Abstract
We tested whether signaling pathways induced by systemin, oligosaccharide elicitors (OEs), and ultraviolet (UV)-B radiation share common components in Lycopersicon peruvianum suspension-cultured cells. These stress signals all induce mitogen-activated protein kinase (MAPK) activity. In desensitization assays, we found that pretreatment with systemin and OEs transiently reduced the MAPK response to a subsequent treatment with the same or a different elicitor. In contrast, MAPK activity in response to UV-B increased after pretreatment with systemin and OEs. These experiments demonstrate the presence of signaling components that are shared by systemin, OEs, and UV-B. Based on desensitization assays, it is not clear if the same or different MAPKs are activated by different stress signals. To identify specific stress-responsive MAPKs, we cloned three MAPKs from a tomato (Lycopersicon esculentum) leaf cDNA library, generated member-specific antibodies, and performed immunocomplex kinase assays with extracts from elicited L. peruvianum cells. Two highly homologous MAPKs, LeMPK1 and LeMPK2, were activated in response to systemin, four different OEs, and UV-B radiation. An additional MAPK, LeMPK3, was only activated by UV-B radiation. The common activation of LeMPK1 and LeMPK2 by many stress signals is consistent with the desensitization assays and may account for substantial overlaps among stress responses. On the other hand, MAPK activation kinetics in response to elicitors and UV-B differed substantially, and UV-B activated a different set of LeMPKs than the elicitors. These differences may account for UV-B-specific responses.
Plants have developed sophisticated defensive and protective responses to the various forms of stress they encounter in their environment. It has become increasingly evident that the underlying stress signaling pathways overlap and interact substantially. Although there is a remarkable specificity for stress signal perception, the signaling pathways and the resulting responses often appear to be rather unspecific. On an evolutionary scale, this may provide a basis for rapid and versatile adaptations to changing environmental challenges. For an individual plant, this may allow for an efficient response to several stressors present at the same time, a scenario plants often encounter in their environment.
The systemin-mediated wound response in tomato plants is a well-investigated stress response. Systemin, an 18-amino acid wound-signaling peptide, is produced at wound sites in response to attack by herbivorous insects, and is perceived by the systemin receptor SR160, a Leu-rich repeat receptor kinase (Scheer and Ryan, 2002). Systemin is required for the systemic expression of defensive proteinase inhibitor (PI) genes (for review, see Ryan, 2000) via production of the long-distance wound signal jasmonic acid (Lee and Howe, 2003). PIs may also function in pathogen responses because PI genes are expressed in response to oligosaccharide elicitors (OEs; Walker-Simmons and Ryan, 1984; Doares et al., 1995; Howe et al., 1996; Ramonell et al., 2002). OEs are general (nonhost-specific) elicitors derived from fungal or plant cell wall material and released in response to fungal, oomycyte, or insect attack. The elicitors are perceived by specific cell surface receptors but largely activate the same signaling components as systemin, including ion fluxes (Felix and Boller, 1995; Moyen et al., 1998; Lecourieux et al., 2002), phospholipase A2 (Narváez-Vásquez et al., 1999), NADPH oxidase (Low and Merida, 1996; Orozco-Cárdenas et al., 2001), jasmonate (Doares et al., 1995; Howe et al., 1996), and ethylene (O'Donnell et al., 1996). PIs are known to interfere with digestive proteases in insect guts. A dysfunctional PI defense system has been shown to severely compromise the resistance of tomato plants against herbivorous insects (Orozco-Cardenas et al., 1993). Consistent with their synthesis in response to pathogen-derived elicitors, PIs have been shown to possess antimicrobial properties (Pautot et al., 1991; Linthorst et al., 1993; Terras et al., 1993; Giudici et al., 2000).
Responses to biotic stressors often overlap with responses to abiotic stress such as UV-B radiation (280–320 nm), a portion of solar radiation that reaches the surface of the earth and is well known to cause damage to cellular macromolecules in plants (Jansen et al., 1998; Mazza et al., 1999). UV-B coordinates the regulation of sets of defense-related and other genes that are activated via different signaling pathways involving reactive oxygen species, salicylic acid, jasmonate, and ethylene (Green and Fluhr, 1995; A.-H.-Mackerness et al., 1999, 2001; Brosché et al., 2002). We had previously shown that UV-B activates components of the systemin signaling pathway and provided evidence that UV-B may activate the systemin receptor SR160 (Yalamanchili and Stratmann, 2002). It is unlikely that receptor activation by UV-B is limited to the systemin receptor; other membranebound receptors may also be activated. Receptor activation in response to UV radiation is well known in animal epidermal cells where a range of growth factor/cytokine receptors are activated by UV-B (Sachsenmaier et al., 1994; Herrlich and Böhmer, 2000). For the epidermal growth factor receptor (EGFR), it was suggested that oxidative UV stress leads to inactivation of a receptor Tyr phosphatase, which results in increased intrinsic EGFR activity (Gross et al., 1999). Alternatively, UV may activate a Src Tyr kinase that, in turn, activates EGFR via extrinsic phosphorylation (Kitagawa et al., 2001). A nonspecific ligand-independent receptor activation in plant cells would be expected to result in the engagement of different receptor-mediated signaling pathways. This hypothesis is supported by our previous work showing that a UV-B pretreatment of L. peruvianum cells transiently desensitized a subsequent response to systemin, chitosan, or β-glucan (Yalamanchili and Stratmann, 2002; J. W. Stratmann, unpublished data). Moreover, in tomato plants, UV-B radiation potentiated the response to a subsequent wounding (Stratmann et al., 2000b), and UV-C radiation induced PI synthesis in a jasmonate-dependent manner (Conconi et al., 1996).
On the transcriptome scale, DNA microarray technology revealed extensive overlaps in gene expression patterns among various stress responses, indicating the existence of an extensive network of regulatory interactions (Durrant et al., 2000; Reymond et al., 2000; Schenk et al., 2000; Desikan et al., 2001; Brosché et al., 2002; Ramonell et al., 2002). Conversely, other sets of genes were found to be specifically induced by only one treatment, indicating the existence of specific signaling routes in addition to shared pathways. It will be a challenging task for the future to unravel how specificity and commonality among stress responses are established by signal transduction processes.
Many stress signals are relayed via a mitogen-activated protein kinase (MAPK) module consisting of three functionally interlinked kinases. In Arabidopsis, approximately 60 MAPK kinase kinases (MAPKKK) have been identified based on sequence homology to known MAPKKKs. In contrast, only 10 putative MAPKK were identified. MAPKKs are activated by MAPKKKs and in turn activate approximately 20 putative MAPKs (Ichimura et al., 2002; Jonak et al., 2002). MAPK substrates have yet to be identified in plants. In yeast and metazoans, they include transcription factors and cytoplasmic proteins such as phospholipase A2 (Lin et al., 1993). Based on their relative number, MAPKKKs may be activated by stress signals in a specific manner. In contrast, MAPKKs and MAPKs often integrate different input signals by acting as points of convergence or divergence. For example, the alfalfa (Medicago sativa) MAPKKs, SIMKK and PRKK, can activate two or three MAPKs, and a specific MAPK, SIMK, is activated by SIMKK and PRKK (Cardinale et al., 2002). Similar signaling patterns were found in Arabidopsis (Asai et al., 2002) and tobacco (Nicotiana tabacum; Yang et al., 2001).
