Abstract
Screening immediate-early responding genes during the hypersensitive response (HR) against tobacco mosaic virus infection in tobacco (Nicotiana tabacum) plants, we identified a gene encoding ornithine decarboxylase. Subsequent analyses showed that other genes involved in polyamine biosynthesis were also up-regulated, resulting in the accumulation of polyamines in apoplasts of tobacco mosaic virus-infected leaves. Inhibitors of polyamine biosynthesis, α-difluoromethyl-ornithine, however, suppressed accumulation of polyamines, and the rate of HR was reduced. In contrast, polyamine infiltration into a healthy leaf induced the generation of hydrogen peroxide and simultaneously caused HR-like cell death. Polyamine oxidase activity in the apoplast increased up to 3-fold that of the basal level during the HR, and its suppression with a specific inhibitor, guazatine, resulted in reduced HR. Because it is established that hydrogen peroxide is one of the degradation products of polyamines, these results indicate that one of the biochemical events in the HR is production of polyamines, whose degradation induces hydrogen peroxide, eventually resulting in hypersensitive cell death.
One of the early events during the hypersensitive response (HR) in pathogen-attacked plants is the production of reactive oxygen intermediates, including superoxide (O2–), hydrogen peroxide (H2O2), hydroxyl radical (OHÿ), and others. Reactive oxygen intermediates restrict pathogens by strengthening cell walls through oxidative cross-linking (Bradley et al., 1992), by directly attacking pathogens (Levine et al., 1994), and by acting as signal molecules to induce defense responses such as rapid hypersensitive cell death (Hammond-Kosack and Jones, 1996; Alvarez et al., 1998). This latter event prevents pathogens from spreading from the site of entry. To examine the molecular mechanisms underlying HR in tobacco (Nicotiana tabacum) plants, an experimental system with the tobacco mosaic virus (TMV) and intact leaves of a tobacco cultivar carrying the resistant (N) gene has often been used. When tobacco plants carrying the N gene are inoculated with TMV and incubated at 30°C, at which temperature the N gene dose not function, viral particles multiply. When transferred to 20°C (temperature shift), the N gene is activated, resulting in lethal HR (Gianinazzi, 1970). The system is simple and synchronizable, making it suitable to examine signal transduction pathways active in the HR.
Polyamines are small, positively charged aliphatic amines at cellular pH values and therefore bind to negatively charged molecules, including nucleic acids, acidic phospholipids, and proteins (Cohen, 1998). Consequently, they modulate DNA-protein (Shah et al., 1999), and protein-protein interactions (Thomas et al., 1999). Common natural polyamines include putrescine, spermidine, and spermine, along with related minor compounds and conjugated forms. Their pathway of biosynthesis is well established (Kumar et al., 1997). The first rate-limiting step is catalyzed by Orn decarboxylase (ODC), which converts Orn into putrescine. Putrescine is then successively converted to spermidine and spermine by spermidine synthase and spermine synthase, respectively, with addition of propylamino groups derived from decarboxylated S-adenosyl-Met, which was generated through a reaction involving S-adenosyl-Met decarboxylase. In addition to this major pathway, another pathway to putrescine from Arg has been proposed, catalyzed by the Arg decarboxylase-producing intermediates, agmatine and N-carbamoyl putrescine. Genes encoding these enzymes have been cloned from various organisms (Walden et al., 1997).
In animal cells, polyamines are considered to have specific roles in embryonic development (Kusunoki and Yasumasu, 1978), in control of the cell cycle (Alm et al., 2000), in carcinogenesis (Seiler et al., 1998), and in immune system functions (Seiler and Atanassov, 1994). A recent study also suggested polyamines to be linked with cell proliferation and likely with apoptosis as well (Thomas and Thomas, 2001). This is based on the observation that the gene encoding ODC is activated during apoptosis induced by overexpression of c-Myc, a transcription factor belonging to the Myc oncogene family (Bello-Fernandez et al., 1993), which is important for both cell proliferation and apoptosis (Packham and Cleveland, 1994). A possible role of polyamines in apoptosis was proposed involving the generation of hydrogen peroxide through their degradation by flavin-containing polyamine oxidase (Ha et al., 1997; Bonneau and Poulin, 2000).
In plants, polyamines are also thought to play important roles in growth, development, and stress responses (Walden et al., 1997). For example, the level of polyamines is reported to fluctuate during plant-microbe interactions (Walters, 2000). In tobacco plants infected with TMV, enzymatic activity of ODC and Arg decarboxylase increases during HR, resulting in elevated concentrations of their products and conjugates, mainly in necrotic regions (Martin-Tanguy et al., 1973; Negrel et al., 1984). Also, spermine accumulates in intercellular spaces and induces pathogenesis-related proteins during HR (Yamakawa et al., 1998). Accumulation of polyamines has been observed in tobacco cultivars resistant to TMV, but not in TMV-susceptible counterparts (Marini et al., 2001). However, investigations of polyamine function have been mainly focused on changes in their levels and spectrum, leaving the biological significance to be determined. Although genetic analyses have been conducted at the gene level (DeScenzo and Minocha, 1993; Masgrau et al., 1997), the available information on their impact on the HR is limited.
In this paper, we provide evidence that ODC and therefore polyamines are critical components in induction of hypersensitive cell death during pathogen attack in tobacco plants, and that this is largely based on production of hydrogen peroxide through their degradation by polyamine oxidase.
