Abstract
Plants respond to various stresses by expressing distinct sets of genes. The effects of multiple stresses on plants and their interactions are not well understood. We have discovered that salt stress causes the accumulation of proteinase inhibitors and the activation of other wound-related genes in tomato (Lycopersicon esculentum) plants. Salt stress was also found to enhance the plant's response to wounding locally and systemically. The tomato mutant (def-1), which has an impairment in the octadecanoid pathway, displayed a severe reduction in the accumulation of proteinase inhibitors under salt stress, indicating that salt stress-induced accumulation of proteinase inhibitors was jasmonic acid dependent. The analysis of salt stress in another tomato mutant, spr-1, which carries a mutation in a systemin-specific signaling component, and transgenic tomato plants that express an antisense-prosystemin cDNA, showed that prosystemin activity was not required for the salt-induced accumulation of proteinase inhibitors, but was necessary to achieve maximal levels. These results suggest that a prosystemin independent- but jasmonic acid-dependent pathway is utilized for proteinase inhibitor accumulation in response to salt stress.
Plants in the field are exposed to multiple stresses, and their response to these various stresses determines their capacity to survive. Plants can use multiple signaling pathways and signals to mediate their response; for example, at least four different signaling pathways have been identified for water-deficit stress (Shinozaki and Yamaguchi-Shinozaki, 1997; Xiong et al., 2002). Different forms of stress may activate or utilize the same components, including proteins and other signaling molecules. Transgenic tobacco (Nicotiana tabacum) plants expressing constitutively active NPK1, a mitogen-activated protein kinase kinase kinase (MAPKKK) showed an enhanced tolerance to cold, heat shock, and salt stress (Kovtun et al., 2000). Signaling molecules such as jasmonic acid (JA) are involved in multiple stress responses and development in plants (Creelman and Mullet, 1995, 1997; Turner et al., 2002). However, it is the specific combination of various components of the signaling network coupled with spatial and temporal factors that allows the plant to mount a directed response to any given stress factor.
The phenomenon of cross-tolerance where a plant's resistance to one stress resulted in resistance to another form of stress is a growing area of research (Genoud and Metraux, 1999; Bowler and Fluhr, 2000). Increased tolerances to multiple stresses can involve the interaction between abiotic and biotic stresses. For example, plants pretreated with a sub-lethal dose of ozone or UV irradiation exhibited tolerance to a virulent pathogen (Yalpani et al., 1994; Sharma et al., 1996). Inoculation of Arabidopsis with a rhizobacterium made the plants more tolerant to drought and the pathogen Erwinia carotovora (Timmusk and Wagner, 1999). It has been shown that drought and UV radiation act synergistically to induce protective mechanisms in pea (Pisum sativum) and wheat (Triticum aestivum) plants (Alexieva et al., 2001). Grasses infected with endophytic fungi display enhanced tolerance to a variety biotic and abiotic stresses (Bacon et al., 1997; Malinowski and Belesky, 2000). Currently, very little is known on how different stresses interact with one another, or how their interaction will effect the plant's ability to respond to its environment or a given stress.
Wounding in tomato (Lycopersicon esculentum) plants is a well-characterized stress response. Tomato plants respond to mechanical wounding or herbivorous insect attack by inducing the synthesis of a wide array of defense-related proteins at the wound site and systemically throughout the distal portions of the plant (Bergey et al., 1996). Wounding causes the release and mobilization of an 18-amino acid polypeptide hormone called systemin (Pearce et al., 1991) and other wound-signaling peptides (Ryan et al., 2002). The binding of systemin to a cell surface receptor kinase (Scheer and Ryan, 2002) causes alterations in ion transport (Felix and Boller, 1995; Schaller and Oecking, 1999), the formation of hydrogen peroxide (Orozco-Cárdenas and Ryan, 1999; Orozco-Cárdenas et al., 2001), the activation of an MAPK (Stratmann and Ryan, 1997; Stratmann et al., 2000a), an increase of intracellular calcium (Moyen et al., 1998) and calmodulin (Bergey and Ryan, 1999), and the activation of a phospholipase A2 (Lee et al., 1997; Narváez-Vásquez et al., 1999). The phospholipase acts on plant membranes to release linolenic acid, which is subsequently converted to the biologically active oxylipins, including 12-oxo-phytodienoic acid and JA via the octadecanoid pathway (for review, see Vick and Zimmerman, 1984; Farmer and Ryan, 1992; Peña-Cortés et al., 1993; Blechert et al., 1995; Doares et al., 1995a, 1995b; Bergey et al., 1996). In addition to the elevated levels of oxylipins, wounding induces the synthesis of ethylene, which appears to act synergistically with JA to induce the expression of the defense genes (Felix and Boller, 1995; O'Donnell et al., 1996).
The wound response in tomato plants has been shown to be inhibited as well as activated by other stresses. The signaling molecule salicylic acid produced in response to infection with biotrophic pathogens was shown to inhibit the synthesis of proteinase inhibitors in response to wounding and herbivory (Doherty et al., 1988; Peña-Cortés et al., 1993; Doares et al., 1995b). However, when tomato plants were challenged with the pathogen Pseudomonas syringae pv. tomato (Pst), the causal agent for bacterial speck disease, it increased plants resistance to chewing insects (Bostock et al., 2001). This increased resistance may be due to elevated levels of JA present in plant tissues as a result of the infection by the necrotrophic pathogen (Pieterse and van Loon, 1999). The exposure of tomato seedlings to a form of abiotic stress, UVC radiation, resulted in a JA-mediated activation of wound-related genes and the synthesis of proteinase inhibitors (Conconi et al., 1996). However, when tomato plants were exposed to UVB/UVA radiation, they did not accumulate proteinase inhibitors, but showed a strong potentiation of the systemic induction of proteinase inhibitor synthesis in response to wounding (Stratmann et al., 2000b). It has also been reported that the wound-inducible defense gene Leu aminopeptidase is activated by water deficit, salinity, and abscisic acid (Chao et al., 1999).
In this report, we have investigated the effects of salt stress on the wound response cascade in tomato plants. Salt stress alone was found to induce wound-related genes and this gene activation was mediated via the octadecanoid pathway. The salt-dependent activation of the octadecanoid pathway was found to be independent of the wound prohormone, prosystemin, but prosystemin (PS) activity was necessary to achieve maximal accumulation of proteinase inhibitors. In addition, salt stress was found to strongly enhance the plant's ability to respond to wounding. This analysis describes how an abiotic stress activates the wound response in tomato, and provides further insight into defense gene signaling and the interaction between stress responses in plants.