As a first attempt to unravel the stress signaling network in Lycopersicon peruvianum cells, we focused on stress-responsive MAPKs. We had previously shown that a range of biotic and abiotic stress signals such as a rapid hydraulic signal generated by wounding, systemin, the signaling peptide RALF, UV-B radiation, and the OEs polygalacturonic acid (PGA), chitosan (oligoglucosamine), and β-glucan all induce 48-kD kinase activity in tomato (Lycopersicon esculentum) plants and L. peruvianum suspension-cultured cells. The kinase became rapidly activated, phosphorylated myelin basic protein (MBP), and its activation was associated with Tyr phosphorylation of the kinase. This indicated that it belongs to the MAPK family of protein kinases (Stratmann et al., 1997, 2000a, 2000b; Pearce et al., 2001). In L. esculentum suspension-cultured cells, an MAPK-like enzyme of similar size was also activated by chitin fragments (chitotetraose), xylanase, osmotic shock (Felix et al., 2000), nonmetabolizable sugars (Sinha et al., 2002), a Fusarum oxysporum lycopersici elicitor preparation, voltage application, (Link et al., 2002a), and heat (Link et al., 2002b).
Our analysis of The Institute for Genomic Research (Rockville, MD) tomato gene index (http://www.tigr.org/tdb/tgi/lgi/) revealed at least 11 tentative consensus sequences corresponding to different MAPKs. To understand if and how specificity in stress responses may be established at the level of MAPKs, it is important to identify interactions among stress signaling pathways. Moreover, individual MAPKs responding to particular stress signals must be identified to find out if multiple stress signals converge on a particular MAPK or if different MAPK cascades are activated by different forms of stress. Here, we provide evidence for extensive overlaps between stress signaling pathways by showing that a pretreatment with systemin or OEs affects the MAPK response to a different subsequently supplied elicitor and UV-B. Consistent with these results, we identified two tomato MAPKs that became activated by all stress signals tested. An additional MAPK was activated only by UV-B radiation.
RESULTS
Time-Course Analysis of MAPK Activity in Response to Elicitors
To identify differences among elicitor-induced MAPK pathways in L. peruvianum cells, we performed a time-course analysis for MAPK activity in response to systemin and four OEs using in-gel kinase assays. In response to all elicitors, MAPK activity reached a maximum at 10 min after application (Fig. 1). The activity induced by systemin was more prolonged than in response to the OEs and declined to background levels after 90 to 120 min. The MAPK activity induced by OEs declined to background levels within 1 h. The duration of the response to chitin and β-glucan was consistently shorter than in response to chitosan and PGA.
Figure 1.
Time-course analysis of MAPK activity induced by systemin and OEs. L. peruvianum cells were left untreated or were supplied with systemin (3 nm) or the OEs chitin (100 μm chitotetraose), chitosan (1.7 μg mL–1), β-glucan (10 μg mL–1), and PGA (830 μg mL–1). At the times indicated, samples were quick-frozen and assayed for MAPK activity in in-gel kinase assays. Radioactive bands were visualized by phosphoimaging. Arrows with numbers indicate the apparent molecular masses of systemin- and OE-responsive MAPKs.
Elicitor Pretreatment Affects the Response to a Second Treatment with the Same Elicitor
Using desensitization assays, we had shown previously that systemin pretreatment transiently prevented a subsequent activation of systemin-responsive MAPK by systemin (Yalamanchili and Stratmann, 2002). We tested whether such a refractory state would also be caused by the OEs chitosan, chitotetraose (chitin), β-glucan, and PGA. L. peruvianum cells pretreated with an elicitor at time 0 were treated a second time with the same elicitor 30, 60, 90, and 120 min later (Fig. 2). Ten min after the last treatment, at maximal MAPK activity levels in control cells (see Fig. 1), cells were assayed for MAPK activity by an in-gel kinase assay. At this time, the kinase activity in control samples treated only at time 0 (lane 2) had declined to near background levels (lane 1). As expected, cells treated only at the time of the second treatment showed high levels of kinase activity (lane 3). Cells treated consecutively at 0 and 30 min showed a strongly reduced kinase response to the second treatment (compare lanes 3 and 4, 30 min). The response to the second treatment after an initial pretreatment gradually recovered, and 120 min after the initial treatment, the response to the second treatment was almost as strong as the one induced by the same treatment without initial pretreatment (compare lane 4 with lane 3, all times). The recovery time course for chitin was more prolonged, but was faster than the recovery in systemin-treated cells, which took at least 3 h (Yalamanchili and Stratmann, 2002). The fastest recovery was observed after consecutive PGA treatments (approximately 60 min). This indicated that OEs activate a signaling pathway that undergoes a period of transiently reduced responsiveness after the initial stimulation.
Figure 2.
MAPK activity in response to consecutive OE treatments. L. peruvianum suspension-cultured cells were pretreated with the OEs chitin (100 μm chitotetraose), chitosan (1.7 μg mL–1), β-glucan (10 μg mL–1), or PGA (830 μg mL–1), or were left untreated. At 30, 60, 90, and 120 min thereafter, treated and untreated cells were left untreated or were supplied a second time with the same OE. Ten minutes thereafter, cells were quick-frozen and assayed for MAPK activity. Samples corresponding to a given time point (lanes 1–4) were taken at the same time. Lane 1, Untreated control. Lane 2, MAPK activity in response to the pretreatment. Lane 3, MAPK activity in response to the second treatment without pretreatment. Lane 4, MAPK activity in response to the second treatment after a pretreatment.
Elicitor Pretreatment Affects the Response to a Second Treatment with a Different Elicitor or UV-B Radiation
The refractory behavior of signaling pathways can be used to examine potential interactions among different signaling pathways. A pretreatment with a particular elicitor that transiently affects the MAPK response to a subsequent treatment with a different stress signal indicates that certain signaling compounds are activated by both elicitors. L. peruvianum cells were initially treated with OEs or systemin. Thirty minutes later, when the responsiveness of the cells was strongly reduced (Fig. 2), the cells were treated a second time with the same elicitor or with a different elicitor. The experimental setup is as shown in Figure 2. Cells were assayed at maximal MAPK activity levels, 10 min after the second treatment (see Fig. 1). The full response (100%) was defined as the response to the second treatment alone. Figure 3A shows that pretreatment with chitosan, chitotetraose, or β-glucan strongly diminished the response to a subsequent treatment with all four OEs (approximately 10% of the full response). A pretreatment with systemin or PGA reduced the response to all four OEs more moderately (30%–50% of the full response). On the other hand, the OEs reduced the response to systemin only weakly (50%–60% of the full response) or hardly at all in the case of PGA. Controls, in which the cells were treated consecutively with the same elicitor, confirmed the results shown in Figure 2. In the experiments involving consecutive treatments with different elicitors, the responsiveness to the second treatment recovered over time (data not shown).
Figure 3.
MAPK activity in response to consecutive treatments with systemin, OEs, and UV-B. The experimental setup was as in Figure 2. A, L. peruvianum cells were pretreated with the OEs chitosan (CHO), chitotetraose (CH4), β-glucan (GLU), or PGA, with systemin (SYS), or were left untreated. Elicitor concentrations were as indicated in Figure 2. At 30 min thereafter, treated and untreated cells were left untreated or were supplied a second time with a different elicitor. Ten minutes thereafter, cells were quick-frozen and assayed for MAPK activity. The increase in MAPK activity in response to the second treatment after a pretreatment was expressed as percentage of the full response (100%), defined as the response to the second treatment without a pretreatment. B, Cells were pretreated as described in A or were left untreated. At 30 min thereafter, treated and untreated cells were left untreated or were irradiated with UV-B for 5 min. Ten and 90 min after the start of irradiation, cells were frozen and assayed for MAPK activity. The increase in MAPK activity was expressed as in A.
