Abstract
Elevated glucose concentrations stimulate the transcription of the pre-proinsulin (PPI), L-type pyruvate kinase (L-PK), and other genes in islet beta cells. In liver cells, pharmacological activation by 5-amino-4-imidazolecarboxamide riboside (AICAR) of AMP-activated protein kinase (AMPK), the mammalian homologue of the yeast SNF1 kinase complex, inhibits the effects of glucose, suggesting a key signaling role for this kinase. Here, we demonstrate that AMPK activity is inhibited by elevated glucose concentrations in MIN6 beta cells and that activation of the enzyme with AICAR prevents the activation of the L-PK gene by elevated glucose. Furthermore, microinjection of antibodies to the α2- (catalytic) or β2-subunits of AMPK complex, but not to the α1-subunit or extracellular stimulus-regulated kinase, mimics the effects of elevated glucose on the L-PK and PPI promoter activities as assessed by single-cell imaging of promoter luciferase constructs. In each case, injection of antibodies into the nucleus and cytosol, but not the nucleus alone, was necessary, indicating the importance of either a cytosolic phosphorylation event or the subcellular localization of the α2-subunits. Incubation with AICAR diminished, but did not abolish, the effect of glucose on PPI transcription. These data suggest that glucose-induced changes in AMPK activity are necessary and sufficient for the regulation of the L-PK gene by the sugar and also play an important role in the regulation of the PPI promoter.
Keywords: L-type pyruvate kinase, insulin, promoter
Glucose is the primary fuel for many organisms. The ability of single cells and whole animals to respond to change in glucose availability is of particular importance for survival. Thus, in yeast, glucose induces expression of genes involved in its own metabolism and represses the expression of proteins involved in the utilization of alternative carbon sources (1). In mammals, blood glucose concentration normally is maintained between a narrow range, irrespective of exogenous glucose ingestion. In the liver, glucose (in the presence of insulin) induces expression of genes encoding glucose transporters and glycolytic and lipogenic enzymes and represses genes of the gluconeogenic pathway (2–4). Similarly, in islet beta cells, glucose activates several of the same genes, as well as activating transcription of the pre-proinsulin (PPI) gene (5). The mechanisms of glucose repression and glucose activation of gene expression are relatively well understood in yeast, where the sucrose nonfermenting 1 protein kinase (SNF1) complex is activated by glucose removal and phosphorylates transcription factors involved in gene repression, such as Mig1 (1, 6–8). Little is known about the glucose-signaling pathway in mammals (9), although a recent study revealed that glucose removal also activates the mammalian homologue of the SNF1 complex, AMP-activated protein kinase, in two islet beta cell derived lines, HIT-T15 and INS-1 (10).
The L-type pyruvate kinase gene (L-PK) is expressed in the liver, pancreatic beta cells, the small intestine, and the proximal renal tubule (11, 12). L-PK gene transcription is regulated positively by glucose and insulin and negatively by glucagon and cAMP, both in liver (13) and, in the case of glucose, islet beta cells (11, 14). The glucose response unit appears to be located between nucleotides −183 and −119 with respect to the cap site (15, 16). Within this region a site between nucleotides −165 and −154, termed L4, confers glucose responsiveness (17). This site consists of two E boxes and has been shown to bind the nuclear factors USF1 and USF2 (upstream stimulatory factors 1 and 2; refs. 18 and 19), which are essential for a normal response of L-PK gene transcription to glucose (14, 20). More recently, it was shown that the orphan nuclear receptor COUPTF-II (chicken ovalbumin upstream promoter transcription factor II) also binds this region and could act as a repressor (21). Adjacent to the L4 box is a binding site (L3) for the ancillary factor HNF4 (hepatocyte nuclear factor 4; refs. 18 and 22). Other data have suggested a role for sterol response element-binding protein (23), although the involvement of this factor is disputed (24).
The nature of the molecules that signal the availability of glucose to the L-PK transcriptional machinery is also controversial. A dephosphorylation/phosphorylation cycle is likely to be involved, because protein phosphatase inhibitors block the effects of glucose on L-PK (B.D. and A.K., unpublished data) and fatty acid synthase transcription (25). It is also clear that glucose must enter the cell and be phosphorylated to glucose 6-phosphate (26, 27) and possibly must also be metabolized into pentose phosphate cycle intermediates (28).
The regulation of the PPI gene promoter is also complex and may involve the interplay of at least eight separate transcription factors (29). The effects of glucose appear to be mediated via a region located 212–198 bp upstream of the transcriptional start point of the human gene, termed the A3 box (29), and may involve the transcription factor pancreatic duodenum homeobox protein (IPF-1/STF-1/IUF-1/IDX-1) (30, 31).