RESULTS
Salt Stress Induction of Proteinase Inhibitor II (Inh II) Accumulation
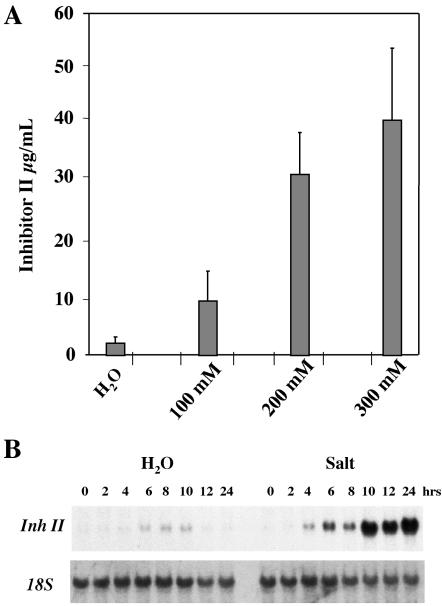
We wanted to determine how salt stress would affect the wound response in tomato plants. Salt stress was chosen because it is an abiotic stress that is initially perceived in the roots, in contrast to wounding that is inflicted on the leaves. The roots of 15-d-old soil-grown tomato seedlings were treated with different concentrations of NaCl and were assayed 24 h later for proteinase inhibitor accumulation. Figure 1A shows an NaCl concentration-dependent accumulation of Inh II. Plants exposed for 24 h to 100 mm NaCl produced a weak accumulation of Inh II, nearly 10 μg mL-1 leaf juice. Tomato seedlings treated with 200 mm NaCl produced Inh II levels of 30 μg mL-1 leaf juice, similar to levels that are found in the systemic unwounded leaf in response to wounding (see Fig. 5). Inhibitor levels in plants treated with 300 mm NaCl were about four times higher as in plants treated with 100 mm NaCl. These results demonstrate that salt stress alone induces the accumulation of the Inh II protein. The plants exposed to 100 and 200 mm NaCl stress for 24 h appeared to be a slightly darker green than untreated control plants, but otherwise looked normal. However, plants exposed to 300 mm NaCl displayed signs of necrosis on the leaves.
Figure 1.
Protein accumulation and transcript levels of Inh II in response to 24 h exposure to salt stress. Fifteen-day-old tomato seedlings were treated with different concentrations of NaCl as indicated or water. A, The bars represent the levels of proteinase inhibitor II accumulation in unwounded leaves 24 h after treatment. The data represents a minimum of six independent experiments showing similar results (six plants/treatment). B, RNA-blot analysis of Inh II gene expression in response to salt stress. Total RNA was extracted from the leaves that were harvested at the times indicated (three plants/time point). Twenty micrograms of total RNA was loaded per lane. The RNA was hybridized with 32P-labeled Inh II DNA. 18S rRNA was used as loading control.
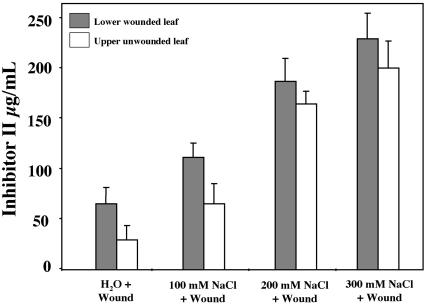
Figure 5.
Inh II accumulation in response to salt stress and wounding. Fifteen-day-old tomato seedlings were watered with different concentrations of NaCl (described above) or water. Immediately after treatment, the lower leaf was subjected to a single wound across the mid-vein and leaves were assayed 24 h later for accumulation of proteinase inhibitors. The bars represent the levels of Inh II accumulation in the wounded lower leaf (shaded bars) and upper unwounded leaf (white bars) 24 h after treatment. The data represents a minimum of six independent experiments showing similar results (six plants/treatment).
To determine the kinetics for salt-induced activation of the Inh II gene, a gel-blot analysis of Inh II transcript levels was performed in plants exposed to 200 mm NaCl over a 24-h period. As shown in Figure 1B, the analysis of Inh II transcript levels revealed a biphasic activation of the gene. After 4 h of exposure to salt stress, a weak activation of the Inh II gene was observed in the leaves. The transcript levels increased significantly by 6 h and appeared to wane after 8 h of exposure. This was followed by a dramatic increase in the gene activation after 10 h of exposure that was maintained throughout the remainder of the 24-h time course. In the water control plants, we observed only minimal activation of the Inh II gene in the leaves 4 h after watering the roots. This activation increased slightly over the next few hours, but disappeared after 12 h.
Effects of Prolonged Exposure to Salt Stress on the Induction of Wound-Response Genes
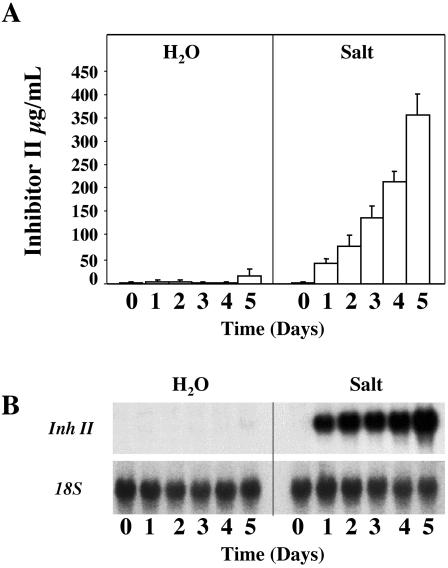
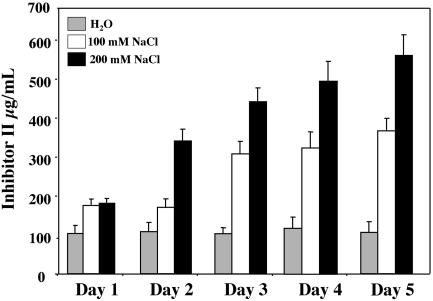
Because a limited exposure to salt stress alone induced the accumulation of Inh II in young tomato plants, we wanted to investigate the effects of prolonged exposure to salt stress on the activation of wound-response genes. Fifteen-day-old plants were watered daily with 200 mm NaCl solution over a 5-d period. At 24-h intervals, leaf tissue was collected and assayed for Inh II accumulation and RNA gelblot analysis. As shown in Figure 2A, plants subjected to continual watering with 200 mm NaCl showed a steady increase of nearly 50 μg mL-1 leaf juice per day of Inh II accumulation over the first 4 d of exposure. On the 5th d, we observed a 2-fold increase in total Inh II concentration in the leaf, increasing almost 200 μg mL-1 in a 24-h time period. This dramatic increase in Inh II concentration in 1 d was equal to the combined accumulation of the first 4 d and it was consistently observed during the course of our analysis. It should be noted that after the 5-d treatment period, the salt concentration in the soil might have accumulated to levels higher than 200 mm. Plants exposed to water alone showed limited Inh II accumulation over the 5-d period; this accumulation was most likely due to handling or watering of the plants.
Figure 2.