We had previously shown that UV-B activates 48-kD MAPK activity in L. peruvianum cells. A 5-min UV-B irradiation period resulted in a prolonged biphasic MAPK response with two peak activities at 10 and 90 min after the start of irradiation (Yalamanchili and Stratmann, 2002). To test if the elicitors would affect the MAPK response to UV-B radiation, L. peruvianum cells were pretreated with a particular elicitor and 30 min later were irradiated with UV-B for 5 min. The effects of an elicitor pretreatment at both peaks was tested by assaying MAPK activity 10 and 90 min after start of UV-B irradiation (i.e. 40 or 120 min after pretreatment, respectively). The four OEs caused an approximately 50% reduction in the MAPK response to UV-B when measured 10 min after start of irradiation (Fig. 3B). Systemin had a weaker effect on UV-B (approximately 25% reduction), consistent with our previous results (Yalamanchili and Stratmann, 2002). Surprisingly, MAPK activity was not reduced, but rather increased above the response to UV-B alone when MAPK activity was assayed 90 min after start of irradiation (Fig. 3B). The synergistic effect was very strong after systemin pretreatment, with a UV-induced MAPK activity of 300% ± 75% of the full response to UV-B alone. OE pretreatment resulted in UV-B responses of approximately 150% of the full response (for PGA, statistically not significant). This indicates that the two MAPK activities at 10 and 90 min are regulated differently. Taken together, the data suggest a substantial interaction among signaling pathways for systemin, OEs, and UV-B.
Cloning of Three Tomato MAPKs with Homology to Known Stress-Responsive MAPKs
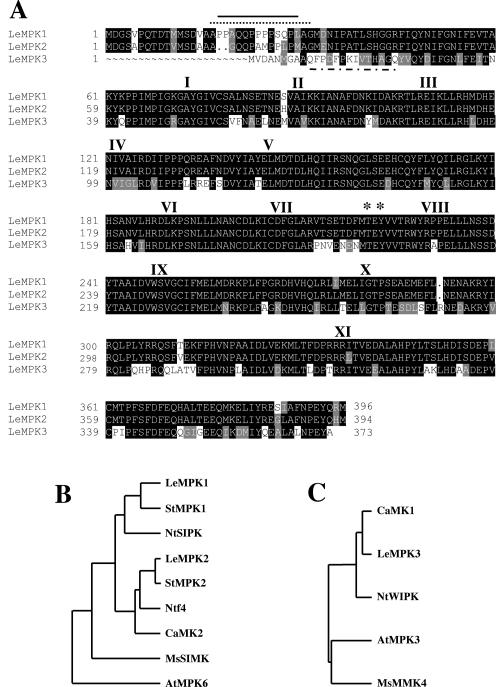
In the previous experiments, MAPK activation served as a marker response to identify interactions among elicitor- and UV-B-induced signaling pathways. However, multiple MAPKs with a similar apparent Mr may have been activated in these experiments. To distinguish between individual MAPKs, we cloned tomato MAPKs, generated member-specific antibodies, and performed immunocomplex kinase assays. MAPKs were cloned based on homology to the two major stress-responsive MAPKs in tobacco, SIPK and WIPK (Seo et al., 1995; Zhang and Klessig, 1997). Three full-length cDNAs were obtained from a L. esculentum leaf cDNA library. The deduced amino acid sequence of the three putative kinases revealed the 11-kinase subdomain consensus sequence and the dual phosphorylation TEY motif common to most plant MAPKs (Fig. 4A). They were named LeMPK for Lycopersicon esculentum MAPK. LeMPK1, LeMPK2, and LeMPK3 have predicted molecular masses of 45.5, 45.2, and 42.8 kD, respectively and consist of 396, 394, and 373 amino acid residues, respectively. LeMPK1 and LeMPK2 are 95.4% and 89.2% identical at the amino acid and nucleotide level, respectively.
Figure 4.
Amino acid sequence of three L. esculentum MAPKs. A, The deduced amino acid sequences of LeMPKs 1, 2, and 3 were aligned. Identical and conserved amino acids are shaded in black and gray, respectively. Dots represent gaps. Roman numerals represent the 11 conserved kinase subdomains. Asterisks show the Thr and Tyr residues in the TEY phosphorylation motif. The solid line, the dotted line, and the dashed line represent the sequences used as antigenic peptides to generate specific antibodies against LeMPKs 1, 2, and 3, respectively. B, A phylogenetic tree shows the relationship among the A2 group of plant MAPKs (Ichimura et al., 2002). C, A phylogenetic tree shows the relationship among the A1 group of plant MAPK (Ichimura et al., 2002). The tree was created by the GrowTree method from the GCG Wisconsin Package. Accession numbers: StMPK1, AB062138; StMPK2, AB062139; NtSIPK, AAB58396; Ntf4, Q40532; CaMK2, AF247136; MsSIMK, X66469; AtMPK6, S40472; CaMK1, AF247135; NtWIPK, BAA09600; AtMPK3, Q39023; and MsMMK4, X82270.
LeMPKs are highly homologous to putative orthologs of closely related solanaceous species, including tobacco SIPK and WIPK, and less homologous to putative orthologs from Arabidopsis (Brassicacea) and alfalfa (Fabaceae; Fig. 4, B and C).
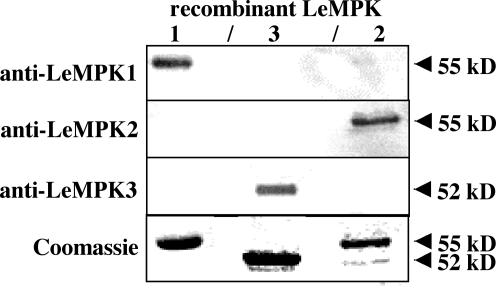
Antibodies Specifically Recognize Recombinant LeMPKs
To identify individual MAPKs, polyclonal antibodies were raised against short peptides corresponding to divergent amino acid stretches at the N-termini of the three LeMPKs (Fig. 4A). To test the specificity of the antisera, recombinant LeMPKs were expressed as His-tagged fusion proteins in Escherichia coli, affinity purified, and separated by SDS-PAGE. The His-tag and linker sequences added 6 kD to the molecular mass of the recombinant proteins. The recombinant proteins showed an altered mobility on SDS-polyacrylamide gels with an apparent molecular mass of 55 kD (LeMPKs 1 and 2) and 52 kD (LeMPK3; Fig. 5, Coomassie). They were specifically recognized by the antibodies raised against the corresponding antigenic peptides (Fig. 5). There was no crossreactivity, despite some sequence identity between the antigenic peptides of LeMPK1 and LeMPK2. Affinity-purified antibodies produced the same results.
Figure 5.
Anti-LeMPK antibodies specifically recognize LeMPKs. Polyclonal antibodies were raised against the N-termini of LeMPKs shown in Figure 4. Recombinant LeMPKs were expressed in E. coli and affinity purified. Five hundred nanograms of each recombinant LeMPK was analyzed by immunoblotting on three separate membranes with anti-LeMPK1, anti-LeMPK2, and anti-LeMPK3 antisera. Antibody-LeMPK immunocomplexes were visualized using an alkaline phosphatase-coupled colorimetric assay. On a separate gel, recombinant LeMPKs were stained with Coomassie Brilliant Blue. Arrowheads with numbers indicate the apparent molecular masses of the recombinant proteins.
Multiple Stress Signals Activate LeMPK1 and LeMPK2
To match a stress signal with a corresponding MAPK, L. peruvianum cells were supplied with systemin, or the OEs chitotetraose, chitosan, β-glucan, and PGA. Ten minutes later, at maximal MAPK activity (see Fig. 1), cells were harvested and assayed in immunocomplex kinase assays. Cell extracts were immunoprecipitated with anti-LeMPK antisera. In controls, antisera were incubated with antigenic peptides corresponding to LeMPKs 1, 2, and 3 before the addition of extracts. Immunoprecipitated proteins were tested for their ability to phosphorylate the artificial MAPK substrate MBP. Figure 6 shows that active LeMPK1 and LeMPK2 were immunoprecipitated in a highly specific manner. Only the peptide against which the respective antibody was raised functioned as a competitor. This proved that the antibodies specifically recognize the respective corresponding LeMPKs in immunocomplex kinase assays.