RESULTS
Accumulation of Transcripts for Polyamine Biosynthetic Enzymes
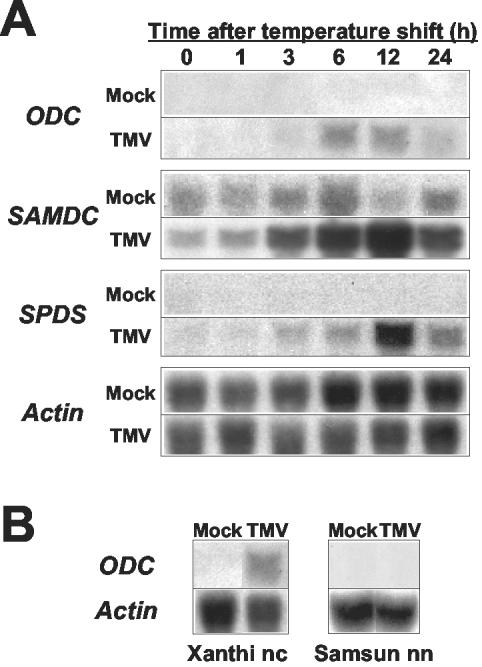
Using the intact tobacco-TMV system, we compared the mRNA populations in TMV-infected wild-type tobacco plants (Nicotiana tabacum cv Xanthi nc) carrying the N gene, whose product confers resistance against TMV, before and after temperature shift, by fluorescence differential display (Yoda et al., 2002). Screening identified a particular gene encoding ODC (Yoda et al., 2002), which converts Orn to putrescine in polyamine biosynthesis (Walters, 2000). To determine whether other genes involved in polyamine biosynthesis might also be activated during the HR, RNA gel-blot analysis was performed with cDNA probes for sperimidine synthase and S-adenosyl-Met decarboxylase together with one for ODC. The results clearly showed that transcripts for all of them accumulated after temperature shift, reaching maximum levels after about 12 h (Fig. 1A). Subsequent RNA gel-blot analysis using tobacco cv Samsun nn plant, which does not carry the N gene, and therefore is susceptible to TMV, showed that transcripts for ODC were not induced after temperature shift (Fig. 1B). These results indicated that expression of ODC is under the control of the N gene and suggested de novo production of polyamines during the HR.
Figure 1.
Transcript accumulation of polyamine biosynthesis-related genes. Healthy leaves of wild-type plants with (tobacco cv Xanthi nc; A and B) or without (tobacco cv Samsun nn) N gene (B) were harvested, inoculated with TMV or mock, and maintained in an incubator at 30°C for 48 h and then at 20°C for appropriate time intervals as indicated in terms of post temperature shift (h). Total RNAs were isolated, and RNA gel-blot analysis was conducted with 32P-labeled probes for ODC, sperimidine synthase (SPDS), and S-adenosyl-Met decarboxylase (SAMDC). Experiments were repeated twice to confirm similar results. As an internal standard for RNA loading, the transcript level for the actin gene was estimated. Accession numbers: ODC, AF233849; SAMDC, AF033100; and SPDS, AB006692.
Inhibition Effect of Polyamine Synthesis
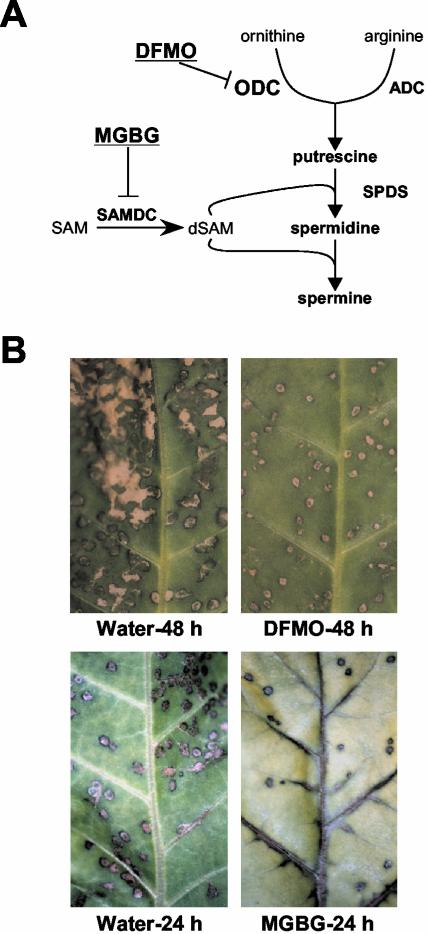
The relationship between polyamines and the HR was then examined using two inhibitors of polyamine biosynthesis; α-difluoromethyl-Orn (DFMO) as an irreversible inhibitor of ODC and methylglyoxalbis(guanylhydrazone) (MGBG) as a competitive inhibitor of S-adenosyl-Met decarboxylase (Fig. 2A). When detached leaves were inoculated with TMV in the presence of DFMO, necrotic lesions became much smaller in comparison with those of the controls (Fig. 2B). Similarly, when detached leaves were inoculated with TMV in the presence of MGBG, the size and number of lesions also became smaller and less than those of the controls, respectively (Fig. 2B). Because TMV was found to proliferate almost equally in infected leaves in the presence or absence of either DFMO or MGBG by RNA gel-blot analyses (data not shown), small lesions were not due to variation in the quantity of TMV particles. Thus, results from two independent pharmacological experiments strongly indicated that polyamines contribute to hypersensitive cell death.
Figure 2.
Effects of DFMO and MGBG on lesion formation during HR. A, Polyamine biosynthesis pathway in plants and inhibition sites by DFMO and MGBG. ADC, Arg decarboxylase; dSAM, decarboxylated S-adenosyl-Met; SAM, S-adenosyl-Met. B, Size of necrotic lesions. Detached leaves were treated with water, 10 mm DFMO, or 10 mm MGBG at the time of TMV infection (48 h before temperature shift). Lesions developed 48 h after temperature shift in the presence of DFMO, and those developed 24 h after temperature shift in the presence of MGBG. Controls inoculated in the absence of drugs are shown in the left panels.