Protein accumulation and transcript levels of Inh II in unwounded tomato plants in response to long-term exposure to salt stress. A, Fifteen-day-old tomato seedlings were treated continuously with 200 mm NaCl or water over a 5-d period. The bars represent the levels of Inh II accumulation in unwounded leaves after the total number of days (indicated above) the plant was exposed to salt stress. The data represent five independent experiments showing similar results (six plants/time point). B, RNA-blot analysis of Inh II gene expression in response to continual treatment of 200 mm NaCl over a 5-d period. Total RNA was extracted from the leaves that were harvested at the times indicated above (three plants/time point). Twenty micrograms of total RNA was loaded per lane. The RNA was hybridized with 32P-labeled Inh II DNA probe. 18S rRNA was used as loading control.
As shown in Figure 2B, the increased accumulation of Inh II mRNA correlated directly with the increase in the levels of Inh II synthesized in the leaf. The steady increase of Inh II accumulation over the first 4 d can be explained by the constant high level of Inh II gene activation due to the continual exposure to salt stress. On d 5, the roughly 2-fold increase of Inh II protein accumulation correlated directly to a similar increase of 1.75-fold in the level of Inh II transcript in the leaves.
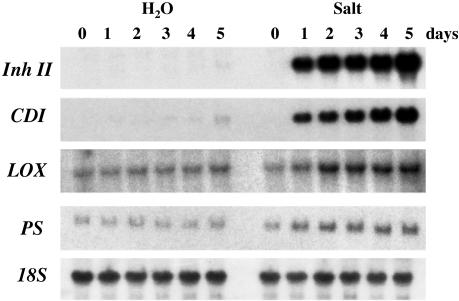
To gain further insight into the mechanisms leading to the dramatic increases in Inh II accumulation in leaves exposed to salt stress, we examined the patterns of expression of additional wound-related genes. Inh II and cathepsin D inhibitor (CDI) are defense-related genes that reflect the magnitude of the plant's wound response. PS and lipoxygenase (LOX) are signaling-related genes (Ryan, 2000). The LOX protein is an enzyme in the octadecanoid pathway involved in the conversion of linolenic acid to JA. The gel-blot analysis in Figure 3 shows the increases in transcript levels of the defense-related genes Inh II and CDI, as well as the wound-signaling genes PS and LOX in response to 5 d of continual exposure to 200 mm NaCl. As previously shown in Figure 2B, Inh II transcripts accumulated to a high level after 1 d and maintained a constant level of transcription until the 5th d, where we observed a 1.7-fold increase in transcript level. In contrast, CDI, showed a more gradual linear increase in expression level over the 5-d time course, also reaching its maximal level on the 5th d of exposure. The signaling genes LOX and PS showed elevated transcript levels after the initial 24 h of exposure to salt as compared with the untreated controls. These levels were maintained over the remainder of the time course.
Figure 3.
RNA-blot analysis of wound-related genes in unwounded plants in response to prolonged salt stress. Fifteen-day-old tomato seedlings were treated continuously with 200 mm NaCl (Salt) or water over a 5-d period. Total RNA was extracted from the leaves of plants that were harvested at the times indicated above (three plants/time point). Twenty micrograms of total RNA was loaded onto each lane. The RNA was hybridized with 32P-labeled Inh II, CDI, PS, and LOX probes. 18S rRNA was used as loading control. The salt and water control treatments were analyzed on the same membrane.
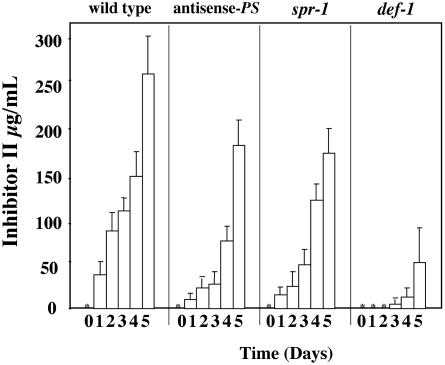
Because salt stress resulted in elevated transcript levels of the signaling genes PS and LOX, we wanted to investigate the roles of PS and the octadecanoid pathway in mediating this response. To better define the signal transduction pathway mediating the salt stress activation of the defense-related genes, we examined the accumulation of Inh II in transgenic antisense-PS plants and the mutant plants, spr-1 and def-1. These plants were constantly exposed to 200 mm NaCl over a 5-d period. The transgenic antisense-PS (McGurl et al., 1992) and the spr-1mutant plants (Howe and Ryan, 1999; Lee and Howe, 2003) disrupt PS activity and are dramatically impaired in the systemic accumulation of proteinase inhibitors in response to wounding. The antisense-PS plant's phenotype is the result of the suppression of PS expression, whereas the spr-1 mutant is defective in a signaling step that couples systemin perception to activation of the octadecanoid pathway. The def-1 mutant has been shown to have an impairment in the octadecanoid pathway, severely compromising its ability to respond to wounding (Howe et al., 1996).
As shown in Figure 4, wild-type plants exposed to 200 mm NaCl, displayed a steady increase in the accumulation of Inh II over the first 4 d of exposure. On the 5th d, a nearly 2-fold increase in total Inh II concentration was observed in the leaves, as previously shown in Figure 2A. The antisense-PS and spr-1 plants showed a similar pattern of accumulation, but were approximately 50% of wild-type levels. However, in the spr-1 mutant, the 2-fold increase in Inh II concentration was observed on d 4, rather than d 5 as was seen in wild-type and antisense-PS plants. The def-1 plants showed no Inh II accumulation during the first 2 d of exposure, and on d 3 and 4, Inh II accumulation was less than 10% of levels observed in wild-type plants at the same time points. Similar to wild-type and antisense-PS plants, the def-1 plants also displayed a 2- to 3-fold increase in Inh II accumulation on the 5th d of exposure.
Figure 4.
The accumulation of the Inh II protein in response to prolonged exposure to salt stress in unwounded antisense-PS, spr-1, and def-1 plants. Fifteen-day-old tomato seedlings of wild-type, antisense-PS, spr-1, and def-1 plants were continuously treated with 200 mm NaCl over a 5-d period. The bars represent the levels of Inh II accumulation observed in unwounded leaves after the total number of days (indicated above) the plant was exposed to salt. Asterisks represent zero Inh II accumulation. The data represents three independent experiments showing similar results (six plants/time point).
Enhancement of Wound-Inducible Proteinase Inhibitor Accumulation 24 h after Wounding and Salt Stress
To investigate how salt stress would affect the wound response, the roots of 15-d-old soil-grown tomato seedlings (two-leaf stage) were treated with different concentrations of NaCl. Immediately after salt treatment, the lower leaf was subjected to a single wound across the mid-vein, and leaves were assayed 24 h later for accumulation of proteinase inhibitors. Figure 5, shows a salt concentration-dependent enhancement of wound-induced Inh II accumulation. Wounded plants exposed to 100 mm NaCl produced nearly double the amount of Inh II in the wounded and systemic leaves when compared with water/wound control plants. In plants treated with 200 mm NaCl, the wounded leaves exhibited an increase in Inh II synthesis that was 3-fold higher than was found in leaves that had been wounded without salt treatment. The enhancement of wound-inducible Inh II accumulation was found to be even more pronounced in the systemic unwounded leaves, in which Inh II levels increased 5- to 6-fold in plants treated with 200 mm NaCl when compared with the systemic leaves of wounded nonsalt-treated control plants. Plants subjected to 300 mm NaCl stress had increases in Inh II accumulation that approached 4-fold in wounded leaves and 6-fold in systemic leaves as compared with controls.