We have shown previously, using the activator 5-amino-4-imidazolecarboxamide riboside (AICAR), that AMP-activated protein kinase (AMPK), the mammalian homologue of the yeast SNF1 complex, probably is involved in the regulation of glucose-responsive genes in mammals (32, 33). AMPK kinase and AMPK are the upstream and downstream components, respectively, of a highly conserved protein kinase cascade (7, 34). AMPK is now considered as a key metabolic master switch (35), regulating carbohydrate and fat metabolism in response to change in cellular energy charge, being activated in an ultrasensitive manner (36) by a fall in ATP/AMP ratio. The enzyme exists as a heterotrimer of α- (catalytic), β- (adapter), and γ- (regulatory) subunits (7). At least two isoforms of each subunit have been described (37). Unlike α1 complexes, complexes containing the α2 isoform of the catalytic subunit are found in both the nucleus and the cytoplasm (10).
To investigate directly the role of individual AMPK isoforms in the regulation of the L-PK and PPI genes in islet beta cells, we now have combined the use of AICAR with single-cell antibody microinjection and dynamic imaging of luciferase reporter constructs (14, 38). These studies suggest that regulation of the AMPK α2-subunit may be necessary and sufficient to explain the effects of glucose on L-PK gene expression in beta cells, and also plays an important, but not exclusive, role in the control of insulin promoter activity.
Experimental Procedures
Materials.
Protein G-agarose was from Boehringer Mannheim. Reverse transcriptase and Taq DNA polymerase were from GIBCO/BRL. Beetle luciferin was from Promega and coelanterazine was from Molecular Probes. AICAR and other reagents were from Sigma and BDH.
Plasmids.
pLPK.LucFF and pΔL4.LPK.LucFF contained, respectively, nucleotides −183 to +10 and −148 to +10 of the rat L-PK promoter fused immediately upstream of humanized firefly luciferase cDNA (plasmid pGL3 basic; Promega) (14). Plasmid pINS.LucFF contained nucleotides −260 to −60 of the human insulin promoter fused upstream of the herpes simplex minimal thymidine kinase promoter and firefly luciferase cDNA (31). The expression plasmid for Renilla luciferase (pRL.CMV) was purchased from Promega.
Antibodies.
Sheep antibodies raised against rat AMPK-α1 and α2- (39) and β2-subunits (40) were produced as described. Polyclonal antibody against extracellular stimulus-regulated kinase was purchased from Santa Cruz Biotechnology. Each antibody was affinity-purified and dialyzed extensively before use.
Cell Culture.
MIN6 cells were used between passages 20 and 30 and grown in DMEM containing 15% (vol/vol) heat-inactivated FCS, 30 mM glucose, 2 mM glutamine, 100 mM 2-mercaptoethanol, 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified atmosphere at 37°C with 5% CO2 unless specified otherwise.
Immunocytochemistry.
Cells were fixed with 4% (vol/vol) paraformaldehyde before probing with primary sheep antibodies vs. AMPK α1 and α2 (39) (1:40) and revealed with tetramethyl rhodamine isothiocyanate-conjugated anti-sheep IgG (1:500). Optical sections were obtained by laser-scanning confocal microscopy, using a Leica DM/IRBE inverted microscope (×40 oil-immersion objective) (41).
RNA Isolation, cDNA Synthesis, and PCR Amplification.
Total RNA was isolated from MIN6 cells by lysis in guanidinium thiocyanate, followed by phenol extraction (42). First-strand cDNA synthesis was performed as described (43). Oligonucleotide primers for L-PK (PKS3 and PKS5) and β-actin mRNAs were used as described (12). The entire coding region of PPI was amplified with primers 1 and 3 given in ref. 41. Radioactive PCR amplification (25–30 cycles; annealing temperature, 59°C) was performed in a final volume of 50 μl containing 250 ng of cDNA, 200–300 ng of each primer, 2.5 mM MgCl2, 10% DMSO, 1 unit of Taq polymerase, and 0.05 μCi of [α-32P]dCTP. mRNAs were quantified by analyzing 10 μl of radioactive PCR products on 5% nondenaturant polyacrylamide gels with PhosphorImager (Molecular Dynamics).
Extraction and Assay of AMPK Activity.
MIN6 cells in monolayer were scraped into ice-cold lysis buffer [50 mM Tris⋅HCl, pH 7.4 at 4°C/250 mM sucrose/50 mM NaF/1 mM sodium pyrophosphate/1 mM EDTA/1 mM EGTA/1 mM DTT/1% (vol/vol) Triton X-100/complete protease inhibitor mixture; Boehringer Mannheim]. Extracts were centrifuged (13,000 × g, 5 min at 4°C), and protein concentration was determined with DC Protein Assay Reagent (Bio-Rad). This protocol has been found to release >90% of the total cellular (i.e., nuclear plus cytosolic) content of each AMPK isoform into the supernatant of similar beta cell lines (I.P.S., unpublished results, and see ref. 10). Extracts were kept at −80°C until AMPK immunoprecipitation and enzyme assay. AMPK was immunoprecipitated from 100 μl of cell extract with either sheep anti-α1 or anti-α2 antibodies. AMPK activity was measured (44) in 10 μl of crude extract or immunoprecipitate by using “SAMS” peptide (the synthetic peptide HMRSAMSGLHLVKRR) and [γ-32P]ATP (specific activity, 1,000 cpm/pmol).