Polyamine Accumulation in Apoplasts
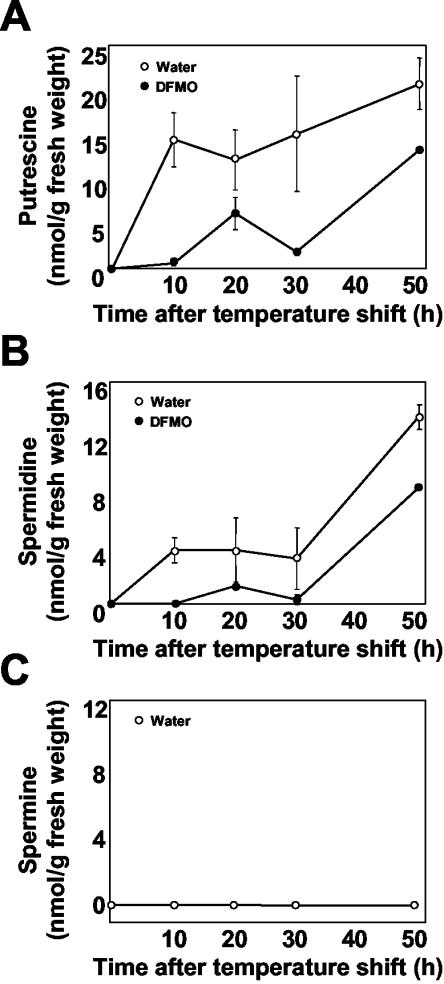
Because the above-described experiments indicated polyamines to accumulate during the HR, the amounts of polyamines were directly measured in TMV-infected leaves. Preliminary estimation of polyamine levels showed no change in either mock-treated or TMV-inoculated leaves after temperature shift (data not shown). However, because spermine was reported to accumulate in the intercellular spaces during HR against TMV infection (Yamakawa et al., 1998), the amounts of polyamines in apoplast were then examined. No polyamines were detectable in apoplast of healthy uninoculated leaves and mock-treated leaves at any time point (data not shown), indicating that temperature shift (low temperature) did not induce accumulation of polyamines. In contrast, putrescine and spermidine began to increase in apoplasts after temperature shift in TMV-infected leaves (Fig. 3), reaching approximately 20 and 12 nmol, respectively, per gram fresh weight after 50 h (Fig. 3, A and B). Their accumulation was suppressed to less than one-half of these values by DFMO treatment (Fig. 3, A and B). Spermine was not detectable under the experimental conditions employed (Fig. 3C). These observations indicated that polyamines are one of the causative or triggering elements for HR onset.
Figure 3.
Accumulation of polyamines in apoplast. Time course of polyamine accumulation in apoplast of TMV-infected leaves with (black circle) or without DFMO (white circle) treatment. At the indicated time points after temperature shift, apoplastic fluid was extracted and benzoylated with benzoyl chloride. The benzoylated samples were separated and quantified for putrescine (A), spermidine (B), and spermine (C) by HPLC. Average values and sds are from three independent experiments each with one individual sample.
Induction of Hydrogen Peroxide and Cell Death by Polyamines
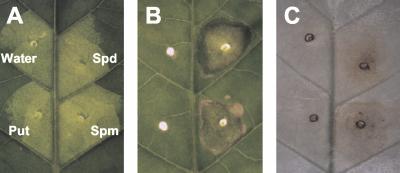
In animal cells, it is known that hydrogen peroxide, which is produced through oxidation of polyamines, plays a critical role in apoptosis (Parchment, 1993; Lindsay and Wallace, 1999). To determine whether this is also the case for the HR in tobacco plants, each polyamine was infiltrated into apoplast of a healthy leaf (Fig. 4A). Two days after infiltration, infiltrated areas with spermidine and spermine were collapsed due to cell death, whereas water treatment and putrescine showed no effects (Fig. 4B). When duplicated samples were then stained with diaminobenzidine (DAB), the generation of large amounts of hydrogen peroxide was visually observed to have accumulated in collapsed areas (Fig. 4C). These results suggested that polyamines are catabolized in the apoplast during the HR, resulting in the generation of hydrogen peroxide.
Figure 4.
Induction of cell death and generation of hydrogen peroxide by polyamines. A, Infiltration of chemicals. Indicated polyamines at the concentration of 10 mm were infiltrated into a healthy tobacco leaf. As the control, water was also infiltrated (Water). Tested samples were putrescine (Put), spermidine (Spd), and spermine (Spm). B, Cell collapse at infiltration sites. Photograph was taken 48 h after infiltration. C, Detection of hydrogen peroxide. 3,3′-Diaminobenzidine (DAB) solution was infiltrated 6 h after the first infiltration and incubated for further 6 h. Sample leaf was briefly boiled in ethanol and observed for hydrogen peroxide, which is seen as reddish-brown color.
Polyamine Degradation by Polyamine Oxidase
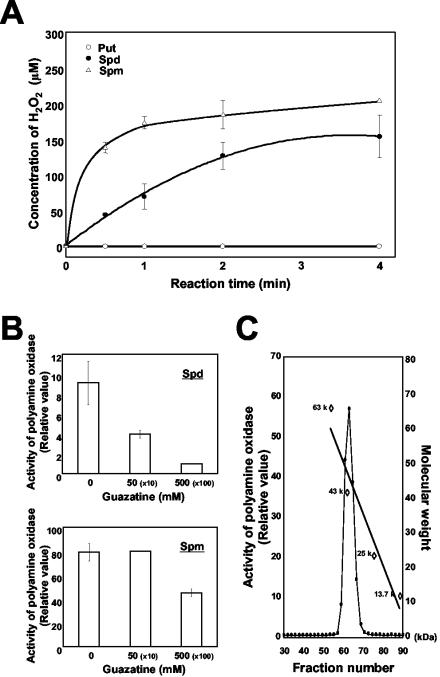
Putrescine (diamine), spermidine, and spermine (polyamines) are oxidized by diamine oxidase and polyamine oxidase, respectively, yielding hydrogen peroxide (Sebela et al., 2001). Hence, an apoplast fluid from healthy leaves was assayed for these enzymatic activities. The results indicated that, whereas putrescine remained intact, spermidine and spermine were efficiently degraded in the presence of the crude extract (Fig. 5A). The enzymatic activity was subsequently examined in the presence of a polyamine oxidase-specific competitive inhibitor, guazatine. Results showed that when spermidine was used as the substrate, guazatine reduced the enzymatic activity up to 50% and 10% of the control by 10- and 100-fold excess concentrations to the substrate, respectively (Fig. 5B). When spermine was used as the substrate, however, guazatine was less effective, showing little inhibition at 10-fold excess and a 40% reduction at 100-fold excess concentrations to the substrate, respectively (Fig. 5B). The difference might result from spermine being the most preferable substrate for the enzyme in vitro (Fig. 5A). Further analyses by fractionating the apoplastic fluid through a molecular sieve revealed the molecular size of protein having the activity to be approximately 50 kD (Fig. 5C). This size agrees with those reported for polyamine oxidase from barley (Hordeum vulgare; Radova et al., 2001) and maize (Zea mays; Tavladoraki et al., 1998). On the basis of these observations, it was concluded that polyamine oxidase was in fact responsible for release of hydrogen peroxide.