Salt Stress Increases Systemic mRNA Levels of Wound-Related Genes
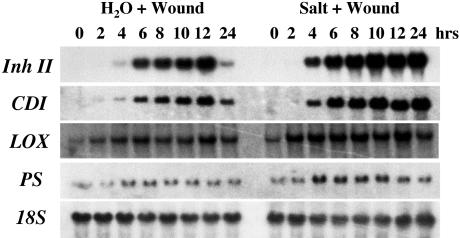
To further investigate the salt-enhanced systemic wound response, the transcript levels of wound-related genes were investigated in the upper unwounded leaves over a 24-h time frame. As shown in Figure 6, wounding of the control seedlings resulted in a typical systemic wound response, with Inh II and CDI transcripts beginning to accumulate 2 to 4 h after wounding, reaching a maximum 12 h after wounding, and then declining during the remainder of the time course. Whereas the basal levels of PS and LOX showed a transient increase over the same period. In comparison, plants exposed to both salt stress and wounding exhibited significantly higher levels of mRNA induction. The salt-stressed/wounded plants displayed increased systemic Inh II and CDI transcript levels 4 h after treatment, which continued to increase over the remainder of the time course. Relative transcript levels for the signaling genes LOX and PS were higher in the salt-stressed/wounded plants than in the water/wounded control plants. However, their levels decreased at 24 h in contrast to the defense-related genes.
Figure 6.
RNA-blot analysis of the systemic induction of wound-related genes in response to salt stress and wounding. Fifteen-day-old tomato seedlings were watered with 200 mm NaCl (Salt) or water. Immediately after treatment, the lower leaf was subjected to a single wound across the mid-vein. The upper unwounded (systemic) leaves were harvested at the times indicated and were immediately frozen in liquid nitrogen for RNA extraction. Twenty micrograms of total RNA was analyzed by northern blot. RNA blots were hybridized with 32P-labeled DNA probes (see Fig. 3) as indicated. 18S rRNA was used as loading control.
Comparison of Inh II Accumulation after Wounding and Salt Stress for Wild-Type, Antisense-PS, def-1, and spr-1 Tomato Plants
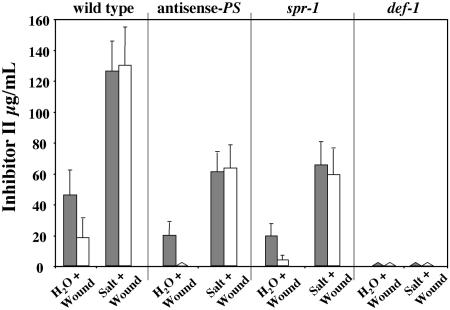
Because salt stress together with wounding enhanced the systemic expression of the signaling genes, we wanted to further investigate the roles of PS and the octadecanoid pathway in mediating this enhanced response. As shown in Figure 7, antisense-PS and spr-1 plants have a severely reduced systemic response (unwounded leaves) to water/wounding when compared with wild-type plants, but did show a significant accumulation of Inh II in the wounded (local) leaf, approximately 50% of wild-type levels, whereas def-1 plants show no Inh II accumulation in the wounded or unwounded leaves.
Figure 7.
The accumulation of the Inh II protein in response to salt stress and wounding in antisense-PS, spr-1, and def-1 plants. Fifteen-day-old tomato seedlings of wild-type, antisense-PS, spr-1, and def-1 mutant plants were treated with 200 mm NaCl or water. Immediately after treatment, the lower leaf was subjected to a single wound across the mid-vein and leaves were assayed 24 h later for accumulation of proteinase inhibitors. The bars represent the levels of Inh II accumulation in the wounded lower leaf (shaded bars) and upper unwounded leaf (white bars) 24 h after treatment. The data represent a minimum of three independent experiments showing similar results (six plants/treatment).
When the wild-type, antisense-PS, and spr-1 plants were exposed to 200 mm NaCl and wounding, we observed a 3-fold increase in the levels of Inh II protein in the wounded leaves when compared with the plants wounded without salt treatment. Surprisingly, salt stress and wounding in the antisense-PS and spr-1 mutants resulted in the accumulation of Inh II proteins in the unwounded leaves in contrast to water/wounded plants. Although the systemic accumulation of Inh II was restored, in antisense-PS and spr-1 plants, the overall accumulation of Inh II as a result of salt stress and wounding, both locally and systemically, remained roughly 50% of the levels observed in the wild-type plants. These Inh II levels observed systemically in the unwounded leaves of the antisense-PS and spr-1 plants were higher than what would be expected from just salt stress alone (Fig. 4). No Inh II accumulation was observed in def-1 plants in the wounded or unwounded leaves when subjected to salt treatment and wounding, indicating that an intact octadecanoid pathway is critical for the salt-induced enhancement of the wound response.
PS Maximizes Salt Stress-Induced Accumulation of Inh II
The reduced salt stress-induced Inh II accumulation observed in the antisense-PS and the PS insensitive spr-1 mutant plants suggested that PS activity is required to obtain maximal accumulation of Inh II in response to salt stress. To further investigate the role of PS in this response, we decided to examine the effects of salt stress in transgenic tomato plants overexpressing the PS cDNA under the control of the 35S promoter (sense-PS; McGurl et al., 1994). The sense-PS plants show a constitutive wound phenotype accumulating high levels of wound-related proteins (Bergey et al., 1996). Because prolonged exposure to salt stress alone was found to increase the level of wound-related transcripts such as PS, we wanted to determine whether salt stress could further increase the levels of Inh II accumulation in the absence of wounding in the sense-PS plants. Fifteen-day-old sense-PS plants were continually exposed to salt stress over a 5-d period. Each day, plants were assayed for accumulation of Inh II protein. Results in Figure 8 show that 24 h of exposure to 100 or 200 mm NaCl resulted in roughly two times the level of Inh II observed in water-treated control plants. Over the remaining 4 d of the time course, the salt-stressed sense-PS plants displayed a steady increase in accumulation of Inh II when compared with the untreated controls. After 5 d of treatment with 100 or 200 mm NaCl, sense-PS plants increased their accumulation of Inh II to three times or nearly six times (600–800 μg mL-1 leaf juice) the levels found in the untreated control, respectively.
Figure 8.
The accumulation of Inh II protein in unwounded transgenic plants overexpressing PS in response to prolonged exposure to salt stress. Fifteen-day-old tomato seedlings overexpressing the PS cDNA were continuously treated with salt over a 5-d period. The bars represent the levels of Inh II accumulation observed in unwounded leaves at the times indicated, untreated water control (gray bars), 100 mm NaCl (white bars), and 200 mm NaCl (black bars). The data represent three independent experiments showing similar results (six plants/time point).