Microinjection and Luciferase Imaging.
Microinjection was performed (14) at plasmid concentrations of 0.1 (LucFF-based vectors) and 0.05 (pRL.CMV) mg⋅ml−1 and total antibody concentration of 1–1.2 mg⋅ml−1 (Bradford assay) (45). Details of single-living cell photon-counting imaging of firefly and Renilla reniformis luciferase activities are as given in previous publications (14, 31, 38). Individual experiments involved injection of 100–200 separate cells per condition, with an efficiency of 5–20% productive injection, assessed by expression of R. reniformis luciferase activity.
Statistical Analysis.
Data are given as mean ± SEM of three to five individual experiments. Comparisons between means were done by using Student's t test for paired data using Microsoft excel software.
Results
Subcellular Distribution and Activity of AMPK Isoforms in MIN6 Cells.
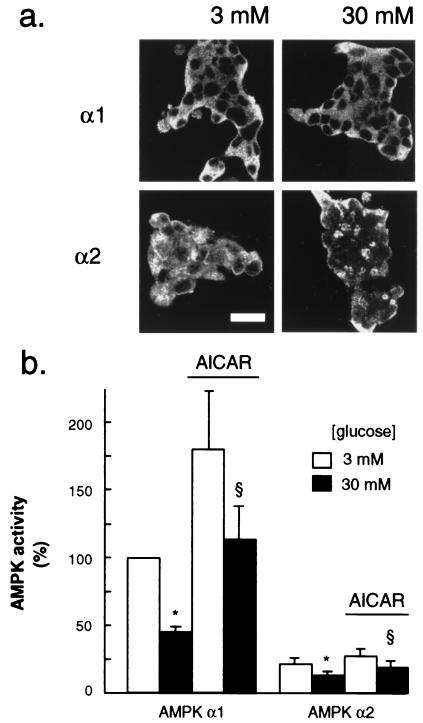
We first examined the presence and the subcellular localization of α1 and α2 AMPK isoforms in fixed MIN6 beta cells. Immunolabeling of cells and confocal microscopic examination (Fig. 1a) showed an exclusive cytosolic localization for α1, whereas α2, which was stained much more weakly, was present both in the cytosol and the nucleus. These distributions were unaffected by culture at either 3 or 30 mM glucose (Fig. 1a).
Figure 1.
Immunolocalization of AMPK isoforms in MIN6 beta cells (a) and regulation of AMPK activity by glucose and AICAR (b). (a) Cells were incubated for 24 h at the indicated glucose concentrations before probing for AMPK α1 and α2 isoforms as detailed in Experimental Procedures. Shown are clusters of 30–40 cells; the dark areas in the center of each cell, most evident after probing with the anti-AMPK α1 antibody, correspond to the position of the nuclei, as identified in bright-field images (not shown). (Bar = 20 μm.) (b) After incubation for 6 h at the indicated glucose concentration, each isoform of AMPK activity was assayed after immunoprecipitation as given in Experimental Procedures. Data are normalized to activity vs. AMPK α1 in extracts cultured at 3 mM and represent the means ± SEM of four separate experiments. P < 0.05 for the effect of 30 mM glucose (*) and 200 μM AICAR (§).
We next examined the modulation by glucose of AMPK activity in MIN6 cells. Assayed either in crude extracts or after immunoprecipitation of the specific isoforms, AMPK activity was decreased by raising the glucose concentration from 3 to 30 mM and activated by a 6-h culture with 200 μM AICAR. Thus, in crude extracts, a 30 ± 10% (P < 0.05; n = 4 separate experiments) decrease in total AMPK activity was elicited at 30 vs. 3 mM glucose. At 30 mM glucose, 200 μM AICAR increased total AMPK activity by 19 ± 6.6% (n = 4 separate experiments) and by 35 and 45% in two separate experiments performed at 3 mM glucose. Examined after immunoprecipitation from cells maintained in 3 mM glucose, the activity of the α2 AMPK isoform was 22 ± 5% of the α1 activity (mean ± variation of two separate preparations). The effects of glucose and AICAR on the immunoprecipitated AMPK isoforms were similar to but more marked than those apparent in crude extracts, probably reflecting the removal of other, glucose/AICAR-insensitive SAMS peptide (the synthetic peptide HMRSAMSGLHLVKRR) kinases (Fig. 1b) (44).
Regulation of Endogenous L-PK Gene Expression in MIN6 Beta Cells: Effects of Glucose and AICAR.