Figure 5.
Identification of polyamine oxidase. A, Time course analysis of polyamine oxidase activity. Apoplastic fluid from wild-type healthy leaves was extracted and subjected to assay at the indicated time (h). Concentrations of hydrogen peroxide generated in the reactions containing 10 μL of apoplastic fluid and 5 mm of the indicated substrate were estimated. Average values and sds are from two independent experiments, each with duplicated samples. B, Inhibition of enzymatic activity by guazatine. Apoplast fluid from wild-type healthy leaves was treated with the indicated concentrations of guazatine for 30 min before polyamine oxidase activity was assayed. The numbers in brackets represent ratios of inhibitor to substrate. Average values and sds are from two independent experiments, each with duplicated samples. C, Fractionation of polyamine oxidase. Apoplastic fluid from healthy leaves was fractionated by gel filtration. Polyamine oxidase activity of each fraction was measured. The relative molecular mass (Mr) was estimated from the standard curve obtained from marker proteins of albumin (67 kD), ovalbumin (43 kD), chymotrypsinogen (25 kD), and ribonuclease A (13.7 kD).
Polyamine Oxidase Activity during HR
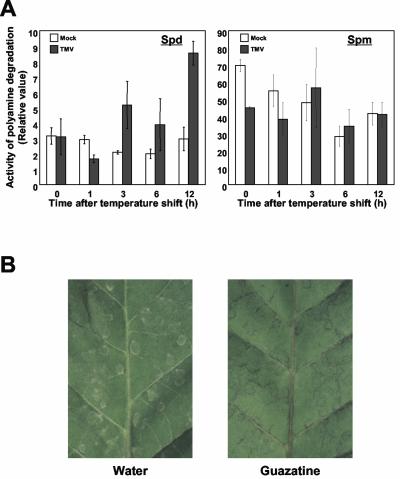
The activity profile of polyamine oxidase in apoplasts during HR was then examined. When spermidine was employed as the substrate, a constant level of polyamine oxidase activity was found to be present in mock-treated leaves (Fig. 6A). In TMV-inoculated leaves, however, the level began to increase, reaching up to 3-fold that of the control 12 h after temperature shift (Fig. 6A). In contrast, the activity was apparently constant during HR when spermine was used as the substrate (Fig. 6A). These results suggested that polyamines are readily degraded whenever they are supplied to apoplast, and that, even so, degradation activity is enhanced by further induction of polyamine oxidase on recognition of TMV. The catalytic activity, however, was dependent on the substrate: approximately 10-fold higher with spermine than with spermidine (Fig. 6A). Putrescine was not degraded (data not shown), consistent with the finding that it was not catabolized by apoplastic fluid (Fig. 5A). To confirm the function of polyamine oxidase in planta, effects of its inhibition during HR were examined. Healthy leaf cuttings were inoculated with TMV and pretreated with guazatine 12 h before temperature shift. The formation of necrotic lesions was weak and incomplete (Fig. 6B), supporting the idea that polyamine oxidase plays a critical role during hypersensitive cell death.
Figure 6.
Polyamine oxidase activity in planta and effects of its suppression. A, Polyamine oxidase activity in apoplast. Apoplastic fluids from leaves inoculated with TMV (black bar) or mock (white bar) were extracted at the indicated time period after temperature shift (h) and were subjected to polyamine oxidase activity assay. The concentration of hydrogen peroxide generated in the reaction containing 10 μL of apoplastic fluid and either 5 mm spermidine (left panel) or 5 mm spermine (right panel) was estimated. Average values and sds are from three independent experiments, each with one individual sample. Experimentally measured values were statistically tested and confirmed to be P ≤ 0.02. B, Inhibition of necrotic lesion formation by polyamine oxidase inhibition. Detached leaves were treated with 10 mm guazatine 12 h before temperature shift. Lesions developing 24 h after temperature shift in the absence (water) and presence (guazatine) of the inhibitor are shown in the panels.
DISCUSSION
The present paper documents that polyamines produced during the HR can serve as direct substrates for hydrogen peroxide production, which could contribute to induction of hypersensitive cell death.