DISCUSSION
Wounding in tomato plants is a well-characterized stress response. Tomato plants respond to mechanical wounding and herbivore attack by inducing the local and systemic synthesis of a wide variety of defense-related proteins, including proteinase inhibitors (Bergey et al., 1996; Ryan, 2000). The levels of accumulation of wound-inducible proteinase inhibitors in the leaves reflects the magnitude of the defense response. The activation and enhancement of the wound response by UV irradiation prompted us to investigate the effect of another abiotic stress, salt on the wound response. Our analysis revealed that a 24-h exposure to salt stress alone was able to induce the accumulation of the wound-inducible Inh II protein in a dose-dependent manner (Fig. 1).
Prolonged exposure to salt stress over a 5-d period resulted in the accumulation of high levels of proteinase inhibitors (Fig. 2). Salt-induced Inh II levels were as high as 350 to 450 μg mL-1 leaf juice, in contrast to a typical strong wound response that produces proteinase inhibitor levels of 100 to 120 μg mL-1 leaf juice. These increased levels were even more dramatic in sense-PS plants, obtaining levels of almost 600 μg mL-1 in leaf juice. This extremely high level in sense-PS plants is probably even higher because the concentrations of Inh II are at the limiting range of the radial immunodiffusion assay. All plants treated with 200 mm NaCl for 5 d grew slowly, with darker green leaves and were roughly one-half the size of untreated plants. It has been shown that prolong exposure to salt stress and drought results in lower water content in the leaves of tomato plants (Cuartero and Fernandez-Muñoz, 1999). We used northern-blot analysis to determine whether the increase in Inh II protein concentration was a result of an up-regulation in the gene expression or as a result of diminished water content in the leaf (Fig. 2). Although it is likely that dehydration of the plant contributes to elevated Inh II concentration in the leaf, the sustained high levels of Inh II transcript over the 5 d shown in Figure 2B suggests active protein synthesis. In addition, the 1.75-fold increase in Inh II transcript level on d 5 correlated very closely with the increase in protein accumulation observed in the leaf. Therefore, the extremely high Inh II concentrations observed in the leaves of the salt-treated plants were probably a result of a combination of factors: a high level of gene activation and protein synthesis, diminished water content in the leaf, and protein turnover during the 5-d period.
Northern-blot analysis revealed that salt stress elevated the transcript levels for the wound-signaling gene LOX, suggesting the involvement of the octadecanoid pathway in mediating the response. This was further confirmed when the def-1 mutant subjected to salt stress showed a severe impairment in the accumulation of the Inh II protein as compared with similarly treated wild-type plants (Fig. 3), and its failure to respond to both wounding and salt stress (Fig. 7). The def 1 mutant is impaired in the octadecanoid pathway, compromising its ability to accumulate JA in response to wounding and elicitors (Howe et al., 1996). This indicates that an intact octadecanoid pathway is necessary for the salt-induced accumulation of the wound-inducible Inh II protein. However, when exposed to salt stress, the def-1 mutant did accumulate low levels of Inh II protein, 10% to 20% of wild-type levels. Since the def-1 mutant has been described as a “leaky” mutant (Howe et al., 1996), the observed Inh II accumulation is likely due to either low-level JA biosynthesis or the action of another component associated with water stress, such as abscisic acid (Peña-Cortés et al., 1989, 1996; Wasternack et al., 1996).
In addition to salt stress, we found that wilting alone also induced the accumulation of proteinase inhibitors, and this response was also mediated via the octadecanoid pathway (data not shown). These data support the findings from a microarray analysis in Arabidopsis showing that a subset of wound-inducible genes was activated in dehydrating leaves (Reymond et al., 2000). However, because the analysis was conducted on excised leaves and not in whole plants, it was unclear if the increased transcript levels resulted from dehydration or a wound stimulus.
To further investigate the effects of salt stress on activation of wound-related genes, we investigated how salt stress that is initially perceived in the roots would affect the transmission of the wound signal in the leaves. This allowed us to examine the interaction of two distinct stresses that are initially perceived in different tissues and at distant locations. The data in Figure 5 show that tomato plants subjected to wounding and salt stress exhibit an enhanced response to wounding when compared with plants subjected to wounding alone. This salt-induced enhancement of the wound response occurs in the local wounded leaves, but was found to be more pronounced in the systemic unwounded leaves.
The increased accumulation of Inh II due to wounding and salt treatment cannot be explained solely by the additive effect of salt stress and wounding alone. We found that the simultaneous exposure to 100 mm NaCl and wounding increased Inh II levels an average of 60% higher in the wounded leaves, and 50% higher in the systemic leaves from what we would have expected if it was merely an additive effect. This enhancement was even more pronounced in plants subjected to 200 mm NaCl stress, where the increases were found to be nearly 100% higher in wounded and 180% in systemic leaves above the expected values for an additive effect. These data indicated that salt stress not only activates the wound response, but it also strongly enhances the response. These results are similar to the effects shown by tomato plants subjected to wounding and UVB/A irradiation (Stratmann et al., 2000b).
PS was another wound-signaling gene that was found to be up-regulated by salt stress. However, we showed that PS activity was not required for salt stress-induced accumulation of Inh II protein. This finding was based on the analysis of the effect of salt stress on the spr-1 mutant and antisense-PS tomato plants. The transgenic antisense-PS plant has a strongly suppressed PS expression (McGurl et al., 1992), resulting in a substantially reduced ability to systemically accumulate proteinase inhibitors in response to wounding, whereas the spr-1 tomato mutant is defective in PS perception and the production of a systemic wound signal (Lee and Howe, 2003). Exposure of the spr-1 mutant and antisense-PS tomato plants to salt stress resulted in a significant accumulation of Inh II, roughly 50% of wild-type levels. These results suggested that PS was not necessary for salt-induced accumulation of the Inh II protein. To further investigate the role of PS in the salt-induced accumulation of proteinase inhibitors, we subjected plants overexpressing the cDNA for PS (sense-PS) to salt stress. Sense-PS plants exhibit a constitutive wound phenotype, synthesizing and accumulating wound-related proteins to high levels (McGurl et al., 1994; Bergey et al., 1996). Just as with wounding, we wanted to examine if salt treatment could further enhance the accumulation of Inh II in sense-PS plants. We found that sense-PS plants exposed to salt stress had dramatically increased levels of Inh II in the leaves (Fig. 8). These results combined with data that showed reduced levels of Inh II in the spr-1 and antisense-PS plants (Fig. 4) indicate that although PS activity is not required for salt activation of wound response genes, it is necessary to achieve maximum levels of accumulation.
When we investigated the effects of salt stress on wounding in the spr-1 and antisense-PS backgrounds, we were surprised to discover that salt stress had appeared to restore the systemic accumulation of Inh II, although at 50% the level observed in wild-type plants (Fig. 7). The systemically accumulated Inh II levels cannot be explained by just salt stress induction alone because the Inh II levels were at least two times higher than what is observed in the unwounded salt treated controls (Figs. 1 and 4). However, it is unclear how this restoration of the systemic response occurs.
