7.4, KCl in mM (cis/trans) was (1000/100).
Figure 2.
Representative bilayer recordings and I-V curves showing modulation of single-channel currents through the wt-αHL pore by cyclodextrin adapters. (a), Current recording in the presence of 40 μM βCD added to the trans side of the bilayer. The chambers contained 10 mM K phosphate (pH 7.5), with cis, 1000 mM KCl; trans, 200 mM KCl. The transmembrane potential is indicated. The thin line indicates zero current. (b), Current recording in the presence of 40 μM s7βCD added to the trans side. Other conditions as in a. (c), I–V curves for αHL (□), αHL·βCD (○), and αHL·s7βCD (●) based on recordings made with cis: 1000 mM KCl; trans, 200 mM KCl. Reversal potentials (Vr) are marked by arrows. (d), I–V curves for αHL (□), αHL·βCD (○), and αHL·s7βCD (●) based on recordings made with cis: 200 mM KCl; trans, 1000 mM KCl. (e), I–V curves recorded to test cation selectivity in 10 mM phosphate buffer (pH 7.5): □ and ■, αHL and αHL·s7βCD with cis: 1000 mM KCl; trans, 1000 mM NaCl; ○ and ●, αHL and αHL·s7βCD with cis: 1000 mM NaCl; trans, 1000 mM KCl.
In addition, the effect of βCD on the mutant M113N was examined. M113N binds the cyclodextrin far more tightly than wt-αHL (16). The almost nonselective M113N pore (PK+/PCl− = 0.68–0.87) became highly anion selective with βCD bound (PK+/PCl− = 0.046–0.079) (Table 1). The mutant M113N/L135N (16) gave similar results (data not shown). The effect of γ-cyclodextrin (γCD) which contains eight glucose units, on the selectivity of the wt-αHL pore was also tested. γCD enhanced the anion selectivity of the pore (PK+/PCl− = 0.38), but to a lesser extent than βCD (PK+/PCl− = 0.23–0.25) (Table 1).
The Anionic Adapter Hepta-6-sulfato-β-cyclodextrin (s7βCD) Creates a Cation-Selective αHL Pore.
We wished to test the effects of a charged adapter on the ion selectivity of αHL. Many commercially available derivatives of βCD are complex mixtures of regioisomers with different extents of substitution. Therefore, we tested hepta-6-sulfato-β-cyclodextrin (s7βCD, Fig. 1) of 97 mole % isomeric purity. s7βCD bound to the wt-αHL pore from the trans side of the membrane producing a substantial single-channel block (Fig. 2b) (Table 1). The dwell time of s7βCD at pH 7.5 (τ = 846 ± 37 msec at −40 mV, n = 3) was far greater than βCD (τ = 0.84 ± 0.09 msec at −40 mV, n = 8) and, as expected, it was voltage-dependent (data not shown). I–V curves were constructed for currents recorded under both cis/trans and trans/cis KCl gradients (Fig. 2 c and d) and charge selectivities calculated from Vr. The wt-αHL·s7βCD complex is strongly cation selective (PK+/PCl− = 6.7–10) (Table 1). αHL-M113N also became cation selective with s7βCD bound (PK+/PCl− = 4.1–6.1) (Table 1).
We also compared the preference of wt-αHL·s7βCD for Na+ and K+. I–V data were recorded with 1000 mM NaCl in one chamber and 1000 mM KCl in the other (Fig. 2e). Ion selectivities were calculated from Vr and the average values of PK+/PCl− by using the GHK equation expanded to include Na+, K+ and Cl− (1). The permeability ratio, PK+/PNa+ = 0.87, was the same for both cis/trans and trans/cis gradients. The value for the unmodified wt-αHL pore under the same conditions was PK+/PNa+ = 1.0.
Cation-Selective Mutant Pores Become Anion Selective with βCD as an Adapter.