Figure 6.
Systemin and OEs activate LeMPK1 and LeMPK2 in immunocomplex kinase assays. L. peruvianum cells were left untreated (control), or were supplied with systemin or OEs for 10 min (elicitor). Systemin and OE concentrations were as indicated in Figure 1, respectively. Extracts were prepared, incubated with anti-LeMPK1, 2, or 3 antisera (MPK1, 2, 3), and protein G Sepharose. After washing, immunocomplexes were incubated with [γ-32P]ATP and MBP. Phosphorylated radioactive MBP was separated by SDS-PAGE and was visualized by phosphoimaging. To demonstrate specificity of the anti-LeMPK antisera, antisera were preincubated with an excess of competitor peptides derived from the N-termini of LeMPKs 1, 2, and 3 (peptide 1, 2, and 3) before the addition of extracts.
Figure 6 shows that systemin and the four OEs chitosan, chitotetraose, β-glucan, and PGA all activated LeMPK1 and LeMPK2. In contrast, LeMPK3 was not activated by any of the elicitors. When MAPKs in systemin-treated extracts were immuno-complexed and subsequently tested for activity in in-gel kinase assays, similar results were obtained (data not shown), confirming the results obtained in immunocomplex kinase assays. These data show that two highly homologous LeMPKs are coordinately activated by elicitors of plant defense responses and represent two points of convergence for multiple stress signals.
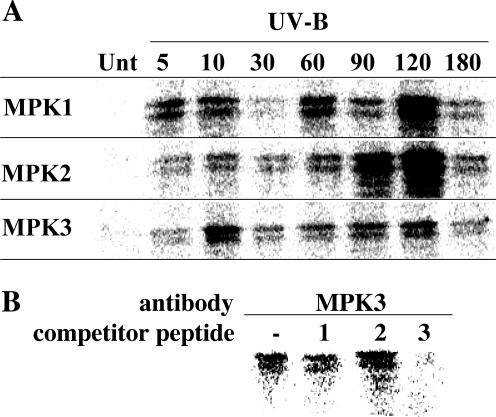
UV-B Coordinately Activates LeMPKs 1, 2, and 3 in a Biphasic Manner
UV-B radiation (280–320 nm) leads to induction of MAPK activity in L. peruvianum cells. The UV-B-induced activation kinetics differed strikingly from the elicitor-induced kinetics by following a prolonged biphasic time course (Yalamanchili and Stratmann, 2002). Using immunocomplex kinase assays, we tested whether different MAPKs would contribute to the overall MAPK activity during the two peak periods. L. peruvianum cells were irradiated for 5 h with UV-B and were assayed for MAPK activity at various times thereafter. We found that UV-B activated not only LeMPKs 1 and 2, but also LeMPK3 (Fig. 7A). All three LeMPKs followed similar time courses, showing an initial peak activity at 5 to 10 min followed by a decrease and a second stronger peak activity at 90 to 120 min. The antigenic peptide corresponding to LeMPK3 interfered specifically with immunoprecipitation of LeMPK3, demonstrating that anti-LeMPK3 antibodies specifically recognize LeMPK3 in immuno-complex kinase assays (Fig. 7B).
Figure 7.
Time-course analysis of LeMPK1, 2, and 3 activity induced by UV-B. A, L. peruvianum cells were left untreated (Unt) or were irradiated for 5 min with UV-B. At the times indicated, samples were quick-frozen and assayed for MAPK activity in immunocomplex kinase assays with anti-LeMPK1, 2, and 3 antisera (MPK1, 2, and 3) as described in Figure 6. A representative experiment is shown; the exact timing and magnitude of the response varied slightly among different experiments. B, To demonstrate specificity of the anti-LeMPK3 antiserum, antiserum was preincubated with an excess of competitor peptides and was thereafter incubated with extract derived from the 10-min UV-B sample shown in A.
DISCUSSION
We had shown previously that diverse biotic and abiotic stress signals lead to a rapid increase of 48-kD MAPK activity. Here, we show that signaling pathways triggered by systemin, OEs, and UV-B radiation substantially overlap and converge at the level of two highly homologous stress-responsive MAPKs, indicating the presence of an extensive stress signaling network in L. peruvianum suspension-cultured cells.
To reveal overlaps among signaling pathways, we exploited a common feature of many signaling processes. Reversibly regulated signaling components are inactivated by degradation or posttranslational modifications after an initial activation. During this refractory period, these components are less responsive to a subsequent stimulation with the same signal (Felix et al., 1998; Meskiene and Hirt, 2000). Later, the inactivating factors may be down-regulated and the signaling components regain their potential to become activated (Fig. 2 and Yalamanchili and Stratmann, 2002). We tested this signaling behavior using desensitization assays and found that systemin, OEs, and UV-B radiation mutually affect their capacity to induce MAPK activity, indicating the presence of signaling components that are activated by all of these stress signals. Systemin- and OE-responsive signaling compounds (see Introduction) may function in similar but separate signaling pathways, tissues, or cellular compartments. The cross-desensitization experiments shown in Figure 3 rule this out and demonstrated that identical signaling components are activated by systemin, OEs, and UV-B. In these desensitization assays, MAPK activation served as a reporter response. MAPKs are reversibly phosphorylated and may undergo a refractory state upon activation. However, conceptually, any reversibly activated component between a receptor and the MAPK may contribute to the refractory periods observed.
Activation of multiple MAPKs may account for the different extents to which stress signals affected each other in desensitization assays. However, in these assays, it was not possible to identify individual MAPKs. Therefore, we cloned and sequenced tomato MAPKs and generated specific antibodies to match a particular stress signal with a particular MAPK. Although MAPKs comprise a family of approximately 20 genes in Arabidopsis (Ichimura et al., 2002; Jonak et al., 2002), there are only three or four MAPKs that are known to be stress responsive in a single plant species. In tobacco, SIPK and WIPK mediate responses to a wide range of stress signals (Zhang and Klessig, 2000). LeMPK3 is highly homologous to the tobacco WIPK. LeMPK1 and LeMPK2 show very high sequence homology (approximately 95%) to SIPK and another tobacco MAPK, Ntf4. LeMPK1 and LeMPK2 belong to the A2 group of plant MAPKs, and LeMPK3 belongs to group A1, according to the classification scheme suggested by Ichimura et al. (2002). Interestingly, only the solanaceous species tobacco, potato (Solanum tuberosum), and tomato possess two highly homologous MAPKs of the A2 group, SIPK/Ntf4, StMPK1/2, and LeMPK1/2, respectively, suggesting a gene duplication event in an ancestral solanaceous species (Fig. 4B). In parsley (Petroselinum crispum), two highly homologous WIPK orthologs have been cloned that are both activated by the Pep-13 elicitor, suggesting that in different angiosperm lineages, different stress-responsive MAPK genes were duplicated (Kroj et al., 2003).
SIPK and LeMPK1/2, or WIPK and LeMPK3, show a high overall sequence homology. However, the N-termini against which SIPK- or WIPK-specific antibodies had been raised (Zhang and Klessig, 1998; Seo et al., 1999) are highly variable, precluding the use of SIPK- or WIPK-specific antibodies to target LeMPKs. Therefore, we raised antibodies that specifically recognized unique N-terminal amino acid stretches of the three LeMPKs, and demonstrated that systemin activates LeMPK1 and LeMPK2 in suspension-cultured L. peruvianum cells. We then showed that all four OEs activated the same two MAPKs as systemin. Based on the intensity of the signals and the almost equal potency of the antibodies as determined in immunoblots (Fig. 5), the magnitude of the LeMPK1 and LeMPK2 response to systemin and OEs appears to be similar. This similarity, and the high sequence homology of LeMPK1 and LeMPK2, suggest that they are functionally redundant. However, it is possible that the two MAPKs have additional specific functions, depending on their interacting proteins and the cell types in which they are expressed. This is exemplified by the tobacco SIPK homolog Ntf4, which is regulated by developmental cues in reproductive tissues (Wilson et al., 1995; Voronin et al., 2001).