What insights can we derive from our analysis of salt stress activation of the wound response with respect to signaling? We found that the initial activation of Inh II gene occurs 4 h after wounding or salt treatment alone. This suggests that the initial salt-induced signal from the root travels (Fig. 1) at the same velocity to the leaf as the wound signal from wounded to systemic leaf (Fig. 6). However, these signals were generated differently. In leaves, a single transient wound across the mid-vein was made, whereas intact roots where exposed to a continual salt stimulus. Another possibility could be that the salt-induced signal travels faster but the concentrations are very low, requiring 4 h to reach levels that can induce the gene. This salt-induced gene activation appears to occur in two phases. One can speculate that the initial signal leading to gene activation originates from the roots (Jackson, 1997), whereas the second may be produced in the leaf. Because the octadecanoid pathway mediates the salt-induced accumulation of Inh II, it follows that the signal generated may be JA or some other oxylipin. There is evidence from other studies that show increased JA accumulation in the roots and leaves of salt-stressed plants (Moons et al., 1997; Wang et al., 2001). These salt-induced increases in JA levels could occur as a by-product of alterations in membrane composition and structure, or as a result of the activation of fatty acid desaturases, which lead to release and accumulation of linolenic acid, the precursor to JA biosynthesis (Khaware et al., 1995; Surjus and Durand, 1996; Nishiuchi and Iba, 1998).
There is evidence suggesting that JA acts as the long-distance wound signal (Zhang and Baldwin, 1997; Li et al., 2002). In addition, the recent analysis of the spr-1 mutant indicates that a synergistic interaction between JA and systemin is necessary to generate and transduce the systemic signal, and that the mutant displayed reduced JA accumulation in wounded leaves (Lee and Howe, 2003). Our results appear to be consistent with the latest findings with respect to wound signaling, and the synergistic interaction of JA and PS.
Based on these recent findings, we can propose a mechanism for accumulation of Inh II in response to salt stress. Salt stress leads to the synthesis and release of JA. JA induces the synthesis of the PS and Inh II proteins. The newly synthesized PS can activate the wound-response pathway (Dombrowski et al., 1999), resulting in the further release of linolenic acid from the membranes leading to JA biosynthesis and the accumulation of additional Inh II and PS, thereby creating a positive feedback loop that amplifies the salt-induced wound response. Coupling this feedback loop with a salt-induced, PS-independent JA biosynthesis results in the accumulation of very high levels of proteinase inhibitors.
Whether the strong induction of the wound response by salt stress is relevant to the success of the plant in the field is not known. In addition, it is unclear if this activation provides the plant any increased tolerance to water-deficit stress. Sense-PS plants were found to survive higher initial concentrations of salt than wild-type plants (J.E. Dombrowski and C.A. Ryan, unpublished data). One possible explanation for their increased survival rate could be that in addition to accumulating wound-related proteins, there is a concurrent activation of water deficit-tolerance genes. In support of this hypothesis, microarray analysis revealed that mechanical wounding activated osmotic stress-related genes in Arabidopsis (Reymond et al., 2000; Cheong et al., 2002). The survivability of the sense-PS plants could also be attributed to the presence of high levels of proteinase inhibitors in the leaves or even PS itself, a highly hydrophilic protein (McGurl et al., 1992), which could function as osmoprotectants.
JA has been implicated in a wide range of stress responses, as well as development in plants (Creelman and Mullet, 1995, 1997; Turner et al., 2002). It has been reported that pretreatment of barley (Hordeum vulgare) seedlings with JA improved their performance to salt stress (Tsonev et al., 1998). Sorbitol stress in barley led to a low and transient rise in JA levels in the leaves (Kramell et al., 2000). Salt stress increased JA levels in the roots of rice (Oryza sativa) plants and in the leaves of Iris hexagona (Moons et al., 1997; Wang et al., 2001). However, it is still unclear exactly what role JA plays in the water-deficit response, and more research is needed to better define its function.
Why would a plant redirect energy and resources to activate a defense pathway and accumulate such high levels of proteinase inhibitors during a period of water deficit? One possible rationale could be that plants under water stress display reduced growth, which results in decreases in the overall biomass for herbivorous insects to feed on. The activation of the wound response by water-deficit stress would protect the plant against defoliating chewing insects during periods of low growth and would preserve limited foliage until water resources are no longer limiting, thereby improving their survivability. Alternatively, the water deficit-mediated activation of the wound response could simply be a serendipitous event due to functional redundancy in stress signaling networks.
We are beginning to discover that responses to stress are not linear pathways, but are complicated integrated circuits involving multiple pathways and specific cellular compartments, tissues, and the interaction of additional cofactors and/or signaling molecules to coordinate a specified response to a given stimulus. The work described here provides another important step toward unraveling the interactions of stress-activated pathways in plants.
MATERIALS AND METHODS
Plant Material
Wild-type tomato (Lycopersicon esculentum cv Castlemart and cv Better Boy), mutants def-1 and spr-1 (cv Castlemart), transgenic tomato plants overexpressing the sense orientation of PS cDNA (cv Castlemart), and the antisense orientation of PS cDNA (cv Better Boy) were grown in peat pots for 17 h at 28°C under >300 μE m-2 s-1light followed by a 7-h, 17°C dark period. Plants at this stage of development displayed two expanded leaves and a small apical leaf.
Plant Treatments and Proteinase Inhibitor Bioassay
Seven-day-old seedlings in peat pots were transferred to plastic trays and were watered at their base to avoid background due to touch response (Stratmann et al., 2000b). Fifteen-day-old plants were subjected to salt stress by watering at their base with 100, 200, or 300 mm of NaCl from 1 to 5 d. In wounding experiments, immediately after salt treatment, the lower leaf was subjected to a single wound with a hemostat perpendicular to the mid-vein located two-thirds in from the tip of the leaf. After 24 h, Inh II concentrations were assayed from expressed leaf juice of treated plants by radial immunodiffusion as previously described (Ryan, 1967). To measure salt-induced Inh II accumulation in unwounded plants, juice was expressed from both leaves of control (water) plants or salt-treated plants immediately after treatment and in 24-h intervals from the time of initial treatment. A minimum of three plants per time point was analyzed for each experiment.
RNA Gel-Blot Analysis
Leaves were harvested at the times indicated in the “Results” and were immediately frozen in liquid nitrogen for RNA extraction. A minimum of three plants per time point was used. Total RNA extractions were performed using Trizol reagent (Life Technologies, Gaithersburg, MD) following manufacturer instructions. Twenty micrograms of total RNA was loaded in 1.4% (w/v) agarose gels for separation, and gel-blot analyses were performed as described in Moura et al. (2001). 32P-labeled DNA probes were generated from cDNAs (described in Ryan, 2000) using the DECAprime II DNA labeling kit (Ambion, Austin, TX), and were used to hybridize the RNA. Gel blots were hybridized with 18S rRNA for loading control. All experiments were repeated at least twice. We subjected the autoradiograms to scanning densitometry (Densitometer SI; Molecular Dynamics, Sunnyvale, CA) as a way to measure changes in the levels of gene expression.