Polyamines in Hypersensitive Cell Death
Recent studies with animal cells revealed that polyamines possess dual
apparently conflicting functions, both preventing and inducing apoptosis
(Thomas and Thomas, 2001). The
prevention is based on the fact that polyamines stimulate growth and promote
passage through the cell cycle. The induction of apoptosis is considered to be
mediated by c-Myc, a potent transactivator of the ODC gene, whose product then
up-regulates polyamine synthesis. For the latter case, polyamines are
considered to be enzymatically catabolized yielding hydrogen peroxide, which
is biologically responsive. Polyamines are degraded by a variety of oxidases,
among which flavin-containing polyamine oxidases and copper-containing diamine
oxidases play major roles (Morgan,
1998,
1999). Polyamine oxidase in
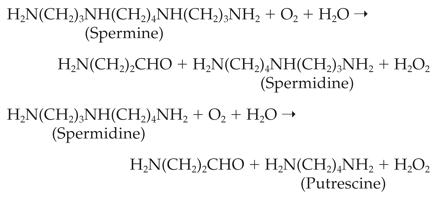
mammalian cells can convert spermine and spermidine, by oxidative cleavage,
back to spermidine and putrescine, respectively, resulting in the formation of
hydrogen peroxide through the following pathway.
In fact, catabolic products of polyamine analogs by polyamine oxidase were reported to induce programmed cell death in animal cells (Ha et al., 1997). It has thus been suggested that, during apoptosis of animal cells, oxidative degradation of polyamines results in production of hydrogen peroxide, which directly induces cell death or indirectly transmits signals leading to this outcome (Parchment and Pierce, 1989; Ha et al., 1997; Bonneau and Poulin, 2000).
In plants, however, the relationship between polyamines and hydrogen
peroxide has so far not been examined in detail. During the HR against
pathogens, ODC activity and polyamines are reported to increase around and/or
in necrotic lesions (Torrigiani et al.,
1997), with accumulation in apoplast of pathogen-infected plants
(Yamakawa et al., 1998;
Walters, 2000). Hydrogen
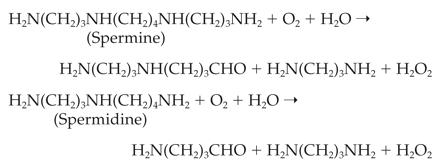
peroxide was shown to be produced through the degradation of polyamines by
polyamine oxidase through the following pathway
(Sebela et al., 2001), which
differs from that of mammalian cells.
Hydrogen peroxide has also been considered to be a causative element for hypersensitive cell death (Levine et al., 1994). Thus, we speculated that the two phenomena might be closely related each other. However, no experimental work has hitherto been performed to support this idea, leaving open questions as to the physiological functions of polyamines during HR and as to the source(s) of hydrogen peroxide in planta. Our current study revealed that a set of genes involved in polyamine biosynthesis is simultaneously up-regulated upon pathogen infection, resulting in rapid production of polyamines. Accumulated polyamines are degraded by polyamine oxidase in apoplast, yielding hydrogen peroxide, which efficiently induces hypersensitive cell death. These findings clearly provide a possible missing link between polyamines and hydrogen peroxide during HR, and shed light on the biochemical events that constitute HR.
Degradation of Polyamines by Polyamine Oxidase
In plant cells, both polyamine oxidase and diamine oxidase are reported to be located in apoplast (Slocum and Furey, 1991; Laurenzi et al., 2001). In the present study, we also found polyamine oxidase activity in apoplast fluid, and we consequently speculated that accumulated polyamines were degraded by this enzyme in apoplast during HR, as in the case with animal cells. The present experimental results clearly substantiate this hypothesis by showing direct associations among polyamine production, their degradation catalyzed by polyamine oxidase, and hydrogen peroxide formation. The time-course analyses suggested that within several hours after elicitation, newly synthesized polyamines are efficiently degraded by polyamine oxidase, releasing hydrogen peroxides in apoplast. A question then arises as to which polyamine serves as the main substrate for polyamine oxidase in apoplasts during HR. Although spermine appeared to be the better substrate than spermidine in vitro, the former was shown to be hardly detected in apoplast, being less than one-twentieth that of the latter under the normal condition (Masgrau et al., 1997). This is consistent with our current results, showing no detectable accumulation regardless of HR, and suggests spermine to be less probable as the main substrate for the enzyme.
In contrast, spermidine was induced, but the level was maintained low during the initial 30 h of HR. Such a profile change is consistent with that of polyamine oxidase activity, showing a constant basal level regardless of the infection and a 3-fold increase by TMV recognition. These results can be best explained by assuming that, upon onset of HR, spermidine is newly produced and transported into apoplast, where it is readily degraded by polyamine oxidase. Putrescine also accumulated during the HR, but the infiltration assay showed that it apparently induced neither hydrogen peroxide nor hypersensitive cell death, suggesting that it has some other function. Taken together, it is conceivable that spermidine may be the main substrate for polyamine oxidase and therefore that the rate limiting step of this system is the production of this polyamine. It is worthy of note that a simple infiltration experiment here facilitated the detection of polyamine oxidase activity in planta. This method is useful for identification of in vivo polyamine and/or diamine oxidase activities and might be generally applicable to other plants.
Alternative Pathways for Hydrogen Peroxide Production
One of the earliest events that occurs after pathogen recognition is activation of NAD(P)H oxidase in plasma membranes (Keller et al., 1998). This results in synthesis of superoxide radicals in apoplast, which spontaneously dismutate to give other active oxygen intermediates, including hydrogen peroxide and hydroxyl radical (Bestwick et al., 1997). The mechanism by which NAD(P)H oxidase contributes to production of hydrogen peroxide temporally and spatially during HR is currently not clear, but such a rapid NAD(P)H oxidase-dependent production of hydrogen peroxide may be necessary to directly dispatch pathogens, to induce cell death of infected and adjacent cells, and to enhance production of defense signal molecules, including salicylic acid (Leon et al., 1995). One such signal molecule, most probably jasmonic acid (Walters et al., 2002), might induce expression of genes involved in polyamine biosynthesis, resulting in accumulation and subsequent hydrogen peroxide generation through degradation in apoplast.