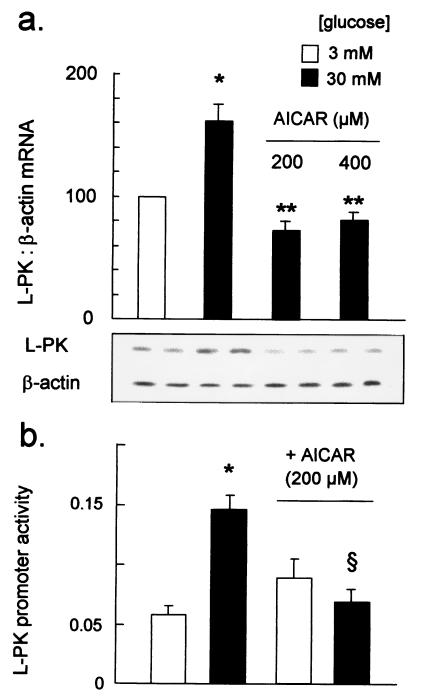
Increasing the glucose concentration from 3 to 30 mM significantly elevated L-PK mRNA levels in MIN6 beta cells after 6 h, as assessed by semiquantitative reverse transcription–PCR (Fig. 2). Incubation with 200 μM AICAR decreased L-PK mRNA levels in cells incubated at 30 mM glucose (Fig. 2a), but was without effect on β-actin (Fig. 2a) or pancreatic duodenum homeobox protein (not shown) mRNA levels.
Figure 2.
Regulation of L-PK gene expression by glucose and AICAR. L-PK mRNA was determined by semiquantitative reverse transcription–PCR, as detailed in Experimental Procedures, with 25 elongation cycles. (Lower) Autoradiograph of PCR products from a single experiment, run in duplicate. (Upper) Data from four separate experiments, *, P < 0.05 for the effect of 30 vs. 3 mM glucose; **, P < 0.01 for the effect of AICAR. (b) L-PK promoter activity was assessed after microinjection of cells with plasmids pLPK.LucFF and pRL.CMV and incubation for 6 h at either 3 or 30 mM glucose, plus 200 μM AICAR, as indicated. Firefly and R. reniformis luciferase activities were measured as detailed in the Experimental Procedures. Data were from five separate experiments, involving 42–70 productively injected cells in each condition. P < 0.05 for the effect of 30 vs. 3 mM glucose (*) and AICAR (§).
Effects of AICAR on L-PK Promoter Activity in Single MIN6 Beta Cells.
L-PK promoter activity was monitored through changes in reporter firefly luciferase activity and normalized to the activity of the constitutive cytomegalovirus promoter, driving Renilla luciferase production (14). Cells incubated for 6 h after microinjection at 30 mM glucose displayed between 2.5- and 10-fold greater L-PK promoter activity than cells maintained at 3 mM glucose (Figs. 2b and 3). Addition of 200 μM AICAR completely abolished the activation of transcription by glucose (Fig. 2b).
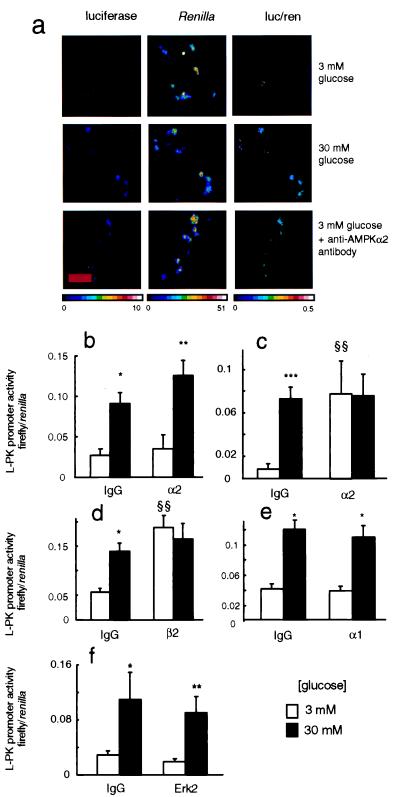
Figure 3.
Effect of anti-AMPK antibody injection on regulation of L-PK promoter by glucose. (a) MIN6 cells were microinjected with plasmids LPK.LucFF and pCMV.RL as detailed in Fig. 2, plus either preimmune IgG or anti-AMPK-α2 IgG (1.0 mg⋅ml−1 each) as shown. In all cases, cells were doubly injected into the cytosol and nucleus and cultured for 6 h at the indicated glucose concentrations before successive imaging of firefly and R. reniformis luciferase activities (see Experimental Procedures and ref. 38). The intensity of cell luminescence is given in pseudocolor (photon⋅pixel−1⋅s−1). The relative activity of the L-PK vs. cytomegalovirus promoters in individual cells is indicated in the ratiometric images (firefly/R. reniformis luciferase activities) which were calculated by using photek software. (Bar = 50 μm.) (b) Effect of nucleus-only injection of anti-α2-antibodies. (c) Same as b, but with nucleus plus cytosolic injection. (d) Same as c, but with anti-AMPK β2 antibodies. (e) Same as c, using anti-AMPK α1-IgG. (f) Same as c, using anti-Erk2 (p42 MAPK) antibodies. Data from three to five separate experiments, with 20–90 productively injected cells per condition. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for the effect of 30 vs. 3 mM glucose; §§, P < 0.01 for the effect of anti-AMPK antibodies.
Microinjection of Anti-α2 AMPK Antibodies Causes an Increase in L-PK Promoter Activity at Low Glucose Concentration.