LeMPK1 and LeMPK2 are activated by systemin and OEs. We found that these LeMPKs were also activated in response to leaf wounding in L. esculentum plants (S. R. Holley and J. W. Stratmann, unpublished data). Activation of the same MAPKs is consistent with a common regulation of wound-response gene expression by wounding, systemin, and OEs. However, it is not known whether systemin and OEs activate additional elicitor-specific genes. The reciprocal effects of the OEs and systemin in desensitization assays indicate the presence of additional signal-specific components. Moreover, some differences among systemin and OEs were noted regarding the duration of MAPK activation (Fig. 1). We also cannot exclude the participation of additional LeMPKs that were not recognized by our antibodies. These differences could potentially result in signal-specific gene expression patterns leading to defense responses tailored to a particular stressor. Magnitude and duration of MAPK activation has been suggested to establish signal specificity in yeast and mammalian cells (Sabbagh et al., 2001) Marshall, 1995). Additional mechanisms, such as tethering of MAPK pathway kinases to scaffolding proteins, are also known to confer signal specificity via MAPK pathways (Breitkreutz and Tyers, 2002; Smith and Scott, 2002; Park et al., 2003).
Homologous MAPKs in different plant species may not perform the exact same function. In photoautotrophic L. esculentum cells, chitosan and PGA activated a MAPK with putative homology to SAMK, an alfalfa ortholog of LeMPK3 (Link et al., 2002a). This discrepancy with our results may be explained by the species difference or by the heterologous anti-SAMK antibodies used by Link et al. (2002a), which may crossreact with LeMPK1/2. In tobacco, it was shown that UV-C radiation activated only SIPK, but not WIPK, the ortholog of the UV-B-responsive LeMPK3 (Miles et al., 2002). On the other hand, WIPK was activated by oligogalacturonides in tobacco cells (Droillard et al., 2000), but LeMPK3 did not respond to oligogalacturonides (PGA). Chitin activated LeMPK1 and LeMPK2 in L. peruvianum cells and the putative ortholog SIMK in alfalfa cells (Cardinale et al., 2000), but it did not activate the putative parsley ortholog PcMPK6 or the LeMPK3 orthologs PcMPK3a and 3b (Kroj et al., 2003). Thus, relatively invariable stress signals such as UV radiation or the general elicitors PGA (oligogalacturonides) and chitin appear to activate different MAPK pathways in different species. Does this result in species-specific responses to the same stressor? Or does a given stress signal use different MAPK conduits in different species to achieve the same general output response?
We had shown previously that the abiotic stressor UV-B strongly desensitized tomato cells to a subsequent elicitation by systemin. Accordingly, an initial systemin treatment reduced the UV-B response, albeit to a lesser degree (Yalamanchili and Stratmann, 2002). In L. peruvianum cells, MAPK activity induced by UV-B follows a biphasic time course. When cells were pretreated with OEs and systemin, UV-B-responsive MAPK activity at the first activity peak was reduced. Surprisingly, MAPK activity at the 90-min peak was increased (150%) above levels induced by UV-B alone (100%), particularly after a pretreatment with systemin (300%). This indicates the presence of shared signaling components that are activated by elicitors of plant defense responses and UV-B radiation. Consistent with these results is our finding that UV-B and elicitors all activate LeMPK1 and LeMPK2. The different effects on the UV-B response measured at 10 and 90 min after irradiation indicate that the two MAPK activity phases are differentially regulated. A possible explanation for the two phases may be the involvement of different MAPKs with different activation kinetics. We found that LeMPK3, in addition to LeMPK1 and LeMPK2, was activated in response to UV-B radiation. However, all three kinases follow a similar biphasic time course (Fig. 7). These data suggest that an initial UV-B signal rapidly and transiently activates all three MAPKs and generates a secondary signal that induces the second activity phase after a brief refractory period. A possible candidate for such a secondary signal would be reactive oxygen species, which are generated in response to many different forms of stress, including UV-B (Green and Fluhr, 1995).
UV-B has multiple effects on plants and is known to activate several signaling pathways (A.-H.-Mackerness et al., 1999; Brosché and Strid, 2003), including the systemin signaling pathway (Yalamanchili and Stratmann, 2002). Interestingly, many Nicotiana longiflora (Solanaceae) genes, which are regulated by chewing Manduca sexta larvae or their oral secretions (Halitschke et al., 2003), were found to be regulated in the same way by UV-B radiation under natural field conditions (Izaguirre et al., 2003). Brosché et al. (2002) also demonstrated overlaps in Arabidopsis gene expression patterns among different forms of stress, including wounding and UV-B. These transcriptome analyses are consistent with several effects of UV-B on stress responses in tomato such as a potentiating effect of UV-B on the wound response in L. esculentum leaves (Stratmann et al., 2000b), engagement of the systemin signaling pathway by UV-B via activation of the systemin receptor SR160 in L. peruvianum cells (Yalamanchili and Stratmann, 2002), the presence of common signaling components for UV-B and elicitors revealed in desensitization assays (Fig. 3), and activation of LeMPK1 and LeMPK2 by UV-B and elicitors (Figs. 6 and 7). Activation of SR160 and other elicitor receptors by UV-B provides a mechanistic explanation for the overlap between UV-B and systemin/wounding responses consistent with the concept of recruitment of defense signaling pathways by UV-B (A.-H.-Mackerness et al., 1999; Yalamanchili and Stratmann, 2002; Brosché and Strid, 2003).
On the other hand, UV-B activates not only the systemin- and OE-responsive LeMPKs 1 and 2, but also LeMPK3. Moreover, the activation kinetics in response to UV-B differed greatly compared with elicitor time courses, suggesting that UV-B regulates additional signaling components and genes that are not regulated by systemin and OEs. Taken together, general and specific UV-B signaling mechanisms may result in a broad gene expression profile, but may also account for UV-specific effects observed in tomato and other plants.
In field experiments, it has been shown that UV-B increased the defensive potential of plants (Ballaré et al., 1996; Mazza et al., 1999b, Zavala et al., 2001) via regulation of insect-responsive genes (Izaguirre et al., 2003). Although the irradiation conditions used in the experiments presented here are not natural, the signaling mechanisms underlying plant defense responses under field conditions may be similar. Thus, a defense response that appears to be activated as a consequence of unspecific UV-B signaling may provide an adaptive advantage for plants.
MATERIALS AND METHODS
Suspension-Cultured Cells
Cultivation conditions for Lycopersicon peruvianum suspension-cultured cells were as described previously (Yalamanchili and Stratmann, 2002).
Stress Treatments
L. peruvianum suspension-cultured cells were supplied with the following concentrations of elicitors in an aqueous solution: 100 μm tetra-N-acetylchitotetraose (chitin), 1.7 μg mL–1 chitosan (from crab shells), 10 μg mL–1 β-glucan (from baker's yeast), and 830 μg mL–1 PGA from orange (oligogalacturonides; all from Sigma, St. Louis), or 3 nm systemin. UV-B irradiation was as described previously (Yalamanchili and Stratmann, 2002). Briefly, two 15-W UV-B lamps (F15T8.UV-B 15W; Ultraviolet Products, San Gabriel, CA) were used for irradiation under room light conditions. The UV-B lamps do not provide light in the UV-C range below 280 nm, but do provide light in the UV-A range above 320 nm (for spectral emission, see Stratmann et al., 2000b). The irradiance was monitored with an UVX Radiometer connected to an UVX-31 sensor (calibration wavelength 310 nm; both Ultraviolet Products). The distance between the lamps and the surface of the medium was adjusted for an UV-B irradiance of 5.4 ± 0.6 mW cm–2 at the surface of the medium. The exact irradiance and thus the dose at the cell surface cannot be determined because UV light is strongly absorbed by the growth medium, and the distance between cell or medium surface and UV lamps varies while cultures are vigorously shaken.
