Abstract
The function of ASR (ABA [abscisic acid]-, stress-, and ripening-induced) proteins remains unknown. A grape ASR, VvMSA, was isolated by means of a yeast one-hybrid approach using as a target the proximal promoter of a grape putative monosaccharide transporter (VvHT1). This promoter contains two sugar boxes, and its activity is induced by sucrose and glucose. VvMSA and VvHT1 share similar patterns of expression during the ripening of grape. Both genes are inducible by sucrose in grape berry cell culture, and sugar induction of VvMSA is enhanced strongly by ABA. These data suggest that VvMSA is involved in a common transduction pathway of sugar and ABA signaling. Gel-shift assays demonstrate a specific binding of VvMSA to the 160-bp fragment of the VvHT1 promoter and more precisely to two sugar-responsive elements present in this target. The positive regulation of VvHT1 promoter activity by VvMSA also is shown in planta by coexpression experiments. The nuclear localization of the yellow fluorescent protein–VvMSA fusion protein and the functionality of the VvMSA nuclear localization signal are demonstrated. Thus, a biological function is ascribed to an ASR protein. VvMSA acts as part of a transcription-regulating complex involved in sugar and ABA signaling.
INTRODUCTION
The ASR proteins, which are induced by abscisic acid (ABA), stress, and ripening, were first described in tomato (Iusem et al., 1993; Amitai-Zeigerson et al., 1994; Rossi and Iusem, 1994). They are characterized as small, basic proteins with strong hydrophilicity as a result of high levels of His, Glu, and Lys. Subcellular fractionation experiments and immunodetection in tomato fruit chromatin fractions suggested that tomato ASR1 is localized to the nucleus (Iusem et al., 1993). This conclusion agrees with the fact that loblolly pine and melon ASR possess a putative nuclear targeting signal at the C terminus (Padmanabhan et al., 1997; Hong et al., 2002). Moreover, these nuclear basic proteins can bind DNA, as demonstrated by DNA gel blot and filter binding experiments for tomato ASR1 (Gilad et al., 1997). Together, these data support the idea that ASRs resemble eukaryotic nonhistone chromosomal proteins (Iusem et al., 1993).
After the cloning of the first ASR gene in tomato, several orthologs were isolated from many different species: dicotyledonous and monocotyledonous plants, grasses, and trees (Maskin et al., 2001). However, no ASR-like gene has been identified in Arabidopsis. All available data suggest that ASR proteins are encoded by small multigene families: five genes in tomato (Rossi et al., 1996; Gilad et al., 1997), four genes in loblolly pine (Chang et al., 1996), and at least three genes in maize (Riccardi et al., 1998). Almost all known ASR genes contain two strongly conserved regions. The first region is a short N-terminal stretch containing six to seven His residues that might constitute a Zn binding site. The second region is a large part of the C-terminal region, corresponding to ∼70 amino acids.
In different species, ASR genes are expressed in various organs, such as the fruit of tomato, pomelo, and apricot (Iusem et al., 1993; Canel et al., 1995; Mbeguie-A-Mbeguie et al., 1997), the roots and leaves of tomato, rice, pine, and maize (Amitai-Zeigerson et al., 1994; Chang et al., 1996; Riccardi et al., 1998; Vaidyanathan et al., 1999), the tubers of potato (Silhavy et al., 1995), and the pollen of lily (Wang et al., 1998). Thus, distinct members of one ASR family may be expressed in different organs, under different conditions, and with different expression patterns (Canel et al., 1995; Maskin et al., 2001). The sequence homology among the family members, including even the 3′ noncoding regions, and their similar sizes hindered studies of the expression of individual members. However, their expression patterns may differ considerably between transcripts and proteins, as described previously for cold-regulated genes of potato (Schneider et al., 1997). Finally, ASR genes seem to be involved in processes of plant development, such as senescence and fruit development, and in responses to abiotic stresses, such as water deficit, salt, cold, and limited light (Schneider et al., 1997; de Vienne et al., 1999; Maskin et al., 2001; Jeanneau et al., 2002).
Variations in abiotic environmental factors (light, water, and temperature) may lead to a significant decrease of photosynthetic efficiency in source tissues and thus to a reduced carbohydrate supply to sink organs. According to a recent report, the response to sugar starvation is one of the adaptive mechanisms of plants to cold and water deficit (Yu, 1999). Sugar starvation also has been described as a component of senescence (Dieuaide et al., 1992). In addition to their roles as major structural components and cell nutrients, sugars may act as potential signals in plant growth and development (Smeekens and Rook, 1997; Gibson, 2000; Smeekens, 2000). Interactions between sugar signaling and ethylene (Zhou et al., 1998), ABA (Arenas-Huertero et al., 2000; Finkelstein and Gibson, 2001), cytokinin (Riou-Khamlichi et al., 1999), and light (Mita et al., 1995) signaling have been established. The crosstalk between sugar signal transduction and some plant hormones has been studied further in Arabidopsis mutants (i.e., sugar-insensitive mutants affected in ABA or ethylene response) (Zhou et al., 1998; Arenas-Huertero et al., 2000; Finkelstein and Gibson, 2001; Gazzarrini and McCourt, 2001; Rook et al., 2001).
As suggested initially by Maskin et al. (2001), ASR proteins might act as downstream components of a common signal transduction pathway involved in the responses of plant cells to environmental factors. However, to our knowledge, there is no precise information available concerning the biological functions of these proteins.
Here, we describe the isolation of a grape ASR gene (VvMSA) by means of the one-hybrid approach, using as a target the proximal promoter of a putative grape monosaccharide transporter (VvHT1), which contains two sugar boxes and is regulated by sugars (Atanassova et al., 2003). We show that VvMSA expression, which is upregulated at early stages of fruit development and at late grape ripening, is inducible by sucrose and that this sugar induction is enhanced strongly by ABA. Furthermore, using two different expression systems, we demonstrate a specific in vitro binding activity of VvMSA to the target VvHT1 promoter and the requirement of two sugar boxes for this interaction. The positive regulation of VvHT1 promoter activity by VvMSA is further confirmed by their coexpression in planta. The study of a yellow fluorescent protein (YFP)–VvMSA fusion protein demonstrates its preferential nuclear localization and the functional role of its intrinsic nuclear localization signal (NLS). Therefore, VvMSA appears to act as part of a complex that regulates the expression of a monosaccharide transporter.
RESULTS
The One-Hybrid Approach
To clone transcription factors involved in the regulation of VvHT1 expression, the yeast one-hybrid approach was developed according to Kim et al. (1997). VvHT1 is highly similar to several monosaccharide transporters (Fillion et al., 1999; Leterrier et al., 2003). During the ripening of grape berries, it exhibits a biphasic expression pattern with a first peak soon after fruit set and a second peak after véraison (Fillion et al., 1999). Its promoter contains several sugar boxes, and its activity is induced by sucrose and glucose treatment (Atanassova et al., 2003). To avoid the isolation of general transcription factors, the proximal 160-bp region of the VvHT1 promoter upstream of the TATA box was chosen as a target. The 160-bp part of the VvHT1 promoter contains two positive sugar-responsive motifs, a perfect “sucrose box 3” encompassing an imperfect SURE1 sequence (Tsukaya et al., 1991; Grierson et al., 1994). This target VvHT1 promoter was fused in front of two reporter genes, HIS3 and LacZ, for expression in yeast. A cDNA expression library from grape berries at the véraison stage was fused to the sequence coding the activation domain of the GAL4 transcription factor of yeast. The use of two reporter genes decreases the number of false-positive clones (Kim et al., 1997).
After transformation of the host strain bearing both reporter constructs with the activation domain/cDNA fusion library and double selection of transformants, 10 positive clones were obtained. Among these positive clones, four displayed similarities with unknown proteins, three were expressed in Arabidopsis, and one was expressed in rice. Five clones shared identity with proteins involved in gene transcription regulation: an AUX/IAA protein, a MADS-box protein, a Ser/Thr protein kinase, a Gly-rich protein, and a histone variant H3.3. The complete cDNAs of all of these clones were obtained, analyzed by sequencing, and submitted to GenBank as new grape sequences, except VvMADS1, which already was known. A 10th clone showed strong identity with a family of proteins induced by ABA, stress, and ripening (ASR) that is known to be expressed in fruits. This clone was selected for further analysis.
Cloning of an ASR Gene from Grape
The ASR homolog was named VvMSA (for Vitis vinifera maturation-, stress-, ABA-induced protein). VvMSA cDNA is 664 bp long and contains a 5′ untranslated region (UTR) of 59 bp, an open reading frame of 450 bp, and a 214-bp 3′ UTR. The predicted polypeptide (pI 5.67) is 149 amino acids long, with a molecular mass of 16.5 kD. This is a small protein containing four hydrophilic domains and similar amounts of His (14.8%, on a frequency basis), Lys (10.7%), and Glu (14.1%). VvMSA shares considerable identity with many ASR proteins from different species (Figure 1). There are two main highly conserved regions: a small N-terminal consensus of ∼18 to 20 amino acids containing a typical stretch of six His residues in an 8–amino acid sequence, and a large C-terminal region of at least 80 amino acids. Checking for specific sequences in VvMSA using the BLOCKS method (http://blocks.fhcrc.org) revealed the presence of two ABA/WDS signatures, which are described in ABA stress– and ripening-induced proteins (Canel et al., 1995) and in water deficit stress–induced proteins (Padmanabhan et al., 1997): 5′-DYRKEEHHKHLEHLGELGVA-3′ and 5′-AGAYALHKKHKSEKDPEHAHKHKIEEEIAAAAA-3′. In addition, the 3′ end of the C-terminal part of VvMSA contains a putative signal for nuclear targeting (5′-KKEAKEEDEEAHGKKHHHLF-3′). PROSCAN (http:// npsa-pbil.ibcp.fr) analysis through PROSITE.BASE indicates that the VvMSA sequence contains three potential sites for three different types of phosphorylation and one site for N-myristoylation.