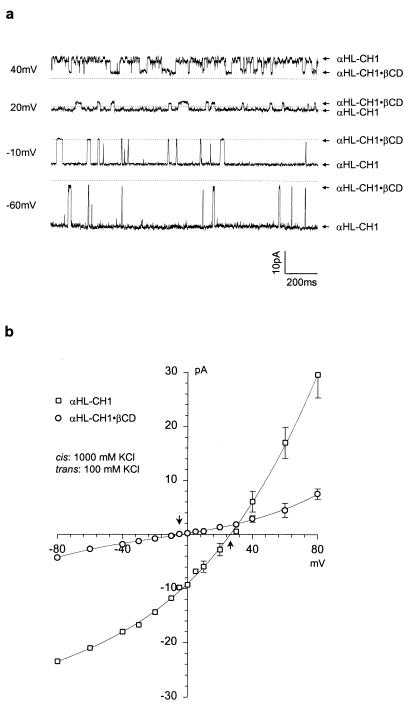
The increase in anion selectivity observed when βCD was used as an adapter for the wt-αHL pore was modest and we wished to determine whether βCD would produce anion selectivity in a cation-selective pore. To this end, we examined αHL-CH1, a chimeric protein that features a transmembrane domain derived from the protective antigen of anthrax toxin fused to the cap domain of αHL. The net charge in the transmembrane barrel of homoheptameric αHL-CH1 is −21, compared with −7 in the wt-αHL barrel, and it is cation selective. The altered barrel in αHL-CH1 retains the site near Met-113, where cyclodextrins are believed to bind (16). βCD indeed converted the cation selectivity of αHL-CH1 (PK+/PCl− = 5.1 at pH 7.4) to weak anion selectivity (PK+/PCl− = 0.82) (Fig. 3 a and b; Table 1). The weakly cation selective mutant E111N/K147N (PK+/PCl− = 1.2) was also examined in the presence of βCD, which converted it to an anion selective pore (PK+/PCl− = 0.15) (Table 1).
Figure 3.
Bilayer recordings and I-V curves showing modulation of single-channel currents through the αHL-CH1 pore by βCD. (a), Current recording in the presence of 40 μM βCD added to the trans side of the bilayer. The chambers contained 10 mM K phosphate (pH 7.4), and 5 μM EDTA, with cis, 1000 mM KCl; trans, 100 mM KCl. (b), I–V curves for αHL-CH1 (□) and αHL-CH1·βCD (○). The data points represent mean values (±SD) from three different experiments for αHL-CH1 and two experiments for αHL-CH1·βCD. Arrows, Vr.
Discussion
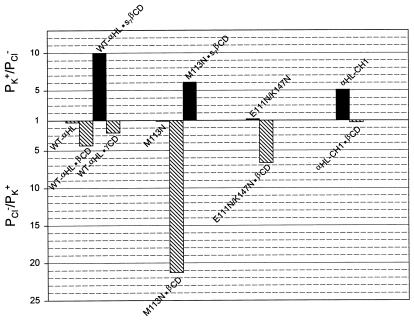
The goal of this study was to determine whether molecular adapters are capable of altering the ion selectivity of a transmembrane pore. We have found that cyclodextrin adapters produce substantial changes in the charge selectivity of αHL. For example, in the most striking examples, the wt-αHL pore equipped with an anionic adapter, s7βCD, was strongly cation selective (PK+/PCl− = 10), whereas a mutant αHL, M113N, equipped with unmodified βCD was strongly anion selective (PK+/PCl− = 0.05) (Fig. 4). In both these cases, a complete block is observed when various guest molecules bind to the adapter within the channel lumen (data not shown). Therefore, it is likely that most of the current flows through the center of the adapter, rather than around its edges. The direction of the salt gradient had a significant effect on PK+/PCl− values (Table 1), which might be attributed to several causes, including differential screening of charged groups, effects on the conformation of the protein and the onset of multi-ion transport conditions. Nevertheless, the general trends are unaffected by these deviations. The cyclodextrin adapters produced no significant discrimination between cations, as manifest in PK+/PNa+ values.