Desensitization Assays
MAPK activity in response to consecutive treatments with two stress signals was assayed using in-gel kinase assays (see below) and was quantified as described previously (Yalamanchili and Stratmann, 2002). All experiments were performed with two sets of cells and were repeated at least three times with different batches of cells.
Cloning of LeMPKs
Degenerate primers (forward: 5′-AAATC/TGCC/TAATGCTTTTGAT-3′; reverse: 5′-CTKGTG/TACA/TACATATTCA/CGTCAT-3′) were designed according to highly conserved sequences among tomato expressed sequence tags with high homology to tobacco (Nicotiana tabacum) WIPK and SIPK. PCR fragments were obtained from a λZAP 35S::prosystemin tomato leaf cDNA library (Heitz et al., 1997). These transgenic plants show a constitutive wound phenotype and accumulate high levels of defense proteins in the leaves (McGurl et al., 1994). The fragments were highly homologous to WIPK or SIPK. Radiolabeled PCR products (DECA prime kit; Ambion, Austin, TX) were used to screen the cDNA library according to standard protocols. Seven clones were isolated and sequenced corresponding to LeMPK1 or 2. To clone the tomato WIPK homolog, 5′- and 3′-untranslated region sequence information was obtained from the above described PCR products to design the following primers: reverse: 5′-CTAAATTTCTATCAATAATGGTTGATCC-3′ and forward: 5′-CTAAATTTCTATCAATAATGGATGCTAATATGGGTGC-3′ (Sigma-Genosys, The Woodlands, TX). They were used in a PCR-based screen of the 35S::PS leaf cDNA library using Pfu Turbo DNA polymerase (Stratagene, La Jolla, CA). After an A-tailing reaction using TaqDNA polymerase (Eppendorf; Brinkmann Instruments, Westbury, NY), the reaction product was cloned into the pGEM-T vector (Promega, Madison, WI) and sequenced. The translation start was determined by comparison with homologous MAPKs and corresponded with the longest open reading frame. Accession numbers are: LeMPK1, AY261512; LeMPK2, AY261513; and LeMPK3, AY261514. GrowTree from the GCG Wisconsin Package (version 10.3; Accelrys, San Diego) was used to create a phylogenetic tree for plant MAPKs from a distance matrix created by Distances using the UPGMA method.
Expression of Recombinant LeMPKs
BamHI and XhoI sites were added via PCR to the 5′ and 3′ ends of the cloned LeMPK genes, respectively. These PCR fragments were ligated inframe into the pET-28a(+) vector (Novagen, Madison, WI). Sequencing confirmed that the His-tag was in frame with the start codon of each LeMPK gene. The constructs were used to transform Escherichia coli BL21(DE3) cells (Novagen). Recombinant protein expression was induced with 1 mm isopropyl β-d-thiogalactopyranoside for 5 h at 37C. The LeMAPK-His tag fusion proteins were purified using a nickel affinity column according to instructions by the manufacturer (Novagen).
Antibody Production and Immunoblot Analysis
The peptides PPAQQPPPPSQPL, AGQQPAMPPLPMAG, and QFPDFPKIVTHAGQ corresponding to the N-termini of LeMPK1, LeMPK2, and LeMPK3, respectively, were synthesized and conjugated to keyhole limpet hemocyanin carrier. Polyclonal antisera were obtained from rabbits immunized with the above peptides, and were affinity purified (Zymed Laboratories, South San Francisco). For immunoblot analysis, 500 ng of each of the recombinant proteins was separated on 10% (w/v) SDS-polyacrylamide gels and was then transferred to polyvinylidene difluoride membranes (Pierce, Rockford, IL) using a semidry blotting apparatus (Bio-Rad, Hercules, CA). After blocking overnight in 10 mm Tris, pH 7.5, 0.9% (w/v) NaCl, and 0.1% (w/v) Tween 20 with 5% (w/v) nonfat dry milk, blots were incubated for 1 h with affinity-purified antibodies (5, 20, and 5 μg, for anti-LeMPK1, 2, and 3, respectively; data not shown) or polyclonal antisera (1:100 dilution). After three washes with 10 mm Tris, pH 7.5, 0.9% (w/v) NaCl, and 0.1% (w/v) Tween 20, the blots were incubated with a goat anti-rabbit alkaline phosphatase-conjugated secondary antibody (Zymed Laboratories; 1:2,000 dilution) for 1 h. The immunocomplexes were visualized by reaction with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium substrate (Zymed Laboratories). Another gel containing the same samples was stained with Coomassie Brilliant Blue R250. Antisera and affinity-purified antibodies produced the same results without crossreactivity. Because antisera were more potent than affinity-purified antibodies, we continued all further experiments with antisera.
Immunocomplex Kinase Assays
Extracts from L. peruvianum cells were obtained as described previously (Stratmann et al., 2000a). Extracts containing 100 μg of total protein (for UV-B experiments, 200 μg) were rotated for 2 h at 4°C with anti-LeMPK1, 2, or 3 antisera (1:200 dilution; in UV-B experiments, 1:100) in immunoprecipitation buffer (10 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm Na3VO4, 1 mm NaF, 10 mm β-glycerophosphate, 1% [w/v] Triton X-100, 0.5% [w/v] Nonidet P-40, 2 mm dithiothreitol, and 1 complete PI tablet [Roche, Indianapolis] 50 mL–1). For reactions with competitor peptides, antibodies were preincubated for 30 min at room temperature with an excess of the peptides against which the antibodies were raised. Approximately 15 μL packed volume of recombinant protein G, immobilized on Sepharose 4B beads (Zymed Laboratories), was added, and incubation continued for another 1 to 2 h at 4°C. The beads were precipitated by a brief centrifugation and were washed two times with immunoprecipitation buffer, one time with immunoprecipitation buffer containing 1 m NaCl, and three times with kinase reaction buffer (without MBP and ATP). Kinase reactions were performed for 8 min at room temperature in 20 μL of kinase reaction buffer (20 mm HEPES, pH 7.5, 15 mm MgCl2, 2 mm EGTA, 1 mm dithiothreitol, 0.25 mg mL–1 MBP, and 25 μm ATP) containing 0.1 μCi [γ-32P]ATP (370 MBq mL–1 and 111 TBq mmol–1). The reaction was stopped by addition of SDS-PAGE sample buffer. After electrophoresis on a 15% (w/v) SDS-polyacrylamide gel, gels were washed three times for 0.5 h in 20% (w/v) isopropanol and were dried. Radiolabeled MBP was visualized by a phosphoimaging system. Preimmune serum was tested accordingly and did not produce any signals (data not shown).
In-Gel Kinase Assays
In-gel kinase assays with MBP as an artificial MAPK substrate were performed as described previously (Stratmann and Ryan, 1997).
Replications
All experiments were repeated at least three times with different batches of cells. Representative experiments are shown.
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner for noncommercial research purposes, subject to the requisite permission from any third-party owners of all or parts of the material. Obtaining any permissions will be the responsibility of the requestor.
Acknowledgments
We thank Dr. Carlos Ballaré for stimulating discussions on interactions between UV-B and herbivore responses in plants.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.024414.