The above experiments suggest that the effect of elevated glucose concentration on L-PK promoter activity may be due to the inhibition of AMPK activity. We therefore determined the effect of directly inhibiting AMPK activity by microinjecting anti-AMPK antibodies into cells then incubated at a low glucose concentration (3 mM). We first investigated the effect of nuclear microinjection of anti-α2 antibodies because this isoform of AMPK displayed significant nuclear localization (Fig. 1a). However, microinjection of the antibody solely into the nucleus was without effect on L-PK promoter activity (Fig. 3b). By contrast, separate injection of the antibody into both the nucleus and the cytosol provoked a powerful activation of L-PK promoter activity in cells incubated in 3 mM glucose to a level indistinguishable from that observed in cells incubated in 30 mM glucose (Fig. 3 a and c). Injection of this antibody into both compartments and subsequent incubation of cells at 30 mM glucose had no additive effect on the L-PK promoter compared with control (preimmune) IgG (Fig. 3c). Similarly, antibody to the anti-β2-subunit of AMPK (and capable of immunoprecipitating both α1 and α2 activities; data not shown) also enhanced L-PK promoter activity 3-fold at 3 mM glucose, but was without significant effect after incubation at 30 mM glucose (Fig. 3d). In contrast, anti-α1 antibody was without significant effect when injected into either the nucleus alone (not shown) or nucleus plus cytosol (Fig. 3e). As observed in previous studies (14), a truncated version of the L-PK promoter lacking the glucose response element (L4 box, plasmid pΔL4.LPK.LucFF) displayed only a small inhibitory effect of the sugar, and this was unaffected by AMPK-α2 antibody microinjection (not shown). Antibodies to extracellular stimulus-regulated kinase (Erk2)/p42 MAP kinase, which is activated by high glucose in beta and MIN6 cells (46, 47), were entirely without effect on the L-PK promoter at either low or high glucose concentrations (Fig. 3f).
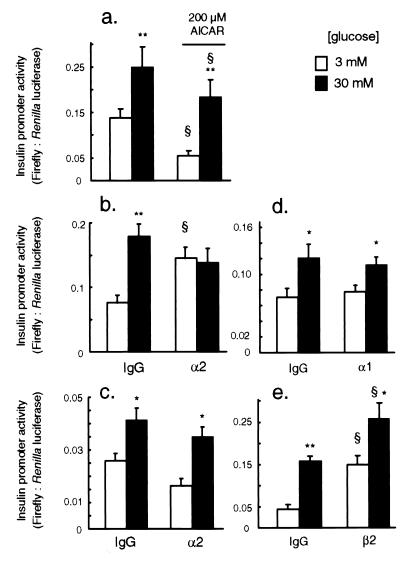
Response of PPI Promoter Activity to Glucose, AICAR, and Microinjection of AMPK Antibodies.
PPI promoter activity was enhanced 1.5- to 3-fold by 30 vs. 3 mM glucose (Fig. 4). Incubation with 200 μM AICAR diminished PPI promoter activity at either 3 or 30 mM glucose, but did not abolish the response to elevated concentrations of the sugar (Fig. 4a). However, microinjection of anti-AMPK α2 antibodies into the nucleus and cytosol provoked a robust stimulation of the activity of the reporter gene in cells maintained at 3 mM glucose, but was without effect in cells incubated at 30 mM glucose (Fig. 4b). By contrast, injection of antibodies to AMPK-α2 into the nucleus alone (Fig. 4c) or anti-AMPK-α1 into the nucleus and cytosol (Fig. 4d) was without effect at either [glucose]. Anti-AMPK-β2 antibodies, injected into the cytosol and nucleus, activated the insulin promoter more than 3-fold at 3 mM glucose and caused a smaller (62%) activation at 30 mM glucose (Fig. 4e).
Figure 4.
Effect of anti-AMPK antibody injection on regulation of the insulin promoter by glucose. Transcriptional analysis was performed as described in Fig. 3, after microinjection with the antibodies indicated. Effects of AICAR (no IgG injected) (a); microinjection of control IgG or anti-AMPK-α2 IgG into the cytosol plus nucleus (b); nucleus-only microinjection of anti-AMPK-α2 IgG (c); microinjection of control IgG, anti-AMPK-α1, or β2 IgG into cytosol plus nucleus, as shown (d and e). Data from three to five separate experiments, 32–105 productively injected cells per condition. *, P < 0.05; *, P < 0.01 for the effect of 30 vs. 3 mM glucose; §, P < 0.05 for the effect of AICAR.
Discussion
In this paper, we have sought direct evidence that inhibition of AMPK activity alone is sufficient to activate the transcription of L-PK and insulin genes in an islet-derived beta cell line and, therefore, could provide a molecular explanation of the activatory effect of glucose. A key strategy in this work has been the direct and acute inhibition of AMPK isoforms in single living cells, achieved through antibody microinjection. Importantly, this technique (i) allows the ready analysis of the behavior of the protein kinase in different subcellular compartments (nucleus and cytosol) and (ii) is unlikely to suffer from “rescue” phenomena, which may occur after the ablation of gene function (for example, by homologous recombination).