Figure 4.
Summary of the charge selectivity data. For cation selective pores PK+/PCl− is shown (dark bars). For anion selective pores, the ratios in Table 1 have been inverted to give PCl−/PK+ (hatched bars).
Ion permeation remains one of the most disputatious areas of theoretical biophysics (2, 32–34). Computational demands place an understanding of permeation beyond present-day exact molecular dynamics simulations (34, 35). The two major practicable approaches, chemical kinetics and diffusion theory, are rich in adjustable parameters and it is not surprising they “explain” most experimental observations. For example, a permeability ratio of 10 amounts to a barrier difference of only 1.4 kcal mol−1, which is readily accommodated. Here is a situation where our ability to measure far exceeds our ability to compute. Therefore, rather than confront the difficulties, we show that our findings are at least consistent with qualitative notions about selectivity.
Ion selectivity clearly depends to a large extent on the dimensions of a pore and the spatial distribution of charges at the entrance to and within the channel lumen (1, 3). A “wide pore” with a radius greater than the Debye length (≈10 Å in 100 mM salt, ≈3 Å at 1000 mM) generally shows weak selectivity because ions in transit interact primarily with water and other ions, rather than with the wall of the lumen (36). In this case, ion selectivities roughly reflect the diffusion coefficients of individual ions in solution. Narrow pores, such as voltage-gated K channels ([diameter (d) = 3 Å]), Na channels (d = 4 Å), and gramicidin A (d = 4 Å) are at the opposite extreme and show not only high charge selectivity, but also substantial discrimination among ions of the same charge. Here, high selectivity arises through dehydration of ions in the channel lumen and coordination by preorganized functional groups in a selectivity filter (1, 37), which in the case of K channels are the oxygen atoms of backbone carbonyls (3, 4). Between the extremes of wide and narrow channels, mid-sized channels, such as the nicotinic acetylcholine receptor and the anion-selective γ-aminobutyric acid type a receptor show high charge selectivity, but low selectivity among ions of the same charge. The selectivity of wide and mid-sized channels can be altered by using mutagenesis to place or alter charged amino acid side chains along the conductive pathway and at its entrance (for recent examples see refs. 11, 15, 38, and 39). Even for highly selective channels electrostatics can provide a prefilter (3, 4).
wt-αHL should be considered a “wide pore” (22). The narrowest internal diameter is ∼14 Å near Met-113, which is close to the cyclodextrin binding site (16, 17). In keeping with the assignment as a wide pore, wt-αHL is of high conductance (658 pS, 1 M NaCl at pH 7.5, −40 mV) and, despite the presence of charged residues throughout the channel lumen (Fig. 1a), charge selectivity is weak (22). Under the conditions used here PK+/PCl− = 0.55–0.79. The selectivity of αHL can be altered by introducing multiple charged side chains into the channel lumen, as seen here with αHL-CH1 (PK+/PCl− = 5.1; 541 pS, 1 M KCl at pH 7.4, −40 mV), in which the net charge of the lower half of the transmembrane barrel is changed from −7 to −21. wt-αHL and αHL-CH1 pores both bound the neutral adapter βCD, which introduces a mid-sized constriction [internal diameter 6.2 Å (40), still sufficient to admit a hydrated ion] as demonstrated by the reductions in conductance: wt-αHL·βCD, g = 240 pS, 1 M NaCl at pH 7.5, −40 mV; αHL-CH1·βCD, g = 109 pS, 1 M KCl at pH 7.4, −40 mV. Both pores are anion selective when the adapter is bound. Therefore, the βCD adapter dominates ion selection as judged by the similar outcomes in both an anion-selective and cation-selective background (Table 1). γCD, which contains eight glucose units, rather than the seven of βCD, is also uncharged, with a larger internal diameter of 7.9 Å (40). As expected, γCD has a lesser effect than βCD on the conductance of the wt-αHL pore (wt-αHL·γCD: g = 328 pS, 1 M NaCl at pH 7.5, −40 mV). The influence on anion selectivity was also reduced (PK+/PCl− = 0.38).