This work was partially supported by the University of South Carolina Research and Productive Scholarship Fund (grant to J.W.S.), and by the National Science Foundation (grant no. IBN 0090766 to C.A.R.).
References
- A-H-Mackerness S, Surplus SL, Blake P, John CF, Buchanan-Wollaston V, Jordan BR, Thomas B (1999) Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana: role of signalling pathways controlled by jasmonic acid, ethylene and reactive oxygen species. Plant Cell Environ 22: 1413–1423 [Google Scholar]
- A-H-Mackerness S, John CF, Jordan B, Thomas B (2001) Early signaling components in ultraviolet-B responses: distinct roles for different reactive oxygen species and nitric oxide. FEBS Lett 489: 237–242 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu W-L, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Ballaré C, Scopel AL, Stapleton AE, Yanovsky M (1996) Solar ultraviolet-B radiation affects seedling emergence, DNA integrity, plant morphology, growth rate, and attractiveness to herbivore insects in Datura ferox. Plant Physiol 112: 161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreutz A, Tyers M (2002) MAPK signaling specificity: it takes two to tango. Trends Cell Biol 12: 254–257 [DOI] [PubMed] [Google Scholar]
- Brosché M, Schuler MA, Connor L, Strid A (2002) Gene regulation by low level UV-B radiation: identification by DNA array analysis. Photochem Photobiol Sci 1: 656–664 [DOI] [PubMed] [Google Scholar]
- Brosché M, Strid A (2003) Molecular events following perception of ultraviolet-B radiation by plants. Physiol Planta 117: 1–10 [Google Scholar]
- Cardinale F, Jonak J, Ligterink W, Niehaus K, Boller T, Hirt H (2000) Differential activation of four specific MAPK pathways by distinct elicitors. J Biol Chem 275: 36734–36740 [DOI] [PubMed] [Google Scholar]
- Cardinale F, Meskiene I, Ouaked F, Hirt H (2002) Convergence and divergence of stress-induced mitogen-activated protein kinase signaling pathways at the level of two distinct mitogen-activated protein kinase kinases. Plant Cell 14: 703–711 [PMC free article] [PubMed] [Google Scholar]
- Conconi A, Smerdon MJ, Howe GA, Ryan CA (1996) The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature 383: 826–829 [DOI] [PubMed] [Google Scholar]
- Desikan R, A-H-Mackerness S, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Syrovets T, Weiler EW, Ryan CA (1995) Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc Natl Acad Sci USA 92: 4095–4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droillard M-J, Thibivilliers S, Cazalé A-C, Barbier-Brygoo H, Lauriére C (2000) Protein kinases induced by osmotic stresses and elicitor molecules in tobacco cell suspensions: two crossroad MAP kinases and one osmoregulation-specific protein kinase. FEBS Lett 474: 217–222 [DOI] [PubMed] [Google Scholar]
- Durrant WE, Rowland O, Piedras P, Hammond-Kosack KE, Jones JDG (2000) cDNA-AFLP reveals a striking overlap in race-specific resistance and wound response gene expression profiles. Plant Cell 12: 963–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Baureithel K, Boller T (1998) Desensitization of the perception system for chitin fragments in tomato cells. Plant Physiol 117: 643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Boller T (1995) Systemin induces rapid ion fluxes and ethylene biosynthesis in Lycopersicon peruvianum cells. Plant J 7: 381–389 [Google Scholar]
- Felix G, Regenass M, Boller T (2000) Sensing of osmotic pressure changes in tomato cells. Plant Physiol 124: 1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudici MA, Regente CM, de la Canal L (2000) A potent antifungal protein from Helianthus annuus flowers is a trypsin inhibitor. Plant Physiol Biochem 38: 881–888 [Google Scholar]
- Green R, Fluhr R (1995) UV-B-induced PR-1 accumulation is mediated by active oxygen species. Plant Cell 7: 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross S, Knebel A, Tenev T, Neininger A, Gaestel M, Herrlich P, Böhmer FD (1999) Inactivation of protein-tyrosine phosphatases as mechanism of UV-induced signal transduction. J Biol Chem 274: 26378–26386 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Gase K, Hui D, Schmidt DD, Baldwin IT (2003) Molecular interactions between the specialist herbivore Manduca Sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata: Microarray analysis reveals that most herbivore-specific transcriptional changes are mediated by fatty acid-amino acid conjugates Plant Physiol 131: 1894–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz T, Bergey DA, Ryan CA (1997) A gene encoding a chloroplast-targeted lipooxygenase in tomato leaves is transiently induced by wounding, systemin, and methyl jasmonate. Plant Physiol 114: 1085–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrlich P, Böhmer FD (2000) Redox regulation of signal transduction in mammalian cells. Biochem Pharmacol 59: 35–41 [DOI] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067–2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaguirre MM, Scopel AL, Baldwin IT, Ballaré CL (2003) Convergent responses to stress: Solar UV-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol 132: 1755–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen MAK, Gaba V, Greenberg BM (1998) Higher plants and UV-B radiation: balancing damage, repair and acclimation. Trends Plant Sci 3: 131–135 [Google Scholar]
- Jonak C, Okresz L, Bögre L, Hirt H (2002) Complexity, cross talk and integration of plant MAP kinase signalling. Curr Opin Plant Biol 5: 415–424 [DOI] [PubMed] [Google Scholar]
- Kitagawa D, Tanemura S, Ohata S, Shimizu N, Seo J, Nishitai G, Watanabe T, Nakagawa K, Kishimoto H, Wada T, Tezuka T, Yamamoto T, Nishina H, Katada T (2001) Activation of extracellular signal-regulated kinase by ultraviolet is mediated through Src-dependent epidermal growth factor receptor phosphorylation: its implication in an anti-apoptotic function. J Biol Chem 277: 366–371 [DOI] [PubMed] [Google Scholar]
- Kroj T, Rudd JJ, Nürnberger T, Gäbler Y, Lee J, Scheel D (2003) Mitogen-activated protein kinases play an essential role in oxidative burst-independent expression of pathogenesis-related genes in parsley. J Biol Chem 278: 2256–2264 [DOI] [PubMed] [Google Scholar]
- Lecourieux D, Mazars C, Pauly N, Ranjeva R, Pugin A (2002) Analysis and effects of cytosolic free calcium increases in response to elicitors in Nicotiana plumbaginifolia cells. Plant Cell 14: 2627–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GI, Howe GA (2003) The tomato mutant spr1 is defective in systemin perception and the production of a systemic wound signal for defense gene expression. Plant J 33: 567–576 [DOI] [PubMed] [Google Scholar]
- Lin L-L, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ (1993) cPLA2 is phosphorylated and activated by MAP kinase. Cell 72: 269–278 [DOI] [PubMed] [Google Scholar]
- Link V, Hofmann MG, Sinha AK, Ehness R, Strnad M, Roitsch T (2002a) Biochemical evidence for the activation of distinct subsets of mitogen-activated protein kinases by voltage and defense-related stimuli. Plant Physiol 128: 271–281 [PMC free article] [PubMed] [Google Scholar]
- Link V, Sinha AK, Vashista P, Hofmann MG, Proels RK, Ehness R, Roitsch T (2002b) A heat-activated MAP kinase in tomato: a possible regulator of the heat stress response. FEBS Lett 531: 179–183 [DOI] [PubMed] [Google Scholar]
- Linthorst HJ, Brederode FT, van der Does C, Bol JF (1993) Tobacco proteinase inhibitor I genes are locally, but not systemically induced by stress. Plant Mol Biol 21: 985–992 [DOI] [PubMed] [Google Scholar]
- Low PS, Merida JR (1996) The oxidative burst in plant defense: function and signal transduction. Physiol Plant 96: 533–542 [Google Scholar]