First, we demonstrate that AMPK activity is stably decreased in MIN6 cells incubated in elevated glucose concentrations. This presumably is caused by the activation of ATP synthesis (48) and consequent fall in AMP levels (36) as already shown in HIT-T15 and INS-1 cells (10). AMPK may be activated by AMP, through a number of mechanisms: (i) direct allosteric activation, (ii) exposing Thr172 to allow phosphorylation by the upstream kinase, AMPK kinase, (iii) decreased dephosphorylation by protein phosphatases, (iv) allosteric activation of AMPK kinase (36, 49–51). Whether the inhibition of AMPK activity by glucose is achieved purely through changes in the intracellular concentration of AMP or whether the direct activation of protein phosphatases also is involved is unclear. In the latter case it is possible that pentose phosphate pathway intermediates may play a role (28) because phosphatase 2A-like proteins are activated by xylulose 5-phosphate (52, 53). In addition, glucose might inhibit the homologous yeast SNF1 kinase complex via formation of a complex with Reg1p and Glc7p, the latter representing the catalytic subunit of protein phosphatase-1 (54).
Regulation of the L-PK Promoter by AMPK.
AICAR, which is taken up by cells and phosphorylated to the AMP analogue, ZMP (49), inhibits the glucose-dependent activation of various glycolytic and lipogenic genes in liver cells (25, 32, 33) and beta cells (this study). However, it remains unclear whether AICAR is absolutely specific for the activation of AMPK (49, 55). Furthermore, the use of AICAR does not provide any information on the relative importance of different AMPK isoforms. By contrast, microinjection of the AMPK antibodies is predicted to lead either to direct inhibition of catalytic activity of the complex, by steric blockage of substrate approach, or blockade of activatory phosphorylation by the upstream AMPK kinase. Alternatively, injection of antibodies may disturb a dynamic process of α2-subunit translocation through the nuclear membrane. Although single-cell assay of AMPKα2 activity was not possible, we consider one (or both) of the two latter mechanisms the likelier. Thus, reactivation of AMPK by purified AMPK kinase is strongly inhibited in vitro by anti-AMPK antibodies (S. Hawley and D.G.H., unpublished results), whereas none of the antibodies used inhibit the activity of AMPK vs. peptide substrates (D.G.H. and I.L., unpublished results). Unfortunately, direct examination of endogenous AMPKα2 distribution after antibody injection into single cells is not feasible.
Strikingly, inhibition through antibody microinjection of the more abundant AMPK α-isoform, α1, had no effect on activated L-PK gene expression. This is consistent with the full effects on L-PK promoter activity seen upon specific inhibition of the α2 isoform (Fig. 3c) and indicates that the α1 and α2 isoforms do not exhibit redundancy with respect to this function. These data contrast with the closely similar substrate specificities of the two AMPK isoforms when examined in vitro (39) and therefore might reflect differences in the subcellular localization/binding of the α1 and α2 AMPK complexes. In particular, an ability of the α2 complex to shuttle between the cytosol and the nucleus may be crucial for the effects of glucose.
Regulation of the Insulin Promoter by AMPK.
The results presented here suggest that, in common with the L-PK promoter, the insulin promoter is regulated through AMPK-dependent mechanisms. That the PPI promoter was still responsive to glucose even in the presence of AICAR may reflect the fact that AMPK activity is inhibited at 30 mM glucose even in the presence of this regulator (Fig. 1b). These data may suggest either (i) additional mechanisms of regulation of AMPK activity by glucose than changes in intracellular adenine nucleotides, (ii) distinct sensitivities of the L-PK and PPI promoters to an AMPK-dependent regulator, or (iii) AMPK-independent mechanisms of regulation of the PPI promoter.
Possible Targets of AMPK.
What may be the nature of the phosphorylated target(s) of AMPK α2? It would appear unlikely that phosphorylation of a known target of AMPK, such as acetyl-CoA carboxylase (56) or hydroxymethyl glutaryl-CoA reductase (7), is involved, because these are phosphorylated efficiently by the α1 isoform of AMPK. Alternative hypotheses therefore are either direct phosphorylation by AMPK of transcription factors or of downstream proteins, which subsequently alter the activity of these factors. A striking observation of the present studies is that inhibitory effects of the α2 antibody are dependent on their presence in the cytoplasm as well as the nucleus (note that injection into the cytoplasm alone was not feasible because of the requirement for plasmid injection into the cell nucleus). This indicates that either (i) inhibition of the phosphorylation of a cytosolic factor by AMPK α2 is critical for the activation of glucose-dependent nuclear events or (ii) AMPK α2 antibodies sequester this isoform, and possibly its target/substrate, as a ternary complex in the cytosol. In this way, nuclear translocation and promoter inactivation would be prevented. In this context it is of interest that both pancreatic duodenum homeobox protein (31, 57) and sterol response element-binding protein (58) are regulated in part through translocation into the nucleoplasm.
Conclusions.