The seven sulfate groups of s7βCD form a negatively charged ring (−7) (Fig. 1b). This adapter greatly reduced the conductance of the wt-αHL pore: wt-αHL·s7βCD, g = 53 pS, 1 M NaCl (pH 7.5), −40 mV. Further, the pore became cation selective, PK+/PCl− = 6.7–10. In this case, electrostatics predominate over the preference of the interior of the cyclodextrin for anions. This interpretation is supported by the finding that the PK+/PCl− value for wt-αHL·s7βCD is highly dependent on the absolute ionic strength (data not shown). It is also in keeping with many studies in the literature in which the introduction by mutagenesis of charged rings at channel entrances or in the lumen, altered charge selectivity in a qualitatively predictable manner. In general, the placement of negatively charged side chains in the lumen favors cation selectivity, whereas positively charged side chains favor anion selectivity, as seen, for example, in the cases of the mitochondrial voltage-dependent anion channel (VDAC) (9), the porin of Paracoccus denitrificans (11), and alamethicin (15).
Interestingly, modified cyclodextrins have been used directly as ion channels. For example, Tabushi and colleagues made a βCD carrying hydrophobic chains on four of the seven 6-positions (41). It was claimed that two of these molecule form a transmembrane pore. In a more extensive study, Lehn and collaborators made a “bouquet” molecule from βCD by attaching seven PEG chains to each face. This molecule showed some of the properties expected of a transmembrane pore (42, 43). In another study, a condensed monolayer of βCD with all seven 6-positions modified with long alkyl chains was deposited on a graphite electrode. The modified surface was permeable to the electroactive marker p-quinone (44). Transport through the central cavity was invoked because it could be blocked with guest molecules. Unfortunately, in none of these cases were clear-cut measurements of charge selectivity made.
In conclusion, we have presented a different way to modulate the ion selectivity of a transmembrane pore. The adapters can be regarded as crude modular selectivity filters. By reducing the dimensions of the channel lumen from “wide” to “mid-sized,” the adapters dominate the charge selectivity of the pore. Unmodified βCD has an affinity for anions, whereas the negatively charged s7βCD rejects anions, allowing cations to pass in preference. Like site-directed mutagenesis, the adapter approach is versatile because various adapters can be used to program the same protein. Furthermore, mutagenesis and the adapter approach can be combined: for example, to increase the dwell time of the adapter on the protein (16). Protein pores with adapters should be useful model systems with which to study the details of ion permeation. In the case of the αHL·βCD system, the protein is of known structure and both the protein and the adapter have sevenfold symmetry. The adapter is not likely to produce any major rearrangements of the protein, although additional structural studies will be required to confirm this and to provide the exact location of the adapter. It may be possible to use a similar approach to alter the activity of other proteins, e.g., to modify the active site of an enzyme. In future work with pores, a great challenge will be to introduce yet greater selectivity; for example, by using an adapter with a ring of carbonyl groups similar to those found in the ionophore valinomycin (45) or eukaryotic potassium channels (4). This simple method to control ion selectivity may also have useful applications in aspects of biotechnology including drug delivery and biosensor design.
Acknowledgments
We thank Carl Miller and John Collier for the PA plasmid and the referees for their suggestions. This work was supported by a Multidisciplinary University Research Initiative (Office of Naval Research) award and grants from the Defense Advanced Research Projects Agency and the Department of Energy.