Figure 1.
Amino Acid Alignment of VvMSA and 22 Known ASR Proteins from Different Species Performed with the CLUSTAL Program.
The comparison of ASR proteins obtained using the CLUSTAL method (DNAstar, Madison, WI) indicated that the closest homologs to VvMSA are pomelo, apricot, peach, and pear ASR clones (Figure 1). The next group of orthologs sharing important identity with VvMSA corresponds to potato and tomato ASR clones. It is followed by a third group, formed mainly by monocotyledonous species (rice, maize, and sugarcane). The fourth and last cluster consists exclusively of the four pine clones.
Determination of VvMSA Gene Number in the Grape Genome
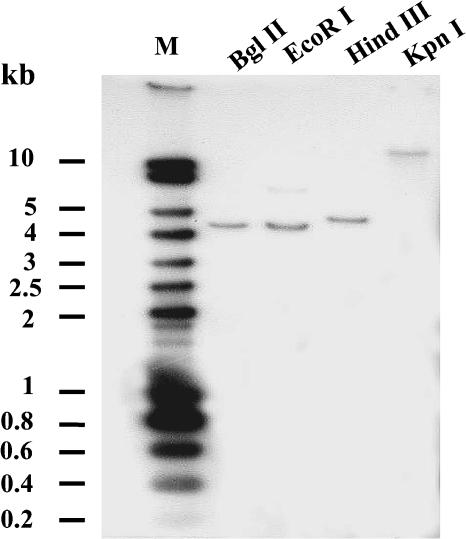
To determine whether VvMSA belongs to a small multigene family, like the majority of known ASR genes in other species, DNA gel blot analysis of grape genomic DNA was performed. After digestion with each of four different enzymes (BglII, EcoRI, HindIII, and KpnI), genomic DNA was hybridized with a probe corresponding to VvMSA cDNA. The presence of a single hybridizing band for DNA digested with each of the tested enzymes strongly suggested that there is only one copy of this ASR gene in the grape genome (Figure 2).
Figure 2.
Gel Blot Analysis of Grape Genomic DNA.
Ten micrograms of genomic DNA was digested with different enzymes: BglII, EcoRI, HindII, or KpnI. The DNA gel blot was hybridized with VvMSA cDNA as an α-32P labeled probe. M, Smart ladder marker DNA.
VvMSA and VvHT1 Expression Are Regulated Developmentally in Grape
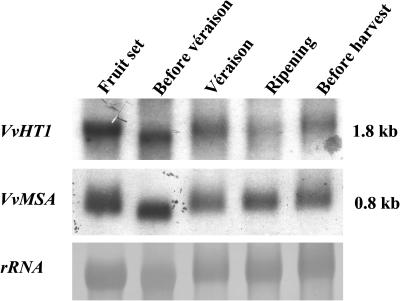
RNA gel blot analysis was used to study the expression of VvHT1 and VvMSA at five different stages of grape development: fruit set, before véraison, véraison, ripening, and harvest (Figure 3). The expression of both genes exhibited nearly similar patterns. The highest amounts of VvHT1 and VvMSA transcripts were found at fruit set and before véraison. Both VvHT1 and VvMSA decreased approximately at véraison and increased slightly during the last stages of ripening.
Figure 3.
VvMSA and VvHT1 Gene Expression during Grape Development.
Gel blot hybridization of RNA from berries at different stages of ripening with VvMSA and VvHT1 probes. Twenty micrograms of total RNA was loaded in each well. Equal loading was checked by staining of 25S rRNA with methylene blue.
Regulation of VvMSA Expression by ABA
To determine whether VvMSA is regulated by ABA, like other ASRs, VvMSA expression in a grape berry cell culture was studied by RNA gel blot analysis. The effects were tested in a medium containing 58 mM sucrose or no sucrose after ABA treatment. VvMSA expression was induced by sucrose, and this induction was enhanced strongly by ABA at 48 and 72 h (Figure 4A). Under our experimental conditions, the ABA effect was consistent only in the presence of sucrose (Figure 4A). In the absence of sucrose, ABA did not affect the amount of VvMSA transcripts.
Figure 4.
ABA Induction of VvMSA and VvHT1 Gene Expression in Grape Berry Cell Suspension.
(A) RNA gel blot analysis of VvMSA messenger accumulation at 48 and 72 h after ABA treatment (10 μM) in either the presence (+S) or absence (−S) of sucrose.
(B) RNA gel blot analysis of VvMSA and VvHT1 transcript amounts at 24, 48, and 72 h after ABA treatment in sucrose-supplemented medium.
Twenty micrograms of total RNA was loaded in each well. VvMSA- and VvHT1-specific labeled probes were used for hybridization. Equal loading was checked by 25S rRNA staining with methylene blue.
Like VvMSA, VvHT1 expression was induced strongly by ABA at 24 h after treatment in the presence of sucrose (Figure 4B). ABA enhanced a strong but transient increase of VvHT1 messengers at 24 h, whereas it induced the VvMSA transcript for at least 72 h after treatment. These results were confirmed when sucrose in culture medium was substituted by glucose (data not shown).
Sucrose Effect on VvMSA and VvHT1 Expression in Grape Berry Cell Suspension
To study the effect of sucrose, 3 days after subculture of the grape berry cell suspension, the cells were washed gently and resuspended without dilution in fresh culture medium containing 58 mM sucrose. This addition of sucrose was followed by a transient increase in the level of VvMSA transcripts after 1 and 4 h of treatment, but this effect disappeared for incubation times up to 24 h. By contrast, the amount of VvHT1 transcripts accumulated gradually and reached a maximal level at 24 h after sucrose addition (Figure 5).
Figure 5.
Time Course of VvMSA and VvHT1 Induction by Sucrose in Grape Berry Cell Suspension.
RNA gel blot analysis of RNA from grape berry cells with VvMSA and VvHT1 probes. The cells were harvested at the times indicated after transfer in fresh culture medium containing 58 mM sucrose. Twenty micrograms of total RNA was loaded in each well. Equal gel loading was confirmed by staining of 25S rRNA.
VvMSA 6xHis-Tagged Protein May Interact with Target VvHT1 Promoter in Vitro
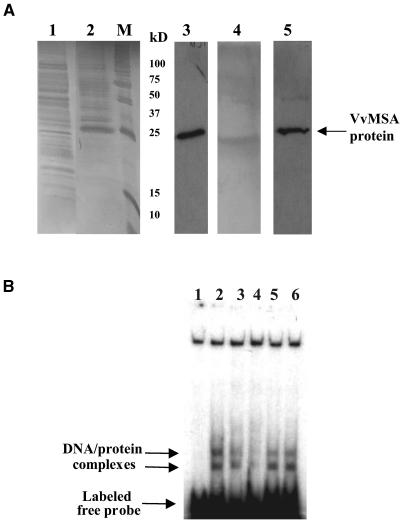
The cloning of VvMSA and the results of the regulation of VvMSA and VvHT1 expression suggested a possible interaction between this protein and the target promoter. To check this presumption, VvMSA cDNA was cloned in the pQE30 expression vector, translated in bacteria as a 6xHis-tagged protein, and purified on nickel–nitrilotriacetic acid agarose (Ni-NTA) . The purified VvMSA protein was revealed by protein gel blot analysis with a RGS-6xHis–specific antibody (Qiagen, Hilden, Germany). The tagged VvMSA protein had an apparent molecular mass of ∼25 kD by 12% SDS-PAGE (Figure 6A), somewhat higher than the predicted mass (16.5 kD). This antibody did not detect any protein in the control M15 bacterial extract.
Figure 6.
Production and DNA Binding Activity of 6xHis-Tagged VvMSA.
(A) SDS-PAGE of 6xHis-tagged VvMSA visualized by silver staining (lanes 1, 2, and 4) and protein gel blot analysis (lanes 3 and 5) with antibody against RGS 6xHis tag. Lane 1, total protein extract of M15 bacterial cells; lane 2, production of VvMSA protein in transformed M15 cells harvested 3 h after isopropylthio-β-galactoside induction; lane M, molecular mass markers; lane 3, 6xHis-tagged VvMSA protein revealed with the anti-RGS 6xHis antibody in a total protein extract of transformed M15 cells harvested 3 h after isopropylthio-β-galactoside induction; lanes 4 and 5, SDS-PAGE (lane 4) and immunostaining (lane 5) of 6xHis-tagged VvMSA after purification on an Ni-NTA affinity column.