All molecular biology procedures and solutions used here were as described by Sambrook et al. (1989)
Acknowledgments
I thank the following: Dr. Clarence A. Ryan (Washington State University, Pullman, WA) for his generous support that enabled me to conduct this research in his laboratory; Dr. Daniel Moura (Washington State University, Pullman, WA) for his invaluable help on the northern-blot analyses and generation of figures; Sue Vogtman (Washington State University, Pullman, WA) for growing and maintaining the plants used in this research; and Dr. Gregg A. Howe (Michigan State University, East Lansing, MI) for his generous gift of the spr-1 mutant.
This research was supported in part by Washington State University College of Agriculture and Home Economics (Project no. 1791), by the National Science Foundation (grant no. IBN 9601099), and by the U.S. Department of Agriculture/Competitive Grants Research Office (grant no. WNP03153).
References
- Alexieva V, Sergiev I, Mapelli S, Karanov E (2001) The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ 24: 1337-1344 [Google Scholar]
- Bacon CW, Richardson MD, White JF (1997) Modification and uses of endophyte-enhanced turf grasses: a role for molecular technology. Crop Sci 37: 1415-1425 [Google Scholar]
- Bergey DR, Howe GA, Ryan CA (1996) Polypeptide signaling for plant defensive genes exhibits analogies to defense signaling in animals. Proc Natl Acad Sci USA 93: 12053-12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergey DR, Ryan CA (1999) Wound- and systemin-inducible calmodulin gene expression in tomato leaves. Plant Mol Biol 40: 815-823 [DOI] [PubMed] [Google Scholar]
- Blechert S, Brodschelm W, Holder S, Kammerer L, Kutchan TM, Mueller MJ, Xia Z-Q, Zenk MH (1995) The octadecanoid pathway: signal molecules for the regulation of secondary pathways Proc Natl Acad Sci USA 92: 4099-4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock RM, Karban R, Thaler JS, Weyman PD, Gilchrist D (2001) Signal interaction in induced resistance to pathogens and insect herbivores. Eur J Plant Pathol 107: 103-111 [Google Scholar]
- Bowler C, Fluhr R (2000) The role of calcium and activated oxygen as signals for controlling cross-tolerance. Trends Plant Sci 5: 241-246 [DOI] [PubMed] [Google Scholar]
- Chao WS, Gu YQ, Pautot V, Bray EA, Walling LL (1999) Leucine aminopeptidase RNAs, proteins, and activities increase in response to water deficit, salinity, and the wound signals systemin, methyl jasmonate, and abscisic acid. Plant Physiol 120: 979-992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong YH, Chang HS, Gupta R, Wang X, Zhu T, Luan S (2002) Transcriptional profiling reveals novel interactions between wounding, pathogen abiotic stress and hormonal responses in Arabidopsis. Plant Physiol 129: 661-677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conconi A, Smerdon MJ, Howe GA, Ryan CA (1996) The octadecanoid signalling pathway in plants mediates a response to ultraviolet radiation. Nature 383: 826-829 [DOI] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE (1995) Jasmonic acid distribution and action in plants: regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci USA 92: 4114-4119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creelman RA, Mullet JE (1997) Biosynthesis and action of jasmonates in plants. Annu Rev Plant Physiol Plant Mol Biol 48: 355-387 [DOI] [PubMed] [Google Scholar]
- Cuartero J, Fernandez-Muñoz R (1999) Tomato and salinity. Sci Hortic 78: 83-125 [Google Scholar]
- Doares SH, Narvaez-Vasquez J, Conconi A, Ryan CA (1995b) Salicylic acid inhibits synthesis of proteinase inhibitors in tomato leaves induced by systemin and jasmonic acid. Plant Physiol 108: 1741-1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doares SH, Syrovets T, Weiler EW, Ryan CA (1995a) Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc Natl Acad Sci USA 92: 4095-4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty HM, Selvendran RR, Bowles DJ (1988) The wound response of tomato plants can be inhibited by aspirin and related hydroxy-benzoic acids. Physiol Mol Plant Pathol 33: 377-384 [Google Scholar]
- Dombrowski JE, Pearce G, Ryan CA (1999) Proteinase inhibitor-inducing activity of the prohormone prosystemin resides exclusively in the C-terminal systemin domain. Proc Natl Acad Sci USA 96: 12947-12952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Ryan CA (1992) Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell 4: 129-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G, Boller T (1995) Systemin induces rapid ion fluxes and ethylene biosynthesis in Lycopersicon peruvianum cells. Plant J 7: 381-389 [Google Scholar]
- Genoud T, Metraux JP (1999) Crosstalk in plant cell signaling: structure and function of the genetic network. Trends Plant Sci 4: 503-507 [DOI] [PubMed] [Google Scholar]
- Howe GA, Lightner J, Browse J, Ryan CA (1996) An octadecanoid pathway mutant (JL5) of tomato is compromised in signaling for defense against insect attack. Plant Cell 8: 2067-2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe GA, Ryan CA (1999) Suppressors of systemin signaling identify genes in tomato wound response pathway. Genetics 153: 1411-1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M (1997) Hormones from roots as signals for the shoots of stressed plants. Trends Plant Sci 2: 22-28 [Google Scholar]
- Khaware RK, Koul A, Prasad R (1995) High membrane fluidity is related to NaCl stress in Candida membranefaciens. Biochem Mol Bio 35: 875-880 [PubMed] [Google Scholar]
- Kovtun Y, Chiu WL, Tena G, Sheen J (2000) Functional analysis of oxidative stress-activated mitogen-activated protein kinase cascade in plants. Proc Natl Acad Sci USA 97: 2940-2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramell R, Miersch O, Atzorn R, Parthier B, Wasternack C (2000) Octadecanoid-derived alteration of gene expression and the “oxylipin signature” in stressed barley leaves: implications for different signaling pathways. Plant Physiol 123: 177-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GI, Howe GA (2003) The tomato mutant spr-1 is defective in systemin perception and the production of a systemic wound signal for defense gene expression. Plant J 33: 567-576 [DOI] [PubMed] [Google Scholar]
- Lee S, Suh S, Kim S, Crain RC, Kwak JM, Nam HG, Lee Y (1997) Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J 12: 547-556 [Google Scholar]
- Li L, Li C, Lee GI, Howe GA (2002) Distinct roles for jasmonate synthesis and action in the systemic wound response of tomato. Proc Natl Acad Sci USA 99: 6416-6421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski DP, Belesky DP (2000) Adaptations of endophyte-infected cool-season grasses to environmental stresses: mechanisms of drought and mineral stress tolerance. Crop Sci 40: 923-940 [Google Scholar]