Abbreviations
- αHL
staphylococcal α-hemolysin
- βCD
β-cyclodextrin
- s7βCD
hepta-6-sulfato-β-cyclodextrin
- wt
wild-type
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hille B. Ionic channels of excitable membranes. Sunderland, MA: Sinauer; 1991. [Google Scholar]
- 2.Andersen O S. J Gen Physiol. 1999;113:763–764. doi: 10.1085/jgp.113.6.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roux B, MacKinnon R. Science. 1999;285:100–102. doi: 10.1126/science.285.5424.100. [DOI] [PubMed] [Google Scholar]
- 4.Doyle D A, Cabral J M, Pfuetzner R A, Kuo A, Gulbis J M, Cohen S L, Chait B T, MacKinnon R. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- 5.Galzi J-L, Devillers-Thiéry A, Hussy H, Bertrand S, Changeux J-P, Bertrand D. Nature (London) 1992;359:500–505. doi: 10.1038/359500a0. [DOI] [PubMed] [Google Scholar]
- 6.Delcour A H. FEMS Microbiol Lett. 1997;151:115–123. doi: 10.1111/j.1574-6968.1997.tb12558.x. [DOI] [PubMed] [Google Scholar]
- 7.Klebba P E, Newton S M C. Curr Op Microbiol. 1998;1:238–247. doi: 10.1016/s1369-5274(98)80017-9. [DOI] [PubMed] [Google Scholar]
- 8.Schirmer T. J Struct Biol. 1998;121:101–109. doi: 10.1006/jsbi.1997.3946. [DOI] [PubMed] [Google Scholar]
- 9.Blachly-Dyson E, Peng S, Colombini M, Forte M. Science. 1990;247:1233–1236. doi: 10.1126/science.1690454. [DOI] [PubMed] [Google Scholar]
- 10.Samartzidou H, Delcour A H. EMBO J. 1998;17:93–100. doi: 10.1093/emboj/17.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saxena K, Drosou V, Maier E, Benz R, Ludwig B. Biochemistry. 1999;38:2206–2212. doi: 10.1021/bi982296f. [DOI] [PubMed] [Google Scholar]
- 12.Bayley H. Curr Op Biotechnol. 1999;10:94–103. doi: 10.1016/s0958-1669(99)80017-2. [DOI] [PubMed] [Google Scholar]
- 13.Lear J D, Schneider J P, Kienker P K, DeGrado W F. J Am Chem Soc. 1997;119:3212–3217. [Google Scholar]
- 14.Qi Z, Sokabe M, Donowaki K, Ishida H. Biophys J. 1999;76:631–641. doi: 10.1016/S0006-3495(99)77231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starostin A V, Butan R, Borisenko V, James D A, Wenschuh H, Sansom M S P, Woolley G A. Biochemistry. 1999;38:6144–6150. doi: 10.1021/bi9826355. [DOI] [PubMed] [Google Scholar]
- 16.Gu L-Q, Braha O, Conlan S, Cheley S, Bayley H. Nature (London) 1999;398:686–690. doi: 10.1038/19491. [DOI] [PubMed] [Google Scholar]
- 17.Song L, Hobaugh M R, Shustak C, Cheley S, Bayley H, Gouaux J E. Science. 1996;274:1859–1865. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 18.Füssle R, Bhakdi S, Sziegoleit A, Tranum-Jensen J, Kranz T, Wellensiek H-J. J Cell Biol. 1981;91:83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krasilnikov O V, Sabirov R Z, Ternovsky V I, Merzliak P G, Muratkhodjaev J N. FEMS Microbiol Immunol. 1992;105:93–100. doi: 10.1111/j.1574-6968.1992.tb05891.x. [DOI] [PubMed] [Google Scholar]
- 20.Bezrukov S M, Vodyanoy I, Brutyan R A, Kasianowicz J J. Macromolecules. 1996;29:8517–8522. [Google Scholar]
- 21.Kasianowicz J J, Brandin E, Branton D, Deamer D W. Proc Natl Acad Sci USA. 1996;93:13770–13773. doi: 10.1073/pnas.93.24.13770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menestrina G. J Membr Biol. 1986;90:177–190. doi: 10.1007/BF01869935. [DOI] [PubMed] [Google Scholar]