It is well known that the oxidative burst after temperature shift in tobacco plants consists of two phases; phase I is rapid and transient, and phase II, arising a few hours after infection, persists for a longer period, up to several hours (Allan et al., 2001). Because of the late expression of genes involved in polyamine biosynthesis and persistent accumulation of their products, we speculate that hydrogen peroxide produced by polyamine degradation may contribute to phase II. However, onset of the HR was not completely suppressed in the presence of guazatine, which substantially reduced polyamine degradation. This implies that some other mechanism(s) might simultaneously operate in generation of hydrogen peroxide at the time of polyamine catabolism. If this is the case, a question arises as to how these sources of hydrogen peroxide, NAD(P)H oxidase, polyamine oxidase, and other(s), are coordinately regulated, and which is more critical for production of hydrogen peroxide during HR. Furthermore, it should be also determined which reactive oxygen intermediates (O2–, H2O2, OHÿ, and others) contribute most to HR. Further investigations are required to address these questions and to substantiate our hypothesis.
It should be mentioned that plants might be capable of using both Orn and Arg for polyamine biosynthesis (Evans and Malmberg, 1989). Transcripts for Arg decarboxylase are also induced during HR (H. Yoda, Y. Yamaguchi, and H. Sano, unpublished data). However, little evidence has so far been presented for an active involvement. Arg decarboxylase from oats (Avena sativa) has been shown to be localized at thylakoid membranes of chloroplasts (Borrell et al., 1995), whereas ODC is generally found in the cytoplasm (Tiburcio et al., 1990). One possibility is that plants are equipped with two alternative pathways for polyamine biosynthesis that function independently in the plant defense network. Another possibility is that Arg decarboxylase regulates polyamine levels in a similar manner as in mammalian cells, in which agmatine, a product of Arg decarboxylase, has been shown to exert effects by inducing an antizyme for ODC (Babal et al., 2001).
Concluding Remarks
It has thus far been a matter of speculation if hydrogen peroxide generated through polyamine degradation contributes to the HR, including hypersensitive cell death. The current study strongly supports this hypothesis by identifying ODC and polyamine oxidase activities and simultaneous generation of hydrogen peroxide during the HR in tobacco plants. To our knowledge, this is the first concrete observation as to the biochemical role of polyamines in the HR. However, the conclusion must be qualified because polyamines and their conjugates have been reported to be multifunctional, raising the possibility that they play some other role(s) even during the HR. Further analyses including their distribution and quantification during the HR will be of help to better understand the mechanism for plant disease resistance.
MATERIALS AND METHODS
Plant Materials and Treatments
Leaves of 2-month-old tobacco plants (Nicotiana tabacum cvs Xhanti nc and Samsum nn) were inoculated with TMV (10 μg mL–1) as previously described (Yoda et al., 2002) and incubated at 30°C under continuous light for 48 h and then at 20°C (temperature shift). For treatment with polyamine biosynthesis inhibitors, 10 mm DFMO (Sigma-Aldrich, St. Louis) and 10 mm MGBG (Sigma-Aldrich) solution, and with the polyamine oxidase inhibitor, 10 mm guazatine, these agents were absorbed at cutting sites of detached leaves immediately after TMV infection (48 h before temperature shift) and 12 h before temperature shift, respectively. Polyamines (10 mm each) were infiltrated into detached leaves (tobacco cv Xhanti nc) with a 1-mL syringe without a needle. Incubation was at 25°C under continuous light. For DAB (Sigma-Aldrich) staining, DAB-HCl solution (1 mg mL–1, pH 3.8) was infiltrated 6 h after the first exposure to polyamine, and incubation was performed for a further 6 h. DAB deposits were visualized after washing leaves in boiled 100% (v/v) ethanol for 15 min to remove chlorophyll.
RNA Isolation and Gel-Blot Analysis
Total RNA was isolated by the acid guanidinium thiocyante-phenolchloroform (AGPC) method (Chomczynski and Sacchi, 1987), and gel-blot analyses were performed as previously described (Yoda et al., 2002). The probe for ODC was synthesized with a pair of specific primers: forward, 5′-GGATGGCCGGCCAAACAATC-3′, and reverse, 5′-TCAGCTTGGATAAGAATAAGCG-3′. Probes for cDNAs encoding spermidine synthase and S-adenosyl-Met decarboxylase were prepared by digesting the plasmid containing each gene with appropriate restriction enzymes.
Polyamine Quantification
To extract polyamines from apoplast of tobacco leaves, 15 leaf-discs (15 mm in diameter) were cut out, weighed, and submerged in water in vacuo. Subsequently, they were subjected to centrifugation to recover apoplastic fluid by placing them in a 10-mL syringe, which was set in a 50-mL Falcon tube. Extracted fluid-containing polyamines were derivatized with benzoyl chloride as described (Flores and Galston, 1982). Subsequent benzoylated polyamines were separated and quantified by HPLC (Waters, Milford, MA) with a reverse-phase (C18) column and an UV detector (254 nm) at room temperature. The solvent system was run isocratically at 64% (v/v) methanol, at a flow rate of 1 mL min–1. Standard curves for estimation were obtained by measuring different known amounts of each polyamine.
Assay for Polyamine Oxidase
Apoplastic proteins from 500 leaf-discs (15 mm in diameter) of healthy tobacco leaves were recovered by centrifugation as described above. This recovery procedure was repeated twice, and second fluid was employed for enzymatic activity. A 10-μL aliquot of recovered fluid was reacted with 5 mm putrescine, spermidine, or spermine for indicated minute(s), and measurement of hydrogen peroxide was performed for 30 s with a luminometer (Lumat LB 9507, EG & G Berthold, Wildbad, Germany) in a 40 μL total volume of 62.5 mm Tris-HCl (pH 8.0) containing 125 μm luminal (Bolwell et al., 1995). For inhibition of polyamine oxidase activity, guazatine was added to 10-μL aliquots of apoplast solution, and mixtures were incubated at room temperature for 30 min before performance of chemiluminescence reactions as described above. Produced hydrogen peroxide was measured using luminol. For assays for TMV or mock-inoculated leaves, 18 leaf discs (15 mm in diameter) were cut out at indicated times after temperature shift and submerged in 10 mm Tris-HCl (pH 8.0) buffer in vacuo. The recovered second fluid was measured in the reaction with each 5 mm polyamines for 1 min as described above.