(B) In vitro binding activity of purified VvMSA to the target VvHT1 promoter fragment. Lane 1, DNA-labeled free probe corresponding to the 160-bp fragment of the VvHT1 promoter; lane 2, DNA/protein complexes formed by the interaction of VvMSA and the labeled 160-bp fragment of the VvHT1 promoter; lanes 3 and 4, competition assays with the unlabeled 160-bp fragment of the VvHT1 promoter at 100- and 200-fold molar excess, respectively; lanes 5 and 6, competition assays with unrelated unlabeled probe (150 bp) at 100- and 200-fold molar excess, respectively.
Purified 6xHis-tagged protein was checked in gel mobility experiments for interaction with the target 160-bp fragment of VvHT1 promoter (Figure 6B). Two complexes were observed in all assays performed with the 6xHis-tagged protein. The same 160-bp fragment, used as unlabeled probe in 100- and 200-fold molar excesses, competed successfully with the labeled fragment, suggesting a specific interaction between the VvMSA protein and the VvHT1 proximal promoter in vitro (Figure 6B, lanes 2 to 4). To confirm the specificity of this interaction, a DNA fragment of the same length with no identity to the target sequence was chosen on the VvHT1 promoter and used as a competitor. Even in 100- and 200-fold molar excesses, this DNA fragment unrelated to the target DNA did not compete with the labeled target sequence (Figure 6B, lanes 5 and 6). However, both complexes displayed different stability levels in the presence of unrelated competitor. Thus, the upper complex partially disappeared, whereas the lower complex was stable even after the addition of 100- and 200-fold molar excess of unrelated unlabeled fragment.
Coupled Transcription and Translation–Produced VvMSA Protein Binds to the 160-bp VvHT1 Promoter
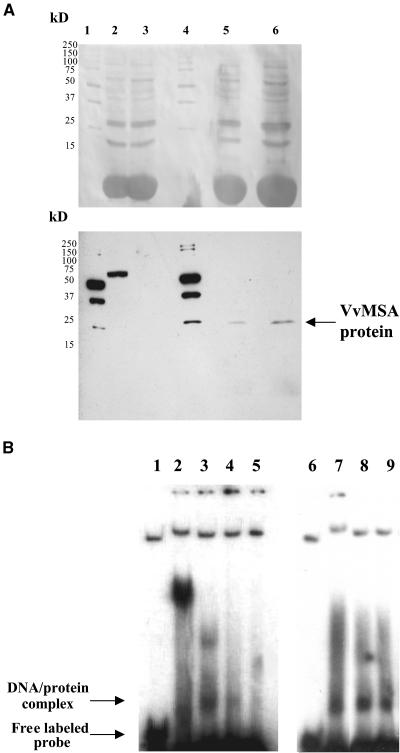
To check for possible interference in DNA binding activity between the N-terminally located 6xHis tag and the N-terminal stretch of six His residues intrinsically present in the VvMSA sequence (at positions +6 to +13 aa; Figure 1), the protein was produced by coupled transcription and translation (TnT) in rabbit reticulocytes. Biotinylated VvMSA protein was revealed by anti-streptavidin antibody in the reaction system supplemented with the VvMSA cDNA, but it was not detected in the free lysate (Figure 7A). The VvMSA protein produced by the TnT system had the same apparent molecular mass as the 6xHis-tagged protein described above (i.e., ∼25 kD).
Figure 7.
Production and DNA Binding Activity of the Native VvMSA Protein.
(A) SDS-PAGE of TnT proteins produced in reticulocyte lysate and visualized after protein gel blot transfer by Ponceau S staining (top gel) and after immunochemical reaction developed using streptavidin-peroxidase and enhanced chemiluminescence (bottom gel). Lane 1, molecular mass marker; lane 2, TnT-positive control based on luciferase detection; lane 3, TnT-negative control (proteins produced in the absence of the VvMSA cDNA); lane 4, molecular mass marker; lanes 5 and 6, TnT proteins produced in the presence of the VvMSA cDNA. Two microliters of TnT reaction mixture was loaded in lanes 2 and 3, 4 μL was loaded in lane 5, and 8 μL was loaded in lane 6.
(B) In vitro binding activity of TnT-produced VvMSA protein to the target fragment of the VvHT1 promoter. Lanes 1 and 6, free DNA-labeled probe corresponding to the 160-bp fragment of the VvHT1 promoter; lane 2, unspecific DNA/protein complex obtained in the control binding assay with proteins produced in the VvMSA cDNA–free TnT system; lanes 3 and 7, complex corresponding to the interaction of the VvMSA protein and the labeled 160-bp fragment of the VvHT1 promoter; lanes 4 and 5, competition assays with the unlabeled 160-bp fragment of the VvHT1 promoter at 100- and 200-fold molar excess, respectively; lanes 8 and 9, competition assays with unrelated unlabeled probe (150 bp) at 100- and 200-fold molar excess, respectively.
Only one complex caused by the interaction between the target 160-bp fragment and TnT-produced VvMSA was apparent in gel mobility-shift assays (Figure 7B). This complex had the same mobility as the lower complex obtained with the 6xHis-tagged protein. Moreover, the complex was competed for completely by the unlabeled 160-bp VvHT1 promoter fragment and was not competed for by the unrelated promoter fragment, both of which were provided at the same molar excess (100- and 200-fold).
Interaction of the VvMSA Protein with Known Consensus Sequences of the VvHT1 Promoter
Double-stranded oligonucleotides containing cis elements of the VvHT1 promoter were used for a more detailed study of motifs recognized specifically by VvMSA. Sequences of one strand of these oligonucleotides are presented in Figure 8A. The first probe, S3S1, corresponds to a 29-bp sequence of the 160-bp target combining two overlapping elements, a complete sucrose box 3 and an imperfect SURE1. The second oligonucleotide, S3, contains the sucrose box 3 motif alone. The third probe, S1, is the perfect consensus sequence of the SURE1 box. The last probe corresponds to a GT-1 element (5′-AGTTTTCCTTGAAAGAAGATTTAATTCA-3′), which is present downstream of the S3S1 combination within the 160-bp target promoter (Villain et al., 1996). All three oligonucleotides carrying only one motif correspond to consensus sequences found in the context of the distal part of the VvHT1 promoter.
Figure 8.
Interaction of VvMSA with Some Consensus Motifs of the VvHT1 Promoter.
(A) Sequences of the positive strain of double-stranded oligonucleotides used in the DNA binding assays. DNA sequences are presented from the 5′ to the 3′ end. cis element sequences are shown in boldface, and when they overlap, the second one is underlined.
(B) S3S1 sequence was used as a labeled probe in all experiments, and competition assays were performed with each of three unlabeled probes at 50- and 100-fold molar excess, as indicated. Lane 1, free S3S1 labeled probe; lane 2, unspecific DNA/protein complexes obtained in a control binding assay with proteins produced in the VvMSA cDNA–free TnT system; lanes 3, 6, and 9, complex corresponding to the interaction of VvMSA and S3S1 labeled probe; lanes 4 and 5, competition assay with S3S1 unlabeled oligonucleotide in 50- and 100-fold molar excess, respectively; lanes 7 and 8, competition assay with S3 unlabeled oligonucleotide in 50- and 100-fold molar excess, respectively; lanes 10 and 11, competition assay with S1 unlabeled oligonucleotide in 50- and 100-fold molar excess, respectively. These gel-shift assay results are representative of three to five independent experiments with similar results.
To test the DNA sequence specificity of protein binding, the interaction of TnT-produced VvMSA protein with labeled S3S1 probe was determined by gel-shift assays in the presence of competitors (Figure 8B). The competitors used were the four distinct unlabeled probes in increasing amounts (i.e., 50- and 100-fold molar excess). The free DNA probe (Figure 8B, lane 1) and its interaction with TnT-expressed proteins in the absence of VvMSA cDNA (Figure 8B, lane 2) were used as controls. In all experiments, only one specific VvMSA/S3S1 complex was observed (Figure 8B, lane 3, arrow). This specific complex is located between two nonspecific complexes and just a little higher than the lower complex in the TnT control, as demonstrated for the TnT-produced VvMSA protein interaction with the 160-bp fragment of the VvHT1 promoter (Figure 7B). In addition, this complex is very strong and appears with the same intensity in the three repetitions involving the positive VvMSA–S3S1 interaction (Figure 8B, lanes 3, 6, and 9), corresponding to competition with the oligonucleotides S3S1, S3, and S1, respectively. This complex was competed for specifically only by the same unlabeled S3S1 probe (Figure 8B, lanes 4 and 5), which corresponds to the target VvHT1 promoter sequence and combines both elements mentioned above. The S3 and S1 oligonucleotides applied individually as unlabeled competitors did not compete efficiently (Figure 8B, lanes 7 and 8 for S3, lanes 10 and 11 for S1). Such a lack of effective competition also was observed with the GT-1 unlabeled sequence (data not shown).