These data indicate that AMPK is a vital regulator of glucose-stimulated gene expression in mammalian islet beta cells. Intriguingly, AMPK appears to have the opposite effect on gene expression in mammalian cells (inhibition) to that of SNF1 in Saccharomyces cerevisiae (activation). Thus, AMPK must inhibit a transcriptional activator (unlike SNF1, which inhibits a repressor, Mig1, in yeast) (8, 59) or activate a repressor. As a result, mutations in the AMPK α2 gene therefore may be important in the development of some forms of hereditary diabetes and could act both through alterations in insulin production (this work and ref. 10) and defective glucose uptake by muscle (35, 55, 60).
Acknowledgments
We thank Alan Leard and Dr. M. Jepson and the Medical Research Council (United Kingdom) Bristol Cell Imaging Facility for technical support. This work was supported by project grants to G.A.R. from the Wellcome Trust, the British Diabetes Association, and the Medical Research Council (United Kingdom), a Wellcome Trust Program grant and BBSRC studentship (I.P.S.) to D.G.H., and grants from the Institut National de la Santé et de la Recherche Médicale (France; to A.K.). I.L. is a Fellow of the Medical Research Council of Canada.
Abbreviations
- AMPK
AMP-activated protein kinase
- AICAR
5-amino-4-imidazolecarboxamide riboside
- L-PK
L-type pyruvate kinase
- PPI
pre-proinsulin
- SNF1
sucrose nonfermenting 1 protein kinase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Johnston M. Trends Genet. 1999;15:29–33. doi: 10.1016/s0168-9525(98)01637-0. [DOI] [PubMed] [Google Scholar]
- 2.Kahn A. C R Seances Soc Biol Fil. 1998;192:813–827. [PubMed] [Google Scholar]
- 3.Rencurel F, Girard J. Proc Nutr Soc. 1998;57:265–275. doi: 10.1079/pns19980041. [DOI] [PubMed] [Google Scholar]
- 4.Scott D K, O'Doherty R M, Stafford J M, Newgard C B, Granner D K. J Biol Chem. 1998;273:24145–24151. doi: 10.1074/jbc.273.37.24145. [DOI] [PubMed] [Google Scholar]
- 5.Docherty K, Clark A R. FASEB J. 1994;8:20–27. doi: 10.1096/fasebj.8.1.8299887. [DOI] [PubMed] [Google Scholar]
- 6.Carlson M. Curr Opin Microbiol. 1999;2:202–207. doi: 10.1016/S1369-5274(99)80035-6. [DOI] [PubMed] [Google Scholar]
- 7.Hardie D G, Carling D, Carlson M. Annu Rev Biochem. 1998;67:821–855. doi: 10.1146/annurev.biochem.67.1.821. [DOI] [PubMed] [Google Scholar]
- 8.Smith F C, Davies S P, Wilson W A, Carling D, Hardie D G. FEBS Lett. 1999;453:219–223. doi: 10.1016/s0014-5793(99)00725-5. [DOI] [PubMed] [Google Scholar]
- 9.Rutter, G. A., Palmer, G. & Tavare, J. M. (2000) News Physiol. Sci, in press. [DOI] [PubMed]
- 10.Salt I P, Johnson G, Ashcroft S J, Hardie D G. Biochem J. 1998;335:533–539. doi: 10.1042/bj3350533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marie S, Diaz-Guerra M-J, Miquerol L, Kahn A, Iynedjian P B. J Biol Chem. 1993;268:23881–23890. [PubMed] [Google Scholar]
- 12.Miquerol L, Lopez S, Cartier N, Tulliez M, Raymondjean M, Kahn A. J Biol Chem. 1994;269:8944–8951. [PubMed] [Google Scholar]
- 13.Vaulont S, Munnich A, Decaux J F, Kahn A. J Biol Chem. 1986;261:7621–7625. [PubMed] [Google Scholar]
- 14.Kennedy H J, Viollet B, Rafiq I, Kahn A, Rutter G A. J Biol Chem. 1997;272:20636–20640. doi: 10.1074/jbc.272.33.20636. [DOI] [PubMed] [Google Scholar]
- 15.Cuif M H, Porteu A, Kahn A, Vaulont S. J Biol Chem. 1993;266:13769–13772. [PubMed] [Google Scholar]
- 16.Decaux J F, Antoine B, Kahn A. J Biol Chem. 1989;264:11584–11590. [PubMed] [Google Scholar]
- 17.Bergot M-O, Diaz-Guerra M J, Puzenat N, Raymondjean M, Kahn A. Nucleic Acids Res. 1992;20:1871–1877. doi: 10.1093/nar/20.8.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaulont S, Puzenat N, Levrat F, Cognet M, Kahn A. J Mol Biol. 1989;209:205–219. doi: 10.1016/0022-2836(89)90273-8. [DOI] [PubMed] [Google Scholar]
- 19.Viollet B, Lefrancois-Martinez A M, Henrion A, Kahn A, Raymondjean M, Martinez A. J Biol Chem. 1996;271:1405–1415. doi: 10.1074/jbc.271.3.1405. [DOI] [PubMed] [Google Scholar]