- McGurl B, Orozco-Cardenas M, Pearce G, Ryan CA (1994) Overexpression of the prosystemin gene in transgenic tomato plants generates a systemic signal that constitutively induces proteinase inhibitor synthesis. Proc Natl Acad Sci USA 91: 9799-9802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurl B, Pearce G, Orozco-Cardenas ML, Ryan CA (1992) Structure, expression, and antisense inhibition of the systemin precursor gene. Science 255: 1570-1573 [DOI] [PubMed] [Google Scholar]
- Moons A, Prinsen E, Bauw G, Montagu M van (1997) Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. Plant Cell 9: 2243-2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura DS, Bergey DR, Ryan CA (2001) Characterization and localization of a wound-inducible type-I serine carboxypeptidase from leaves of tomato plants (Lycopersicon esculentum Mill). Planta 212: 222-230 [DOI] [PubMed] [Google Scholar]
- Moyen C, Hammond-Kosack KE, Jones J, Knight MR, Johannes E (1998) Systemin triggers an increase in cytoplasmic calcium in tomato mesophyll cells: Ca2+ mobilization from intra- and extracellular compartments. Plant Cell Environ 21: 1101-1111 [Google Scholar]
- Narváez-Vásquez J, Florin-Christensen J, Ryan CA (1999) Positional specificity of a phospholipase A activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. Plant Cell 11: 2249-2260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiuchi T, Iba K (1998) Roles of plastid ω-3 fatty acid desaturases in defense response of higher plants. J Plant Res 111: 481-486 [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274: 1914-1918 [DOI] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Narváez-Vásquez J, Ryan CA (2001) Hydrogen peroxide acts as a second messenger for the induction of defense genes in tomato plants in response to wounding, systemin, and methyl jasmonate. Plant Cell 13: 179-191 [PMC free article] [PubMed] [Google Scholar]
- Orozco-Cárdenas ML, Ryan CA (1999) Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway Proc Natl Acad Sci USA 96: 6553-6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce G, Strydom D, Johnson S, Ryan CA (1991) A polypeptide from tomato leaves induces wound-inducible proteinase inhibitor proteins. Science 253: 895-898 [DOI] [PubMed] [Google Scholar]
- Peña-Cortés H, Albrecht T, Prat S, Weiler EW, Willmitzer L (1993) Aspirin prevents wound-induced gene expression in tomato leaves by blocking jasmonic acid biosynthesis. Planta 191: 123-128 [Google Scholar]
- Peña-Cortés H, Prat S, Atzorn R, Wasternack C, Willmitzer L (1996) Abscisic acid-deficient plants do not accumulate proteinase inhibitor II following systemin treatment. Planta 198: 447-451 [Google Scholar]
- Peña-Cortés H, Sánchez-Serrano JJ, Mertens R, Willmitzer L, Prat S (1989) Abscisic acid is involved in the wound-induced expression of the proteinase inhibitor II gene in potato and tomato. Proc Natl Acad Sci USA 86: 9851-9855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CMJ, van Loon LC (1999) Salicylic acid-independent plant defence pathways. Trends Plant Sci 4: 52-58 [DOI] [PubMed] [Google Scholar]
- Reymond P, Weber H, Damond M, Farmer EE (2000) Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell 12: 707-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan CA (1967) Quantitative determination of soluble cellular proteins by radial diffusion in agar gels containing antibodies. Anal Biochem 19: 434-440 [DOI] [PubMed] [Google Scholar]
- Ryan CA (2000) The systemin signaling pathway: differential activation of plant defensive genes. Biochim Biophys Acta 1477: 112-121 [DOI] [PubMed] [Google Scholar]
- Ryan CA, Pearce G, Scheer J, Moura DS (2002) Polypeptide hormones. Plant Cell 14: S251-S264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schaller A, Oecking C (1999) Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell 11: 263-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheer JM, Ryan CA (2002) The system in receptor SR160 from Lycopersicon esculentum is a member of the LRR receptor kinase family. Proc Natl Acad Sci USA 99: 9585-9590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma YK, Leon J, Raskin I, Davis KR (1996) Ozone-induced responses in Arabidopsis thaliana: the role of salicylic acid in the accumulation of defense-related transcripts and induced resistance. Proc Natl Acad Sci USA 93: 5099-5104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K (1997) Gene expression and signal transduction in water-stress response. Plant Physiol 115: 327-334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann J, Ryan CA (1997) Myelin basic protein kinase activity in tomato leaves is induced systemically by wounding and increases in response to systemin and oligosaccharide elicitors. Proc Natl Acad Sci USA 94: 11085-11089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann J, Scheer J, Ryan CA (2000a) Suramin inhibits initiation of defense signaling by systemin, chitosan, and a β-glucan elicitor in suspension-cultured Lycopersicon peruvianum cells. Proc Natl Acad Sci USA 97: 8862-8867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann JW, Stelmach BA, Weiler EW, Ryan CA (2000b) UVB/UVA radiation activates a 48-kDa myelin basic protein kinase and potentiates wound signaling in tomato leaves. Photochem Photobiol 71: 116-123 [DOI] [PubMed] [Google Scholar]
- Surjus A, Durand M (1996) Lipid changes in soybean root membranes in response to salt treatment. J Exp Bot 47: 17-23 [Google Scholar]
- Timmusk S, Wagner EGH (1999) The plant-growth-promoting rhizobacterium Paenibacillus polymyxa induces changes in Arabidopsis thaliana gene expression: a possible connection between biotic and abiotic stress responses. Mol Plant-Microbe Interact 12: 951-959 [DOI] [PubMed] [Google Scholar]
- Tsonev TD, Lazova GN, Stoinova ZG, Popova LP (1998) A possible role for jasmonic acid in adaptation of barley seedlings to salinity stress. J Plant Growth Regul 17: 153-159 [Google Scholar]
- Turner JG, Ellis C, Devoto A (2002) The jasmonate signal pathway. Plant Cell 14: S153-S164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vick BA, Zimmerman DC (1984) Biosynthesis of jasmonic acid by several plant species. Plant Physiol 75: 458-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Mopper S, Hasenstein KH (2001) Effects of salinity on endogenous ABA, IAA, JA and SA in Iris hexagona. J Chem Ecol 27: 327-339 [DOI] [PubMed] [Google Scholar]
- Wasternack C, Atzorn R, Peña-Cortés H, Parthier B (1996) Alteration of gene expression by jasmonate and ABA in tobacco and tomato. J Plant Physiol 147: 503-510 [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK (2002) Cell Signaling during cold, drought, and salt stress. Plant Cell 14: S165-S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N, Enyedi AJ, Leon J, Raskin I (1994) Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 193: 372-376 [Google Scholar]
- Zhang ZP, Baldwin IT (1997) Transport of [2–14C]jasmonic acid from leaves to roots mimics wound-induced changes in endogenous jasmonic acid pools in Nicotiana sylvestris. Planta 203: 436-441 [Google Scholar]