Nuclear Localization of VvMSA
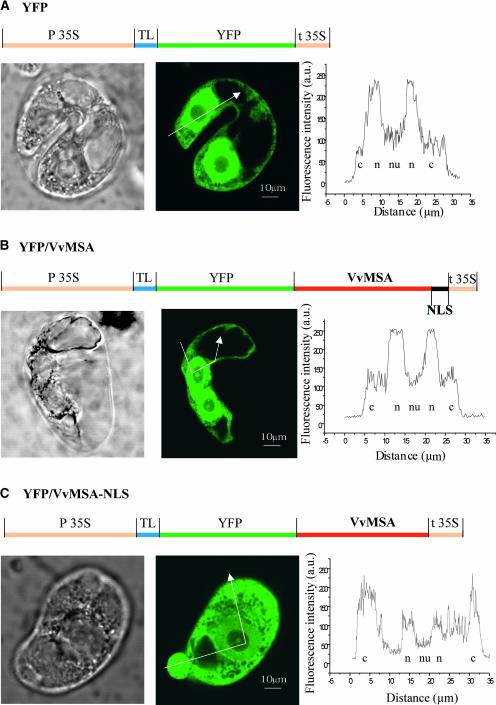
The C-terminal region of VvMSA carries a putative NLS, suggesting the possible trafficking of the protein in the nucleus. To study VvMSA subcellular compartmentalization and the functionality of its NLS, the protein was fused downstream of YFP. The constructs shown in Figures 9A to 9C were used for transient expression experiments in protoplasts of tobacco BY2 cells. The efficiency of the transformation procedure was checked by immunodetection of VvMSA expression in protoplasts (data not shown). The cellular sections presented were chosen after three-dimensional reconstitution of whole BY2 cells because of particularly obvious nuclei. The positive control for nuclear localization was YFP alone (Figure 9A), because of its spontaneous import within the nuclear compartment (Chiu et al., 1996). To create a negative control with a preferential cytoplasmic location, the VvMSA NLS was deleted completely in the YFP-VvMSA NLS fusion protein (Figure 9C). For the YFP protein alone, confocal microscopy revealed greater fluorescence in the nucleus than in the cytoplasm (Figure 9A). The fluorescence caused by the YFP-VvMSA fusion protein also was localized preferentially in the nucleus, and its level clearly was stronger than the residual fluorescence detected in the cytoplasm (Figure 9B).
Figure 9.
Subcellular Localization of the VvMSA Protein.
(A) Subcellular compartmentalization of YFP alone, considered as a positive control for free nuclear targeting.
(B) Preferential nuclear expression of the YFP-VvMSA fusion protein
(C) Strong cytoplasmic localization of the YFP-VvMSA fusion protein, deleted for the VvMSA NLS.
Gene structures of the constructs used are detailed above the corresponding micrographs. From left to right are transmission micrographs, confocal fluorescence images, and profiles of fluorescence intensity obtained for different subcellular compartments as indicated by the arrows. c, cytoplasm; n, nucleus; nu, nucleolus; TL, translational enhancer.
Given that these images were collected using the same values for laser power, photomultiplier gain, iris, and black level, the fluorescence signal in nuclei of YFP-VvMSA–transfected cells appears at least as strong or even stronger than that in nuclei of YFP-expressing cells. By contrast, the YFP-VvMSA NLS fusion protein, which is truncated for the NLS sequence, conferred a clear fluorescence in the cytoplasm and only a slight signal in the nucleus (Figure 9C). In all of the cells observed, no fluorescence signal was detected in nucleoli and vacuoles. The observed differences were confirmed by the profiles of fluorescence intensity in subcellular compartments (Figure 9, right).
Coexpression of the VvMSA Protein and the VvHT1 Promoter in Planta
The interaction between the VvMSA protein and the VvHT1 promoter demonstrated above in vitro was checked further in planta. For this purpose, a coexpression system was developed. The construct of VvMSA cDNA under the control of the 35S RNA promoter of Cauliflower mosaic virus (Figure 10A) was prepared in the binary vector pBI121 (Jefferson et al., 1987) and introduced by electroporation into Agrobacterium tumefaciens strain LBA 4404. The transient expression assays were performed via Agrobacterium-mediated transformation of tobacco leaves in planta. A suspension of Agrobacterium was introduced by infiltration in young leaves of transgenic tobacco plants and transformed in a stable manner with either the promoter VvHT1/GUS or the p35S/GUS reporter gene construct (Atanassova et al., 2003) (Figure 10A). In each of four independent experiments, two different clones per construct were used and two leaves per plant were agroinfiltrated. Three different controls were included: the first corresponded to the Agrobacterium strain free of binary vector carrying VvMSA cDNA; the second consisted of the infiltration buffer free of bacteria; and the last was the blank (i.e., untreated leaf). All of these treatments were applied in parallel to the same leaf, and the sampling corresponded to different areas treated. The expression of VvMSA cDNA in zones of infiltration was revealed by RNA gel blot analysis (Figure 10B).
Figure 10.
In Planta Coexpression and Interaction of VvMSA and the VvHT1 Promoter.
(A) Effector and reporter constructs obtained in pBI121 and pBI101.1 binary vectors, respectively, and introduced in Agrobacterium tumefaciens.
(B) RNA gel blot analysis of VvMSA expression 48 h after infiltration with Agrobacteria carrying VvMSA cDNA of tobacco leaves expressing the pVvHT1-GUS chimerical gene. Two independent tobacco lines were analyzed, and agroinfiltrated areas were compared with buffer-infiltrated areas on the same leaves.
(C) Induction of VvHT1 promoter–conferred GUS activity by VvMSA in two independent tobacco transformants. VvMSA's effect on the VvHT1 promoter (Agro+VvMSA) was compared with that of three different controls: untreated areas of the same leaf (Control), buffer-infiltrated areas of the same leaf (Buffer), and areas of the same leaf infiltrated with the Agrobacterium cell suspension without VvMSA cDNA (Agro). MU, 4-methylumbelliferone.
(D) Absence of VvMSA's effect on 35S promoter–conferred GUS activity in two independent tobacco transformants carrying the p35S/GUS construct.
In both (C) and (D), agroinfiltration results were confirmed in at least four independent experiments for each promoter. Error bars correspond to the se.
The GUS fluorimetric assay (Figure 10C) demonstrated that VvMSA expression induced a 2.5- to 3-fold increase of reporter gene activity conferred by the full-length VvHT1 promoter (2.4 kb) in two independent clones studied (pVvHT1-4 and pVvHT1-14). Furthermore, in the same experimental conditions, the activity of the proximal VvHT1 promoter (0.3 kb), corresponding to the target 160-bp fragment plus the minimal promoter, was upregulated by >10-fold by VvMSA (data not shown). This in planta analysis of the promoter and the trans-acting factor revealed a positive interaction between the studied protein and the target promoter that did not appear in the different control samples. This finding indicates that the observed effect was attributable neither to the presence of Agrobacterium as a biotic factor nor to the infiltration procedure as an abiotic stress. The parallel agroinfiltration of transgenic tobacco plants carrying the GUS reporter gene under the control of the 35S promoter (p35S-2 and p35S-4) did not affect viral promoter-conferred GUS activity (Figure 10D). The latter result confirmed that the regulation of pVvHT1 activity by VvMSA is specific for this promoter.
DISCUSSION
Cloning and Characterization of VvMSA, a Member of the ASR Family
VvHT1 expression is induced by various sugars, including glucose, sucrose, and palatinose, and the promoter of this gene contains several sugar boxes (Atanassova et al., 2003). To identify transcription factors binding to this promoter, we developed a one-hybrid approach and succeeded in the cloning of several cDNAs that encode regulatory proteins, including VvMSA. Although a partial mRNA was present in GenBank (GASR), our work yields a complete cDNA that encodes an ASR protein in grape. The 93% identity between the 3′ UTRs of VvMSA and the GASR partial sequence available in the database also indicates that they correspond to the same gene. No identity was found with the 3′ UTRs of ASR genes from other plant species.
Genomic DNA analysis using the VvMSA cDNA as a probe strongly suggests the presence of a single copy of the ASR gene in grape (Figure 2). The presence of a single copy in grape is in contrast to what has been described in most other species, such as tomato (Iusem et al., 1993), pomelo (Canel et al., 1995), apricot (Mbeguie-A-Mbeguie et al., 1997), and maize (Riccardi et al., 1998).
Several lines of evidence allow us to conclude that VvMSA belongs to the ASR family. Many ASR genes have been isolated in ripening fruit of tomato, pomelo, and apricot (Iusem et al., 1993; Canel et al., 1995; Mbeguie-A-Mbeguie et al., 1997). VvMSA was cloned from a cDNA library corresponding to the onset of grape ripening and is expressed until the time of harvest (Figure 3). As described for other ASRs (Rossi et al., 1998), VvMSA expression is upregulated by ABA. The VvMSA sequence displays identity to all previously characterized ASR sequences (Figure 1). VvMSA shares the main characteristics of ASR (i.e., low molecular mass and high level of hydrophilicity), which indicates that the protein is soluble. Furthermore, the protein sequence contains two ABA/WDS (ABA and water deficit stress) signatures typical of ASR proteins (see Results).
Sugar and ABA in the Regulation of VvMSA and VvHT1 Gene Expression
During grape ripening, VvMSA messengers are accumulated strongly at the stages before véraison (Figure 3), and the signals are present until harvest. Both VvMSA and its target VvHT1 display similar patterns of expression. In grape, which is a nonclimacteric fruit, ABA is the only endogenous hormone whose content increases from véraison to late ripening, in parallel with sugar accumulation (Blouin and Guimberteau, 2000).