Gel Filtration
Apoplast fluid containing polyamine oxidase activity was collected from 500 leaf discs (15 mm in diameter) of healthy tobacco leaves as described above. The collected fluid (about 5 mL) was concentrated to about 0.2 mL by centrifugation with filter devices (CentricutminiU-10, Kurabou, Tokyo), and then subjected to gel filtration on a column of 16/60 Superdex 75 pregrade (Amersham Biosciences, Uppsala) equilibrated with 10 mm Tris-HCl (pH 8.0) containing 0.2 m NaCl. Each 1-mL fraction was collected at a flow rate of 1 mL min–1, polyamine oxidase activity was measured by chemiluminescence using luminol as described above, and fractions containing the highest activity were determined. The relative molecular mass (Mr) was estimated from the standard curve obtained from marker proteins.
Acknowledgments
We are grateful to Drs. Takashi Hashimoto (Nara Institute of Science and Technology) and Kenzo Nakamura (Nagoya University, Japan) for generous gifts of cDNA clones of spermidine synthase and S-adenosyl-Met decarboxylase, respectively. We also thank Drs. Nozomu Koizumi (Nara Institute of Science and Technology) and Malcolm A. Moore (Intermal, Nagoya, Japan) for valuable suggestions and critical reading of the manuscript, respectively.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.024737.
This work was supported by the Research for the Future Program of the Japan Society for the Promotion of Science (grant no. JSPS-RFTF00L01604).
References
- Allan AC, Lapidot M, Culver JN, Fluhr R (2001) An early tobacco mosaic virus-induced oxidative burst in tobacco indicates extracellular perception of the virus coat protein. Plant Physiol 126: 97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm K, Berntsson PS, Kramer DL, Porter CW, Oredsson SM (2000) Treatment of cells with the polyamine analog N,N11-diethylnorspermine retards S phase progression within one cell cycle. Eur J Biochem 267: 4157–4164 [DOI] [PubMed] [Google Scholar]
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92: 773–784 [DOI] [PubMed] [Google Scholar]
- Babal P, Ruchko M, Campbell CC, Gilmour SP, Mitchell JL, Olson JW, Gillespie MN (2001) Regulation of ornithine decarboxylase activity and polyamine transport by agmatine in rat pulmonary artery endothelial cells. J Pharmacol Exp Ther 296: 372–377 [PubMed] [Google Scholar]
- Bello-Fernandez C, Packham G, Cleveland JL (1993) The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci USA 90: 7804–7808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick CS, Brown IR, Bennett MH, Mansfield JW (1997) Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola. Plant Cell 9: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwell GP, Butt VS, Davies DR, Zimmerlin A (1995) The origin of the oxidative burst in plant cells. Free Radic Res 31: 517–532 [DOI] [PubMed] [Google Scholar]
- Bonneau MJ, Poulin R (2000) Spermine oxidation leads to necrosis with plasma membrane phosphatidylserine redistribution in mouse leukemia cells. Exp Cell Res 259: 23–34 [DOI] [PubMed] [Google Scholar]
- Borrell A, Culianez-Macia A, Altabella T, Besford RT, Flores D, Tiburcio AF (1995) Arginine decarboxylase is located in chloroplasts. Plant Physiol 109: 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley DJ, Kjellbom P, Lamb CJ (1992) Elicitor- and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: a novel, rapid defense response. Cell 70: 21–30 [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162: 156–159 [DOI] [PubMed] [Google Scholar]
- Cohen SS (1998) A Guide to the Polyamines. Oxford University Press, Oxford, UK
- DeScenzo RA, Minocha SC (1993) Modulation of cellular polyamines in tobacco by transfer and expression of mouse ornithine decarboxylase cDNA. Plant Mol Biol 22: 113–127 [DOI] [PubMed] [Google Scholar]
- Evans PT, Malmberg RL (1989) Do polyamines have roles in plant development? Annu Rev Plant Physiol Plant Mol Biol 40: 235–269 [Google Scholar]
- Flores HE, Galston AW (1982) Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiol 69: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianinazzi S (1970) Hypersensibilite aux virus, temperatures et proteines solubles chez le Nicotiana tabacum cv. Xanthi-nc. C R Acad Sci Paris D270: 2382–2386 [Google Scholar]
- Ha HC, Woster PM, Yager JD, Casero RA Jr (1997) The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc Natl Acad Sci USA 94: 11557–11562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond-Kosack KE, Jones JD (1996) Resistance gene-dependent plant defense responses. Plant Cell 8: 1773–1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Damude HG, Werner D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10: 255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Altabella T, Taylor MA, Tiburcio AF (1997) Recent advances in polyamine research. Trends Plant Sci 2: 124–130 [Google Scholar]
- Kusunoki S, Yasumasu I (1978) Inhibitory effect of alpha-hydrazinoornithine on egg cleavage in sea urchin eggs. Dev Biol 67: 336–345 [DOI] [PubMed] [Google Scholar]
- Laurenzi M, Tipping AJ, Marcus SE, Knox JP, Federico R, Angelini R, McPherson MJ (2001) Analysis of the distribution of copper amine oxidase in cell walls of legume seedlings. Planta 214: 37–45 [DOI] [PubMed] [Google Scholar]