- MAPK group (Ichimura K, Shinozaki K, Tena G, Sheen J, Henry Y, Champion A, Kreis M, Zhang S, Hirt H, Wilson C et al. (2002) Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7: 301–308 [DOI] [PubMed] [Google Scholar]
- Marshall CJ (1995) Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80: 179–185 [DOI] [PubMed] [Google Scholar]
- Mazza CA, Battista D, Zima AM, Szwarcberg-Bracchitta M, Giordano CV, Acevedo A, Scopel AL, Ballaré CL (1999) The effects of solar UV-B radiation on the growth and yield of barley are accompanied by increased DNA damage and antioxidant responses. Plant Cell Eviron 22: 61–70 [Google Scholar]
- Mazza CA, Zavala J, Scopel AL, Ballaré CL (1999b) Effects of solar UV-B on phytophagous insects: behavioral responses and ecosystem implications. Proc Natl Acad Sci USA 96: 980–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurl B, Orozco-Cardenas ML, Pearce G, Ryan CA (1994) Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc Natl Acad Sci USA 91: 9799–9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meskiene I, Hirt H (2000) MAP kinase pathways: molecular plug-and-play chips for the cell. Plant Mol Biol 42: 791–806 [DOI] [PubMed] [Google Scholar]
- Miles GP, Samuel MA, Ellis BE (2002) Suramin inhibits oxidant signalling in tobacco suspension-cultured cells. Plant Cell Environ 25: 521–527 [Google Scholar]
- Moyen C, Hammond-Kosack KE, Jones J, Knight MR, Johannes E (1998) Systemin triggers an increase of cytoplasmic calcium in tomato mesophyll cells: Ca2+ mobilization from intra- and extracellular compartments. Plant Cell Environ 21: 1101–1111 [Google Scholar]
- Narváez-Vásquez J, Florin-Christensen J, Ryan CA (1999) Positional specificity of a phospholipase A2 activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. Plant Cell 11: 2249–2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell PJ, Clavert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914–1917 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas ML, McGurl B, Ryan CA (1993) Expression of an antisense prosystemin gene in tomato plants reduces resistance toward Manduca sexta larvae. Proc Natl Acad Sci USA 90: 8273–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13: 179–191 [PMC free article] [PubMed] [Google Scholar]
- Park S-H, Zarrinpar A, Lim WA (2003) Rewiring MAP kinase pathways using alternative scaffold assembly mechanisms. Science 299: 1061–1064 [DOI] [PubMed] [Google Scholar]
- Pautot V, Holzer FM, Walling LL (1991) Differential expression of tomato proteinase inhibitor I and II genes during bacterial pathogen invasion and wounding. Mol Plant-Microbe Interact 4: 284–292 [DOI] [PubMed] [Google Scholar]
- Pearce G, Moura D, Stratmann J, Ryan CA (2001) RALF, a 5-kDa ubiquitous polypeptide in plants, arrests root growth and development. Proc Natl Acad Sci USA 98: 12843–12847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramonell KM, Zhang B, Ewing RM, Chen Y, Xu D, Stacey G, Somerville S (2002) Microarray analysis of chitin elicitation in Arabidopsis thaliana. Mol Plant Pathol 3: 301–311 [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA (2000) The systemin signaling pathway: differential activation of plant defensive genes. Biochim Biophys Acta 1477: 112–121 [DOI] [PubMed] [Google Scholar]
- Sabbagh W Jr, Flatauer LJ, Bardwell J, Bardwell L (2001) Specificity of MAP kinase signaling in yeast differentiation involves transient versus sustained MAPK activation. Mol Cell 8: 683–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf HJ (1994) Involvement of growth factor receptors in the mammalian UVC response. Cell 78: 963–972 [DOI] [PubMed] [Google Scholar]
- Scheer J, Ryan CA (2002) The systemin receptor SR160 from Lycopersicon peruvianum is a member of the LRR receptor kinase family. Proc Natl Acad Sci USA 99: 9585–9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk PM, Kazan K, Wilson I, Anderson JP, Richmond T, Somerville SC, Manners JM (2000) Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc Natl Acad Sci USA 97: 11655–11660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Okamoto M, Seto H, Ishizuka K, Sano H, Ohashi Y (1995) Tobacco MAP kinase: a possible mediator in wound signal transduction pathways. Science 270: 1988–1992 [DOI] [PubMed] [Google Scholar]
- Seo S, Sano H, Ohashi Y (1999) Jasmonate-based wound signal transduction requires activation of WIPK, a tobacco mitogen-activated protein kinase. Plant Cell 11: 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha AK, Hofmann MG, Romer U, Kockenberger W, Elling L, Roitsch T (2002) Metabolizable and non-metabolizable sugars activate different signal transduction pathways in tomato. Plant Physiol 128: 1480–1489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith FD, Scott JD (2002) Signaling complexes: junctions on the intracellular information super highway. Curr Biol 12: R32–R40 [DOI] [PubMed] [Google Scholar]
- Stratmann JW, Ryan CA (1997) Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc Natl Acad Sci USA 94: 11085–11089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann J, Scheer J, Ryan CA (2000a) Suramin inhibits initiation of defense signaling by systemin, chitosan and pmg-elicitor in suspension cultured Lycopersicon peruvianum cells. Proc Natl Acad Sci USA 97: 8862–8867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JW, Stelmach BA, Weiler EW, Ryan CA (2000b) UVB/UVA radiation activates a 48-kDa myelin basic protein kinase and potentiates wound signaling in tomato leaves. Photochem Photobiol 71: 116–123 [DOI] [PubMed] [Google Scholar]
- Terras FRG, Schoofs HME, Thevissen K, Osborn RW, Vanderleyden J, Cammue BPA, Broekaert WF (1993) Synergistic enhancement of the antifungal activity of wheat and barley thionins by radish and oilseed rape 2S albumins and by barley trypsin inhibitors. Plant Physiol 103: 1311–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin V, Touraev A, Kieft H, van Lammeren AA, Heberle-Bors E, Wilson C (2001) Temporal and tissue-specific expression of the tobacco ntf4 MAP kinase. Plant Mol Biol 45: 679–689 [DOI] [PubMed] [Google Scholar]
- Walker-Simmons MK, Ryan CA (1984) Proteinase inhibitor synthesis in tomato leaves: induction by chitosan oligomers and chemically modified chitosan and chitin. Plant Physiol 76: 787–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Anglmayer R, Vicente O, Heberle-Bors E (1995) Molecular cloning, functional expression in Escherichia coli, and characterization of multiple mitogen-activated-protein kinases from tobacco. Eur J Biochem 233: 249–257 [DOI] [PubMed] [Google Scholar]
- Yalamanchili RD, Stratmann J (2002) Ultraviolet-B activates components of the systemin signaling pathway in Lycopersicon peruvianum suspension-cultured cells. J Biol Chem 277: 28424–28430 [DOI] [PubMed] [Google Scholar]
- Yang K-Y, Liu Y, Zhang S (2001) Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc Natl Acad Sci USA 98(2): 741–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Scopel AL, Ballaré CL (2001) Effects of ambient UV-B radiation on soybean crops: impact on leaf herbivory by Anticarsia gemmatalis. Plant Ecol 156: 121–130 [Google Scholar]
- Zhang S, Klessig DF (1997) Salicylic acid activates a 48-kD MAP kinase in tobacco. Plant Cell 9: 809–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (1998) The tobacco wounding-activated mitogen-activated protein kinase is encoded by SIPK. Proc Natl Acad Sci USA 95: 7225–7230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Klessig DF (2000) Pathogen-induced MAP kinases in tobacco. Results Probl Cell Differ 27: 65–84 [DOI] [PubMed] [Google Scholar]