This study offers new insight into the role of ABA in the sugar regulation of ASR gene expression. In grape cell suspension, sugar-induced VvMSA messenger accumulation was enhanced strongly by ABA (Figure 4A). Furthermore, the induction of VvMSA mRNA steady state levels by ABA occurs in the presence but not in the absence of sucrose, which means that sucrose is required for the ABA upregulation of this gene. Similarly, recent studies of two sugar-induced genes involved in starch biosynthesis in Arabidopsis (the ApL3 subunit of ADP-glucose pyrophosphorylase and starch-branching enzyme) have shown that ABA strongly enhances their sucrose-induced expression but has no effect in the absence of sucrose (Rook et al., 2001). The existence of Arabidopsis mutants affected in both ABA signaling and sugar sensing also is a strong indication for interactions between these signaling pathways (Gazzarrini and McCourt, 2001).
This ABA enhancement of VvMSA sucrose-induced expression is in good correlation with the ABA upregulation of VvHT1 gene expression, as demonstrated at the transcriptional level (Leterrier, 2002) and at the RNA steady state level in the current study (Figure 4B). All of these results suggest that VvMSA and VvHT1 gene expression may be regulated in a common sugar- and ABA-dependent pathway.
The possible interference of VvMSA with two signal transduction pathways, that of sugar and that of ABA, led us to investigate in more detail the control exerted by sucrose. In grape cell suspension culture, both VvHT1 and VvMSA transcripts are regulated positively by sugar, but with different kinetics. The sudden and strong increase of VvMSA expression preceded the accumulation of VvHT1 transcripts, which demonstrates that the expression of both genes is correlated positively and furthermore that VvMSA may act upstream of VvHT1.
Interaction between VvMSA and the VvHT1 Promoter
Our initial attempts to study the interaction between VvMSA and the VvHT1 promoter fragment of 160 bp in gel mobility assays used the purified 6xHis-tagged protein and suggested the presence of two DNA/protein complexes. These two complexes may correspond to the binding of monomer and dimer forms. The band-shift effect corresponding to the lower complex is nearly doubled for the upper complex (Figure 6B). The His block located at the N-terminal end of VvMSA may mimic a zinc binding structure and may be involved in the formation of dimers, homodimers, or heterodimers. Examples of heterodimerization of plant transcription factors have been described, and among them are many basic domain/Leu zipper proteins, including some that are involved in ABA-inducible gene expression (Riechmann and Ratcliffe, 2000).
Whether the 6-His tag may interfere with VvMSA interactions, either at the level of dimer formation or at the level of DNA binding activity, was studied with further experiments involving TnT-produced VvMSA protein. The protein obtained in vitro bound the proximal VvHT1 promoter fragment and displayed only one complex (Figure 7B). The gel mobility of this complex was comparable to the band shift of the lower 6xHis-tagged VvMSA/pVvHT1 DNA complex. In all competition assays with the same unlabeled fragment or with the unrelated sequence, the specificity of interaction between VvMSA and the VvHT1 promoter was confirmed.
In addition, gel-shift assays performed with oligonucleotides corresponding to consensus sequences found in the target promoter clearly demonstrated that the combination present in the context of the VvHT1 promoter of both elements involved in the positive sugar response (i.e., sucrose box 3 and SURE1) is necessary for specific VvMSA–pVvHT1 interaction in vitro (Figure 8B). Furthermore, in planta coexpression of both of these partners unambiguously showed that VvMSA is involved in the regulation of VvHT1 promoter activity (Figure 10) in a positive and specific way. Although many sugar transporters have now been cloned, their regulation remains poorly understood (Delrot et al., 2000).
Here, the nuclear localization of an ASR protein is shown in vivo by transient expression of different YFP-VvMSA fusion proteins in tobacco BY2 protoplasts (Figures 9A to 9C), thereby confirming the previous assumption that tomato ASR1 may be a nuclear protein (Gilad et al., 1997). Profiles of fluorescence intensity obtained by confocal microscopy clearly lend support to this conclusion. In our conditions of tobacco BY2 cell culture, VvMSA was compartmentalized preferentially in the nucleus, and the residual fluorescent signal in the cytoplasm may reflect the requirement of certain stimuli for this change of compartment, such as alterations in protein phosphorylation state and cooperation with other proteins. For example, VvMSA presents specific sites for phosphorylation by three different kinases, among them casein kinase II, which has been discussed in the literature as a complement of NLS functionality (Kircher et al., 1999). Furthermore, the results showed the functionality of the studied NLS sequence and proved that this signal was required for VvMSA nuclear targeting.
Although VvMSA affects the transcription of VvHT1, it does not show any important similarity to transcription factors already known. Because information is limited regarding the transcription factors involved in fruit development (Giovannoni, 2001), this finding does not exclude the possibility of VvMSA being a transcription factor. The demonstrated nuclear localization of VvMSA and the functionality of its NLS, the DNA binding activity shown in band-shift assays, and the coexpression data obtained in planta allow us to assign VvMSA a role as a transcription-regulating protein. However, VvMSA is expressed at a relatively high level throughout grape ripening. These considerations and the different time courses of VvHT1 and VvMSA induction by ABA (Figure 4B) suggest that VvMSA acts as part of a transcriptional complex with a combinatory effect rather than as a transcription factor acting alone.
Physiological Role of VvMSA
To date, despite the cloning of many ASR genes from different plant species and the molecular characterization of the encoded products, no physiological function has been ascribed to these proteins. A first hypothesis predicted that the small nuclear and basic ASR proteins of tomato were nonhistone chromosomal proteins (Rossi and Iusem, 1994). Nonhistone chromosomal proteins are involved in DNA topology changes, in establishing and maintaining of chromatin higher order structures, and in modulating gene expression (Zlatanova, 1990). Since then, a number of ASR proteins have been characterized, and it has now been reported that they may differ in molecular mass (from 70 to 230 amino acids), in pI (from basic to acidic), in organ and tissue specificity, and in expression regulation. The nuclear localization targeting sequence is not always present in orthologs and even in paralogs of a single multigene family, and its physiological role remains unclear. Therefore, the putative ASR function as nonhistone chromosomal proteins needs to be confirmed experimentally.
Another hypothesis, based on numerous reports indicating the accumulation of ASR proteins in response to various abiotic stresses (Maskin et al., 2001), ascribed them a potential protective role against these stresses (Silhavy et al., 1995). This hypothesis takes into account the high proportion of charged residues in ASR, their high average hydrophilicity, and the predicted helical secondary structure with enhanced accessibility to water molecules. In agreement with this hypothesis, ASR proteins have been reported to be members of the widespread class of hydrophilins, including the seed-specific LEA proteins (Dure et al., 1989; Garay-Arroyo et al., 2000). The similarity between ASR and LEA proteins concerns only the N-terminal conserved repeat, which has been found in some ASRs of wild potato but not in tomato ASR (Silhavy et al., 1995; Maskin et al., 2001). In addition, there is no ASR homolog in Arabidopsis, although this species contains many LEA proteins and the related RAB (responsive to ABA) and DHN (dehydrin) proteins. Therefore, the hypothesis of ASR involvement in seed development requires further investigation.
Together, the data in the literature and the present results allow us to propose the tantalizing hypothesis that some ASR proteins may be involved in the crosstalk between sugar and ABA. Both ABA and sugar are key players in the ripening process in grape (Coombe, 1992), which is triggered at the véraison stage. Our data demonstrate that VvMSA, an ASR member, acts as part of a transcription regulation complex that controls the expression of a monosaccharide transporter homolog and that VvMSA expression itself is under the control of both sugar and ABA. This hypothesis gives further support to the current concept that ascribes a primary role of sugar sensing and signaling in plant responses to abiotic and biotic stresses (Smeekens, 2000).
In conclusion, our study presents experimental evidence for a function of an ASR protein acting as a downstream component of a common transduction pathway for sugar and ABA signals. Further investigations are necessary to identify VvMSA molecular partners in this cascade of signaling.
METHODS
cDNA Library Construction
A cDNA library was produced using mRNA isolated from grape berries (Vitis vinifera) of the variety Ugni blanc at the véraison stage. Total RNA was prepared by ultracentrifugation in a CsCl gradient (Tesnière and Vayda, 1991). Polyadenylated mRNAs were column purified with the Poly (A) Quick mRNA isolation kit from Stratagene. cDNAs were synthesized using a Gibco BRL SuperScript plasmid system with ∼5 μg of poly(A) mRNA. Labeling with α-32P-dCTP was used to monitor the synthesis of first and second strands. All cDNAs longer than 600 bp were ligated as SalI-NotI inserts with pSPORT1 vector (Gibco BRL). Transformation was achieved by electroporation of Electro Max DH10B cells (Gibco BRL), and its efficiency was estimated at 1.2 × 108 plates/μg cDNA. The average size of the inserts was 1 kb.
After plating of Escherichia coli transformants, plasmid DNA was prepared, digested with SalI-NotI, and electrophoresed on a 1% agarose gel. cDNAs ranging in size between 600 bp and 2.5 kb were excised and purified with the Qiex II kit (Qiagen). Two hundred fifty nanograms of these cDNAs and 500 ng of shuttle vector pPC86 were ligated and used to obtain 1.05 × 106 E. coli DH10B transformants. Thus, the cDNA expression library fused downstream of the activation domain of the GAL4 transcription factor was ready to be introduced into yeast.