- Leon J, Lawton MA, Raskin I (1995) Hydrogen peroxide stimulates salicylic acid biosynthesis in tobacco. Plant Physiol 108: 1673–1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583–593 [DOI] [PubMed] [Google Scholar]
- Lindsay GS, Wallace HM (1999) Changes in polyamine catabolism in HL-60 human promyelogenous leukaemic cells in response to etoposide-induced apoptosis. Biochem J 337: 83–87 [PMC free article] [PubMed] [Google Scholar]
- Marini F, Betti L, Scaramagli S, Biondi S, Torrigiani P (2001) Polyamine metabolism is upregulated in response to tobacco mosaic virus in hypersensitive, but not in susceptible, tobacco. New Phytol 149: 301–309 [DOI] [PubMed] [Google Scholar]
- Martin-Tanguy J, Martin C, Gallet M (1973) Presence de composes aromatiques lies a la putrescine dans drives Nicotiana viruses. CR Acad Sci Paris D276: 1433–1435 [Google Scholar]
- Masgrau C, Altabella T, Farras R, Flores D, Thompson AJ, Besford RT, Tiburcio AF (1997) Inducible overexpression of oat arginine decarboxylase in transgenic tobacco plants. Plant J 11: 465–473 [DOI] [PubMed] [Google Scholar]
- Morgan DM (1998) Polyamine oxidases: enzymes of unknown function? Biochem Soc Trans 26: 586–591 [DOI] [PubMed] [Google Scholar]
- Morgan DM (1999) Polyamines: an overview. Mol Biotechnol 11: 229–250 [DOI] [PubMed] [Google Scholar]
- Negrel J, Vallee JC, Martin C (1984) Ornithine decarboxylase activity and the hypersensitive reaction of tobacco to tobacco mosaic virus in Nicotiana tabacum. Phytochemistry 23: 2747–2751 [Google Scholar]
- Packham G, Cleveland JL (1994) Ornithine decarboxylase is a mediator of c-Myc-induced apoptosis. Mol Cell Biol 14: 5741–5747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parchment RE (1993) The implications of a unified theory of programmed cell death, polyamines, oxyradicals and histogenesis in the embryo. Int J Dev Biol 37: 75–83 [PubMed] [Google Scholar]
- Parchment RE, Pierce GB (1989) Polyamine oxidation, programmed cell death, and regulation of melanoma in the murine embryonic limb. Cancer Res 49: 6680–6686 [PubMed] [Google Scholar]
- Radova A, Sebela M, Galuszka P, Frebort I, Jacobsen S, Faulhammer HG, Pec P (2001) Barley polyamine oxidase: characterisation and analysis of the cofactor and the N-terminal amino acid sequence. Phytochem Anal 12: 166–173 [DOI] [PubMed] [Google Scholar]
- Sebela M, Radova A, Angelini R, Tavladoraki P, Frebort I, Pec P (2001) FAD-containing polyamine oxidases: a timely challenge for researchers in biochemistry and physiology of plants. Plant Sci 160: 197–207 [DOI] [PubMed] [Google Scholar]
- Seiler N, Atanassov CL (1994) The natural polyamines and immune system. Prog Drug Res 43: 87–141 [DOI] [PubMed] [Google Scholar]
- Seiler N, Atanassov CL, Raul F (1998) Polyamine metabolism as target for cancer chemoprevention. Int J Oncol 13: 993–1006 [DOI] [PubMed] [Google Scholar]
- Shah N, Thomas T, Shirahata A, Sigal LH, Thomas TJ (1999) Activation of nuclear factor κB by polyamines in breast cancer cells. Biochemistry 38: 14763–14774 [DOI] [PubMed] [Google Scholar]
- Slocum RD, Furey MD (1991) Electron-microscopic cytochemical localization of diamine and polyamine oxidases in pea and maize tissues. Planta 183: 443–450 [DOI] [PubMed] [Google Scholar]
- Tavladoraki P, Schinina ME, Cecconi F, Di Agostino S, Manera F, Rea G, Mariottini P, Federico R, Angelini R (1998) Maize polyamine oxidase: primary structure from protein and cDNA sequencing. FEBS Lett 426: 62–66 [DOI] [PubMed] [Google Scholar]
- Thomas T, Shah N, Klinge CM, Faaland CA, Adihkarakunnathu S, Gallo MA, Thomas TJ (1999) Polyamine biosynthesis inhibitors alter protein-protein interactions involving estrogen receptor in MCF-7 breast cancer cells. J Mol Endocrinol 22: 131–139 [DOI] [PubMed] [Google Scholar]
- Thomas T, Thomas TJ (2001) Polyamine in cell growth and cell death: molecular mechanisms and therapeutic applications. Cell Mol Life Sci 58: 244–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiburcio AF, Kaur-Sawhney R, Galston AW (1990) Polyamine metabolism. In BJ Miflin, PJ Lea, eds, Intermedatory Nitrogen Metabolism. Academic Press, New York, pp 283–325
- Torrigiani P, Rabiti AL, Bortolotti C, Betti L, Marani F, Canova A, Bagni N (1997) Polyamine synthesis and accumulation in the hypersensitive response to TMV in Nicotiana tabacum. New Phytol 135: 467–473 [Google Scholar]
- Walden R, Cordeiro A, Tiburcio AF (1997) Polyamines: small molecules triggering pathways in plant growth and development. Plant Physiol 113: 1009–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters D, Cowley T, Mitchell A (2002) Methyl jasmonate alters polyamine metabolism and induces systemic protection against powdery mildew infection in barley seedlings. J Exp Bot 53: 747–756 [DOI] [PubMed] [Google Scholar]
- Walters DR (2000) Polyamine in plant-microbe interactions. Physiol Mol Plant Pathol 57: 137–146 [Google Scholar]
- Yamakawa H, Kamada H, Satoh M, Ohashi Y (1998) Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection. Plant Physiol 118: 1213–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoda H, Ogawa M, Yamaguchi Y, Koizumi N, Kusano T, Sano H (2002) Identification of early responsive genes associated with the hypersensitive response upon tobacco mosaic virus infection and properties of a WRKY-type transcription factor in tobacco plants. Mol Genet Genom 267: 154–161 [DOI] [PubMed] [Google Scholar]