- 23.Vincent J B, Kirby D M, Nguyen T V, Vigh G. Analyt Chem. 1997;69:4419–4428. doi: 10.1021/ac970418o. [DOI] [PubMed] [Google Scholar]
- 24.Cheley S, Braha O, Lu X, Conlan S, Bayley H. Protein Sci. 1999;8:1257–1267. doi: 10.1110/ps.8.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petosa C, Collier R J, Klimpel K R, Leppla S H, Liddington R C. Nature (London) 1997;385:833–838. doi: 10.1038/385833a0. [DOI] [PubMed] [Google Scholar]
- 26.Bhakdi S, Füssle R, Tranum-Jensen J. Proc Natl Acad Sci USA. 1981;78:5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walker B J, Krishnasastry M, Zorn L, Kasianowicz J J, Bayley H. J Biol Chem. 1992;267:10902–10909. [PubMed] [Google Scholar]
- 28.Braha O, Walker B, Cheley S, Kasianowicz J J, Song L, Gouaux J E, Bayley H. Chem Biol. 1997;4:497–505. doi: 10.1016/s1074-5521(97)90321-5. [DOI] [PubMed] [Google Scholar]
- 29.Montal M, Mueller P. Proc Natl Acad Sci USA. 1972;69:3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zemaitis J F, Clark D M, Rafal M, Scriver N. Handbook of aqueous electrolyte thermodynamics: theory and application. New York, NY: American Institute of Chemical Engineers; 1986. [Google Scholar]
- 31.Krasilnikov O V, Capistrano M-F P, Yuldasheva L N, Nogueira R A. J Membr Biol. 1997;156:157–172. doi: 10.1007/s002329900198. [DOI] [PubMed] [Google Scholar]
- 32.Nonner W, Chen D P, Eisenberg B. J Gen Physiol. 1999;113:773–782. doi: 10.1085/jgp.113.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller C. J Gen Physiol. 1999;113:783–787. doi: 10.1085/jgp.113.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levitt D G. J Gen Physiol. 1999;113:789–794. doi: 10.1085/jgp.113.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakobsson E. Methods. 1998;14:342–351. doi: 10.1006/meth.1998.0589. [DOI] [PubMed] [Google Scholar]
- 36.Kienker P K, Lear J D. Biophys J. 1995;68:1347–1358. doi: 10.1016/S0006-3495(95)80307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenman G, Horn R. J Membr Biol. 1983;76:197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- 38.Kellenberger S, Gautschi I, Schild L. Proc Natl Acad Sci USA. 1999;96:4170–4175. doi: 10.1073/pnas.96.7.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dieckmann G R, Lear J D, Zhong Q F, Klein M L, DeGrado W F, Sharp K A. Biophys J. 1999;76:618–630. doi: 10.1016/S0006-3495(99)77230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeffrey G A, Saenger W. In: Hydrogen bonding in biological structures. Jeffrey G A, Saenger W, editors. Berlin: Springer; 1991. pp. 309–350. [Google Scholar]
- 41.Tabushi I, Kuroda Y, Yokota K. Tetrahedron Lett. 1982;23:4601–4604. [Google Scholar]
- 42.Pregel M J, Jullien L, Lehn J-M. Angew Chem Int Ed Engl. 1992;31:1637–1639. [Google Scholar]
- 43.Pregel M J, Jullien L, Canceill J, Lacombe L, Lehn J-M. J Chem Soc Perkin Trans. 1995;2:417–426. [Google Scholar]
- 44.Odashima K, Kotato M, Sugawara M, Umezawa Y. Analyt Chem. 1993;65:927–936. [Google Scholar]
- 45.Duax W L, Griffin J F, Langs D A, Smith G D, Grochulski P, Pletnev V, Ivanov V. Biopolymers. 1996;40:141–155. doi: 10.1002/(SICI)1097-0282(1996)40:1%3C141::AID-BIP6%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]