Yeast Reporter Constructs
A 160-bp promoter fragment of the VvHT1 gene (Fillion et al., 1999) was cloned in the shuttle vectors pSK1 and pYC7 carrying the HIS3 and LacZ yeast reporter genes, respectively. The proximal region of the VvHT1 promoter was amplified by PCR with sequence-specific primers (forward primer, 5′-TAGAACGGGGAGTTAGAAACAA-3′; reverse primer, 5′-AGCTGTCCCCGATAATATCTAA-3′), which allowed the removal of minimal promoter containing the TATA and CAAT boxes. The PCR product was ligated in pGEM-T Easy vector, and two clones with inserts in the opposite orientation were selected for further cloning. The 160-bp promoter was cloned directionally (5′ → 3′) in the NotI-SpeI sites of pSK1 shuttle vector, a Leu-marked centromeric plasmid, in front of a HIS3 reporter gene under the control of the minimal inactive promoter GAL1. For the second reporter gene construct, the VvHT1 promoter fragment was first excised from pGEM-T Easy as a 5′ → 3′ fragment by SpeI-EcoRI and ligated in the intermediary vector pcDNAII (Invitrogen, San Diego, CA) digested by the same enzymes. The VvHT1 promoter fragment was inserted directionally as a KpnI-XhoI fragment in the pYC7 vector, a URA-marked 2-μm plasmid, in front of the LacZ reporter gene under the control of a minimal inactive CYC1 promoter.
All vectors used, the pPC86 carrying the cDNA library, the pSK1 and pYC7 carrying the reporter genes HIS3 and LacZ, respectively, and the host strain YN954, were kindly provided by Terry Thomas (Texas A&M University, College Station).
Yeast One-Hybrid Cloning System
The reporter yeast strain was constructed by cotransforming strain YM954 with both reporter constructs (5 μg each) using a lithium acetate protocol (Geitz and Woods, 1993). To screen the library, the reporter yeast was transformed with the library DNA. Double screening was applied sequentially as described by Kim et al. (1997). Putative positive yeast clones were grown for 3 days and assayed for β-galactosidase activity colorimetrically according to Breeden and Nasmyth (1985). Yeast plasmid DNA was prepared and transferred to E. coli DH5-α cells by electroporation. Bacterial colonies were screened by transfer to membranes and hybridization with GAL4-specific probe. DNA sequencing was performed by Eurogentec (Seraing, Belgium), and database searches were performed using the Basic Local Alignment Search Tool (BLAST) algorithm.
Genomic DNA Gel Blot Analysis
Genomic DNA was isolated from grape berry cell suspension by phenol extraction after RNase and proteinase K treatments. Ten micrograms of DNA was digested by each of the four enzymes (BglII, EcoRI, HindIII, and KpnI) and blotted onto Hybond N+ membranes by alkaline transfer as described by Amersham Pharmacia Biotech. The VvMSA cDNA was used for hybridization as a 32P-labeled probe. All procedures, hybridizations, and washings were performed at 65°C, and the final washing was performed stringent conditions with 0.1× SSC (1× SSC is 0.15 M NaCl and 0.015 M sodium citrate) and 0.1% SDS.
RNA Gel Blot Analysis
Two different procedures of RNA extraction were performed depending on the plant material used. (1) Total RNA from grape berries of the variety Ugni blanc was extracted according to Davies and Robinson (1996), with an additional step of selective precipitation with 2 M LiCl. (2) Total RNA from grape berry cell suspension (10-mL samples) and from frozen tobacco leaves was isolated by phenol extraction (Howell and Hull, 1978) followed by selective precipitation with 2 M LiCl.
Equal amounts of purified RNA samples were separated by formaldehyde-agarose gel electrophoresis and transferred to Hybond N membranes (Amersham Life Science). RNA gel blots were hybridized with randomly primed 32P probes, and mRNA was quantified using a Storm Bio-Imaging Analyzer (Molecular Dynamics, Sunnyvale, CA).
Grape Berry Cell Suspension, Culture, and Treatments
The grape berry cell suspension derived from Cabernet Sauvignon berries was maintained at 25°C on an orbital shaker (100 rpm) by weekly subculture in a medium supplemented with 58 mM sucrose (Decendit et al., 1996). Three days after subculture, grape berry cells were allowed to settle, washed carefully, and suspended again in fresh medium supplemented with sucrose (58 mM) (Figure 5). For the experiments described in Figure 4, the cell suspension on day 3 of subculture was separated and transferred in four different batches: (1) the same culture medium with sucrose; (2) the same medium with sucrose plus 10 μM abscisic acid (ABA); (3) sucrose-depleted medium; and (4) sucrose-depleted medium supplemented with 10 μM ABA. The cells were sampled at various times after treatment, as indicated in Results, and used for RNA gel blot experiments.
6xHis-Tagged Protein Expression and Purification
To express VvMSA in bacteria, two different constructs were prepared in two QIAexpress pQE vectors (Qiagen). The first was introduced into pQE-30 vector with a 6xHis tag at the N-terminal end, and the second was introduced into the pQE-50 vector without a tag. To clone VvMSA cDNA in the same reading frame as the 6xHis affinity tag, its translation initiation codon was modified (boldface letters) by PCR using the primer 5′-ACGGATCCCTGTCGGAGGAGAA-3′. In parallel, a BamHI restriction site (underlined sequence in the primer above) suitable for cloning was introduced at the 5′ end. PCR-modified cDNA was first digested by NotI and, after a Klenow fill-in reaction, digested sequentially with BamHI and cloned in the BamHI-SmaI sites of pKS plasmid. After this intermediary ligation, the VvMSA cDNA was inserted as a BamHI-EcoRV fragment in BamHI-SmaI of pQE-30 and pQE-50 vectors. For both constructs, the PCR product was checked by sequencing before and after ligation.
The E. coli M15 host strain was transformed with either the pQE-30 or the pQE-50 construct. Luria-Bertani culture medium (100 μg/mL ampicillin and 25 μg/mL kanamycin) was inoculated (1:500) with overnight culture and grown at 28°C with vigorous shaking until an OD600 of 0.6 was reached. After induction with 2 mM isopropylthio-β-galactoside, the culture was incubated for an additional 4 to 5 h, but at 21°C. Cells were harvested at different times to determine the best expression level and then frozen in liquid nitrogen.
Total proteins were extracted under nondenaturing conditions, and VvMSA purification by nickel–nitrilotriacetic acid agarose (Ni-NTA) affinity chromatography and elution were achieved under native conditions according to the recommended QIAexpressionist protocol (Qiagen). After dialysis against the column fixation buffer, VvMSA protein was purified a second time by Ni-NTA affinity chromatography and concentrated by centrifugation in a Centricon tube (3000 D; Pharmacia).
In Vitro Transcription and Translation System
Extracts containing VvMSA protein were prepared by coupled in vitro transcription/translation of 0.75 μg of DNA (pGEM-T Easy vector carrying VvMSA cDNA) using the TnT Coupled Reticulocyte Lysate System at a final concentration of 56% lysate. The immunodetection of coupled transcription and translation (TnT)–produced proteins was achieved by chemiluminescence using the Transcend nonradioactive detection system (Promega).
In Vitro Protein Binding Assay
Electrophoretic mobility-shift assay was performed according to Bernard et al. (2001). Probe DNA (20,000 to 50,000 cpm) was incubated with either 400 ng of Ni-NTA–purified protein or 4 to 7 μL of in vitro translation product in 20 μL of binding reaction mixture containing 1 μg of poly (dI-dC) for 20 min. The reaction mixture was electrophoresed on a 5% polyacrylamide gel (29:1 acrylamide:N′,N-methylene bis-acrylamide). When competition analysis was performed, unlabeled probes were used as competitor in the binding mixture and the reaction was continued for an additional 20 min. The unrelated fragment of the VvHT1 promoter was amplified by PCR with sequence-specific primers (forward, 5′-ACTACGGAAAAATTCGACCC-3′; reverse, 5′-TGGCTCTGATAGGGCTGAAA-3′).
When oligonucleotides were used in band-shift assays, both strains of each synthetic oligonucleotide were hybridized and 5′ end labeled with the T4 polynucleotide kinase and γ-32P-dATP. One and one-half microliter (40,000 to 50,000 cpm) of the labeled probe was added to 18 μL of the binding mixture containing 7 μL of TnT-produced proteins and 1 μg of poly(dI-dC)–poly(dI-dC) as a nonspecific DNA competitor. The incubation mixtures were loaded on 5% polyacrylamide gels (37.5:1 acrylamide:N′,N-methylene bis-acrylamide). After migration in 1× TGE buffer (40 mM Tris, 270 mM glycine, 4 mM EDTA, pH 8.4) at 150 V, the gels were dried and autoradiographed.
YFP-VvMSA Fusion Proteins
To study the subcellular compartmentalization of the VvMSA protein, three different constructs based on a YFP fusion were used. The first one was the pAVA 554 vector (Von Arnim et al., 1998) designed especially as a reporter gene with enhanced yellow fluorescence. Expression of YFP gene expression is driven by a double 35S promoter, and YFP protein synthesis is induced by a viral translation enhancer. The second construct corresponded to the YFP-VvMSA fusion, in which the complete cDNA of VvMSA encompassing the nuclear localization sequence (NLS) was used. To produce this construct, the VvMSA cDNA with a modified ATG codon and a BamHI restriction site introduced at the 5′ end (see the VvMSA sequence used for 6xHis-tagged protein production) was cloned as a BamHI-XbaI (VvMSA stop codon) fragment in the BglII-XbaI sites of pAVA 554. In the third construct, the NLS sequence of VvMSA cDNA was deleted completely by PCR performed with the forward primer mentioned above and the reverse primer 5′-GCTCTAGACTCGTGATGCTCGT-3′, allowing the introduction of the XbaI restriction site (underlined sequence) just upstream of the NLS sequence. The correct sequence of PCR-produced VvMSA, the YFP–VvMSA gene junction, and the stop codon present in the XbaI site were checked by sequencing of both strands of VvMSA in each construct.
Protoplast Preparation and Transformation
Protoplasts were isolated from tobacco BY2 cells cultured as described previously (Atanassova et al., 2003) on the 4th day after subculture in fresh medium. Protoplast preparation and transformation using a polyethylene glycol–based technique were performed essentially according to the method of Neuhaus and Boevink (2001). Overnight digestion of cell walls was achieved with 0.1% pectolyase and 1% cellulase (Seishin, Tokyo, Japan) at 28°C in the dark with gentle shaking. Approximately 7.5 × 105 protoplasts were transformed with 20 μg of plasmid DNA and 20 μg of carrier DNA to a medium containing 40% PEG-6000 (Roth, Saint-Quentin Fallavier, France), 0.1 M Ca(NO3)2, 0.4 M mannitol, and 0.1% Mes, pH 8.0. Observation by confocal microscopy was made 48 h after transformation, and protoplasts were maintained at 26°C in the dark without shaking.
Confocal Imaging
The samples were examined by confocal laser scanning microscopy using a Bio-Rad MRC 1024 microscope equipped with a 15-mW argon-krypton gas laser. The confocal unit was attached to an inverted microscope (IX70; Olympus, Tokyo, Japan). Fluorescence signal collection, image construction, and scaling were performed using the control software (Lasersharp 3.2; Bio-Rad). The YFP protein was excited with the 488-nm blue line, and emission of the dye was collected via a photomultiplier through a 522-nm band-pass filter.
Agroinfiltration
Agrobacteria infiltration was conducted as described by Yang et al. (2000). Agrobacterium tumefaciens strain LBA 4404 containing the binary plasmid pBI121 with VvMSA cDNA was grown at 28°C overnight in YEB liquid medium (5 g L−1 sucrose, 1 g L−1 yeast extract, 10 g L−1 Bactopeptone, 5 g L−1 Gibco beef extract, pH 7.4) supplemented with rifampicin (100 μg/mL) and kanamycin (50 μg/mL). Agrobacteria then were inoculated in 20 mL of induction medium containing AB salts (NH4Cl 18.6 mM; MgSO4, 7H2O, 1.2 mM; KCl 1.9 mM; CaCl2 0.06 mM; FeSO4; 7H2O 0.008 mM), 2 mM phosphate, 1% glucose, 20 mM Mes, pH 5.5, 100 μM acetosyringone, and rifampicin and kanamycin at the same concentrations given above. After overnight culture at 28°C, the bacteria were collected by centrifugation (15 min at 3000g) and washed once in a solution containing 10 mM Mes, pH 5.5, 10 mM MgSO4, and 100 μM acetosyringone. Cells then were resuspended in the same solution, and the OD600 of the suspension was adjusted to 0.8 before infiltration.
Young, nearly expanded leaves of 6-week-old tobacco plants were infiltrated. The infiltrated areas were collected after 48 h and frozen in liquid nitrogen. The GUS fluorimetric assay was performed according to the method of Jefferson et al. (1987).
Upon request, materials integral to the findings presented in this publication will be made available in a timely manner to all investigators on similar terms for noncommercial research purposes. To obtain materials, please contact S. Delrot, serge.delrot@univ-poitiers.fr.
Accession Numbers
The accession number for VvMSA is AF281656. Other accession numbers are AF176655 (GASR) and AJ001062 (VvHT1).
Acknowledgments
We are grateful to Terry Thomas (Texas A&M University, College Station) for the gift of pPC86, pSK1, and pYC7 vectors and of yeast strain YM954, to Matthieu Régnacq (Unité Mixte de Recherche Centre National de la Recherche Scientifique [UMR CNRS] 6161, University of Poitiers, France) for helpful discussions regarding one-hybrid screening, to Marianne Bernard (UMR CNRS 6558, University of Poitiers) for help with the gel-shift assays, to Anne Cantereau (UMR CNRS 6558, University of Poitiers) for confocal microscopy observations, to Marie-Thérèse Bidoyen for technical assistance, and to Bruno Faure for greenhouse plants. Part of this work was supported by the Conseil Régional Poitou-Charentes.
Article, publication date, and citation information can be found at www.plantcell.org/cgi/doi/10.1105/tpc.013854.
References
- Amitai-Zeigerson, H., Scolnik, P.A., and Bar-Zvi, D. (1994). Genomic nucleotide sequences of tomato Asr2, a second member of the stress/ripening-induced Asr1 gene family. Plant Physiol. 106, 1699–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Huertero, F., Arroyo, A., Zhou, L., Sheen, J., and Léon, P. (2000). Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev. 14, 2085–2096. [PMC free article] [PubMed] [Google Scholar]
- Atanassova, R., Leterrier, M., Gaillard, C., Agasse, A., Sagot, E., Coutos-Thévenot, P., and Delrot, S. (2003). Sugar-regulated expression of a putative hexose transport gene in grape. Plant Physiol. 131, 326–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard, M., Dinet, V., and Voisin, P. (2001). Transcriptional regulation of the chicken hydroxyindole-O-methyltransferase gene by the cone-rod homeobox-containing protein. J. Neurochem. 79, 248–257. [DOI] [PubMed] [Google Scholar]
- Blouin, J., and Guimberteau, G. (2000). Maturité. In Maturation et Maturité des Raisins. (Bordeaux, France: Féret), pp. 29–31.
- Breeden, L., and Nasmyth, K. (1985). Regulation of the yeast HO gene. Cold Spring Harbor Symp. Quant. Biol. 50, 643–650. [DOI] [PubMed] [Google Scholar]
- Canel, C., Bailey-Serres, J.N., and Roose, M.L. (1995). Pummelo fruit transcript homologous to ripening-induced genes. Plant Physiol. 108, 1323–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, S., Puryear, J.D., Dias, M.A.D.L., Funkhauser, E.A., Newton, R.G., and Cairney, J. (1996). Gene expression under water deficit in loblolly pine (Pinus taeda L.): Isolation and characterization of cDNA clones. Physiol. Plant. 97, 139–148. [Google Scholar]
- Chiu, W.-L., Niwa, Y., Zeng, W., Hirano, T., Kobayashi, H., and Sheen, J. (1996). Engineered GFP as a vital reporter in plants. Curr. Biol. 6, 325–330. [DOI] [PubMed] [Google Scholar]
- Coombe, B.G. (1992). Research on development and ripening of the grape berry. Am. J. Enol. Vitic. 43, 101–110. [Google Scholar]
- Davies, C., and Robinson, S.P. (1996). Sugar accumulation in grape berries: Cloning of two putative vacuolar invertase cDNAs and their expression in grape berry tissues. Plant Physiol. 111, 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decendit, A., Ramawat, K.G., Waffo, P., Deffieux, G., Badoc, A., and Mérillon, J.M. (1996). Anthocyanins, catechins, condensed tannins and piceid production in Vitis vinifera cell bioreactor cultures. Biotechnol. Lett. 18, 659–662. [Google Scholar]
- Delrot, S., Atanassova, R., and Maurousset, L. (2000). Regulation of sugar, amino acid and peptide plant membrane transporters. Biochim. Biophys. Acta 1465, 281–306. [DOI] [PubMed] [Google Scholar]
- de Vienne, D., Leonardi, A., Damerval, C., and Zivy, M. (1999). Genetics of proteome variation for QTL characterization: Application to drought-stress responses in maize. J. Exp. Bot. 50, 303–309. [Google Scholar]
- Dieuaide, M., Brouquisse, R., Pradet, A., and Raymond, P. (1992). Increased fatty acid β-oxidation after glucose starvation in maize root tips. Plant Physiol. 99, 595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dure, L., III, Crouch, M., Harada, J., Ho, T.-H.D., Mundy, J., Quatrano, R., Thomas, T., and Sung, Z.R. (1989). Common amino acid sequence domains among the LEA proteins of higher plants. Plant Mol. Biol. 12, 475–486. [DOI] [PubMed] [Google Scholar]
- Fillion, L., Ageorges, A., Picaud, S., Coutos-Thévenot, P., Lemoine, R., Romieu, C., and Delrot, S. (1999). Cloning and expression of a hexose transporter gene during the ripening of grape berry. Plant Physiol. 120, 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein, R.R., and Gibson, S.I. (2001). ABA and sugar interactions regulating development: Cross-talk or voices in a crowd? Curr. Opin. Plant Biol. 5, 26–32. [DOI] [PubMed] [Google Scholar]
- Garay-Arroyo, A., Colmenero-Flores, J.M., Garciarrubio, A., and Covarrubias, A.A. (2000). Highly hydrophilic proteins in prokaryotes and eukaryotes are common during conditions of water deficit. J. Biol. Chem. 275, 5668–5674. [DOI] [PubMed] [Google Scholar]
- Gazzarrini, S., and McCourt, P. (2001). Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr. Opin. Plant Biol. 4, 387–391. [DOI] [PubMed] [Google Scholar]
- Geitz, R.D., and Woods, R.A. (1993). Highly efficient transformation with lithium acetate. In Molecular Genetics of Yeast: A Practical Approach, J.R. Johnston, ed (Oxford, UK: IRL Press), pp. 121–134.
- Gibson, S.J. (2000). Plant sugar-response pathways: Part of a complex regulatory web. Plant Physiol. 124, 1532–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilad, A., Amitai-Zeigerson, H., Bar-Zvi, D., and Scolnik, P.A. (1997). ASR1, a tomato water stress-regulated gene: Genomic organization, developmental regulation and DNA-binding activity. Acta Hortic. 447, 441–453. [Google Scholar]
- Giovannoni, J. (2001). Molecular biology of fruit maturation and ripening. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52, 725–749. [DOI] [PubMed] [Google Scholar]
- Grierson, C., Du, J.S., De Torres Zabala, S., Beggs, K., Smith, C., Holsworth, M., and Bevan, M. (1994). Separate cis sequences and trans factors direct metabolic and developmental regulation of a potato tuber storage protein gene. Plant J. 5, 815–826. [DOI] [PubMed] [Google Scholar]
- Hong, S.H., Kim, I.-J., Yang, D.C., and Chung, W.-I.I. (2002). Characterization of an abscisic acid responsive gene homologue from Cucumis melo. J. Exp. Bot. 53, 2271–2272. [DOI] [PubMed] [Google Scholar]
- Howell, S.H., and Hull, R. (1978). Replication of cauliflower mosaic virus and transcription of its genome in turnip leaf protoplasts. Virology 88, 468–481. [Google Scholar]
- Iusem, N.D., Bartholomew, D.M., Hitz, W.D., and Scolnik, P.A. (1993). Tomato (Lycopersicon esculentum) transcript induced by water deficit and ripening. Plant Physiol. 102, 1353–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneau, M., Gerentes, D., Foueillassar, X., Zivy, M., Vidal, J., Toppan, A., and Perez, P. (2002). Improvement of drought tolerance in maize: Towards the functional validation of the ZM-ASR1 gene and increase of water use efficiency by over-expressing C4-PEPC. Biochimie 84, 1127–1135. [DOI] [PubMed] [Google Scholar]
- Jefferson, R.A., Kavanagh, T.A., and Bevan, M.W. (1987). GUS fusions: β-Glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S.Y., Chung, H.-J., and Thomas, T.L. (1997). Isolation of a novel class of bZIP transcription factors that interact with ABA-responsive and embryo-specification elements in the Dc3 promoter using a modified yeast one-hybrid system. Plant J. 11, 1237–1251. [DOI] [PubMed] [Google Scholar]
- Kircher, S., Wellmer, F., Nick, P., Rügner, A., Schäfer, E., and Harter, K. (1999). Nuclear import of the parsley bZIP transcription factor CPRF2 is regulated by phytochrome photoreceptors. J. Cell Biol. 144, 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier, M. (2002). Régulation et Rôle Physiologique du Gène VvHT1 Exprimé Durant la Maturation de la Baie de Raisin. PhD dissertation (Poitiers, France: University of Poitiers).
- Leterrier, M., Atanassova, R., Laquitaine, L., Gaillard, C., Coutos-Thévenot, P., and Delrot, S. (2003). Expression of a putative grapevine hexose transporter in tobacco alters morphogenesis and assimilate partitioning. J. Exp. Bot. 54, 1193–1204. [DOI] [PubMed] [Google Scholar]
- Maskin, L., Gubesblat, G.E., Moreno, J.E., Carrari, F.O., Frankel, N., Sambade, A., Rossi, M., and Iusem, N.D. (2001). Differential expression of the members of the Asr gene family in tomato (Lycopersicon esculentum). Plant Sci. 161, 739–746. [Google Scholar]
- Mbeguie-A-Mbeguie, D., Gomez, R.-M., and Fils-Lycaon, B. (1997). Molecular cloning and nucleotide sequence of a protein from apricot fruit (accession No. U82760) homologous to LEC14B protein isolated from Lithospermum gene expression during fruit ripening (PGR 97–161). Plant Physiol. 115, 1288. [Google Scholar]
- Mita, S., Suzuki-Fujii, K., and Nakamura, K. (1995). Sugar-inducible expression of a gene for β-amylase in Arabidopsis thaliana. Plant Physiol. 107, 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus, J.-M., and Boevink, P. (2001). The green fluorescent protein (GFP) as reporter gene in plant cells. In Plant Cell Biology, C.R. Haves and B. Satiat-Jeunemaitre, eds (Oxford, UK: Oxford University Press), pp. 127–142.
- Padmanabhan, V., Dias, D.M.A.L., and Newton, R.J. (1997). Expression analysis of a gene family in a loblolly pine (Pinus taeda L.) induced by water deficit stress. Plant Mol. Biol. 35, 801–807. [DOI] [PubMed] [Google Scholar]
- Riccardi, F., Gazeau, P., de Vienne, D., and Zivy, M. (1998). Protein changes in response to progressive water deficit in maize. Plant Physiol. 117, 1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechmann, J.L., and Ratcliffe, O.J. (2000). A genomic perspective on plant transcription factors. Curr. Opin. Plant Biol. 3, 423–434. [DOI] [PubMed] [Google Scholar]
- Riou-Khamlichi, C., Huntley, R., Jacqmard, A., and Murray, J.A. (1999). Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283, 1541–1544. [DOI] [PubMed] [Google Scholar]
- Rook, F., Corke, F., Card, R., Munz, G., Smith, C., and Bevan, M.W. (2001). Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J. 26, 421–433. [DOI] [PubMed] [Google Scholar]
- Rossi, M., Carrari, F., Cabrera-Ponce, J.L., Vázquez-Rovere, C., Herrera-Estrella, L., Gudesblat, G., and Iusem, N.D. (1998). Analysis of an abscisic acid (ABA)-responsive gene promoter belonging to the Asr gene family from tomato in homologous and heterologous systems. Mol. Gen. Genet. 258, 1–8. [DOI] [PubMed] [Google Scholar]
- Rossi, M., and Iusem, N.D. (1994). Tomato (Lycopersicon esculentum) genomic clone homologous to a gene encoding an abscisic acid-induced protein. Plant Physiol. 104, 1073–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi, M., Lijavetzky, D., Bernacchi, D., Hopp, H.E., and Iusem, N. (1996). Asr genes belong to a gene family comprising at least three closely linked loci on chromosome 4 in tomato. Mol. Gen. Genet. 252, 489–492. [DOI] [PubMed] [Google Scholar]
- Schneider, A., Salamini, F., and Gebhardt, C. (1997). Expression patterns and promoter activity of the coat-regulated gene ci21A of potato. Plant Physiol. 113, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhavy, D., Hutvágner, G., Barta, E., and Bánfalvi, Z. (1995). Isolation and characterization of a water stress-inducible cDNA clone from Solanum chacoense. Plant Mol. Biol. 27, 587–595. [DOI] [PubMed] [Google Scholar]
- Smeekens, S. (2000). Sugar-induced signal transduction in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 51, 49–81. [DOI] [PubMed] [Google Scholar]
- Smeekens, S., and Rook, F. (1997). Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol. 115, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesnière, C., and Vayda, M.E. (1991). Method for the isolation of high quality RNA from grape berry tissues without contaminating tannins or carbohydrates. Plant Mol. Biol. Rep. 9, 242–251. [Google Scholar]
- Tsukaya, H., Ohshima, T., Naito, S., Chino, M., and Komeda, Y. (1991). Sugar-dependent expression of the CHS-A gene for chalcone synthase from petunia in transgenic Arabidopsis. Plant Physiol. 97, 1414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan, R., Kuruvilla, S., and Thomas, G. (1999). Characterization and expression pattern of an abscisic acid and osmotic stress responsive gene from rice. Plant Sci. 140, 25–36. [Google Scholar]
- Villain, P., Mache, R., and Zhou, D.X. (1996). The mechanisms of GT element-mediated cell type-specific transcriptional control. J. Biol. Chem. 271, 32593–32598. [DOI] [PubMed] [Google Scholar]
- Von Arnim, A.G., Deng, X.-W., and Stacey, M.G. (1998). Cloning vectors for the expression of green fluorescent protein fusion proteins in transgenic plants. Gene 221, 35–43. [DOI] [PubMed] [Google Scholar]
- Wang, C.-S., Liau, Y.E., Huang, J.C., Wu, T.D., Su, C.C., and Lin, C.H. (1998). Characterization of a desiccation-related protein in lily pollen during development and stress. Plant Cell Physiol. 39, 1307–1314. [DOI] [PubMed] [Google Scholar]
- Yang, Y., Li, R., and Qi, M. (2000). In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J. 22, 543–551. [DOI] [PubMed] [Google Scholar]
- Yu, S.-M. (1999). Cellular and genetic responses of plants to sugar starvation. Plant Physiol. 121, 687–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, L., Jan, J.-C., Jones, T.-L., and Sheen, J. (1998). Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc. Natl. Acad. Sci. USA 95, 10294–10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatanova, I. (1990). Histone H1 and the regulation of transcription of eukaryotic genes. Trends Biochem. Sci. 15, 273–276. [DOI] [PubMed] [Google Scholar]