- 20.Vallet V S, Henrion A A, Bucchini D, Casado M, Raymondjean M, Kahn A, Vaulont S. J Biol Chem. 1997;272:21944–21949. doi: 10.1074/jbc.272.35.21944. [DOI] [PubMed] [Google Scholar]
- 21.Lou D Q, Tannour M, Selig L, Thomas D, Kahn A, Vasseur-Cognet M. J Biol Chem. 1999;274:28385–28394. doi: 10.1074/jbc.274.40.28385. [DOI] [PubMed] [Google Scholar]
- 22.Viollet B, Kahn A, Raymondjean M. Mol Cell Biol. 1997;17:4208–4219. doi: 10.1128/mcb.17.8.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foretz M, Pacot C, Dugail I, Lemarchand P, Guichard C, LeLiepvre X, Berthelier-Lubrano C, Spiegelman B M, Kim J, Ferre P, et al. Mol Cell Biol. 1999;19:3760–3768. doi: 10.1128/mcb.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moriizumi S, Gourdon L, Lefrancois-Martinez A M, Kahn A, Raymondjean M. Gene Exp. 1998;7:103–113. [PMC free article] [PubMed] [Google Scholar]
- 25.Foretz M, Carling D, Guichard C, Ferre P, Foufelle F. J Biol Chem. 1998;273:14767–14771. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- 26.Doiron B, Cuif M H, Kahn A, DiazGuerra M J M. J Biol Chem. 1994;269:10213–10216. [PubMed] [Google Scholar]
- 27.Foufelle F, Gouhot B, Pegorier J-P, Perdereau D, Girard J, Ferre P. J Biol Chem. 1992;267:20543–20546. [PubMed] [Google Scholar]
- 28.Doiron B, Cuif M H, Chen R, Kahn A. J Biol Chem. 1996;271:5321–5324. doi: 10.1074/jbc.271.10.5321. [DOI] [PubMed] [Google Scholar]
- 29.German M, Ashcroft S, Docherty K, Edlund H, Edlund T, Goodison S, Imura H, Kennedy G, Madsen O, Melloul D, et al. Diabetes. 1995;44:1002–1004. doi: 10.2337/diab.44.8.1002. [DOI] [PubMed] [Google Scholar]
- 30.Ohlsson H, Karlsson K, Edlund T. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rafiq I, Kennedy H, Rutter G A. J Biol Chem. 1998;273:23241–23247. doi: 10.1074/jbc.273.36.23241. [DOI] [PubMed] [Google Scholar]
- 32.Leclerc I, Kahn A, Doiron B. FEBS Lett. 1998;431:180–184. doi: 10.1016/s0014-5793(98)00745-5. [DOI] [PubMed] [Google Scholar]
- 33.Foretz M, Carling D, Guichard C, Ferre P, Foufelle F. J Biol Chem. 1998;273:14767–14771. doi: 10.1074/jbc.273.24.14767. [DOI] [PubMed] [Google Scholar]
- 34.Hardie D G, Carling D. Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- 35.Winder W W, Hardie D G. Am J Physiol Endocrinol Met. 1999;40:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 36.Hardie D G, Salt I P, Hawley S A, Davies S P. Biochem J. 1999;338:717–722. [PMC free article] [PubMed] [Google Scholar]
- 37.Stapleton D, Mitchelhill K I, Gao G, Widmer J, Michell B J, Teh T, House C M, Fernandez C S, Cox T, Witters L A, et al. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- 38.Rutter G A, Kennedy H J, Wood C D, White M R H, Tavaré J M. Chem Biol. 1998;5:R285–R290. doi: 10.1016/s1074-5521(98)90287-3. [DOI] [PubMed] [Google Scholar]
- 39.Woods A, Salt I, Scott J, Hardie D G, Carling D. FEBS Lett. 1996;397:347–351. doi: 10.1016/s0014-5793(96)01209-4. [DOI] [PubMed] [Google Scholar]
- 40.Thornton C, Snowden M A, Carling D. J Biol Chem. 1998;273:12443–12450. doi: 10.1074/jbc.273.20.12443. [DOI] [PubMed] [Google Scholar]
- 41.Pouli A E, Kennedy H J, Schofield J G, Rutter G A. Biochem J. 1998;333:193–199. [Google Scholar]
- 42.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 43.Akli S, Chelly J, Lacorte J M, Poenaru L, Kahn A. Genomics. 1991;11:124–134. doi: 10.1016/0888-7543(91)90109-r. [DOI] [PubMed] [Google Scholar]
- 44.Salt I, Celler J W, Hawley S A, Prescott A, Woods A, Carling D, Hardie D G. Biochem J. 1998;334:177–187. doi: 10.1042/bj3340177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 46.Khoo S, Cobb M H. Proc Natl Acad Sci USA. 1997;94:5599–5604. doi: 10.1073/pnas.94.11.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frodin M, Sekine N, Roche E, Filloux C, Prentki M, Wollheim C B, Van Obberghen E. J Biol Chem. 1995;270:7882–7889. doi: 10.1074/jbc.270.14.7882. [DOI] [PubMed] [Google Scholar]
- 48.Kennedy H J, Pouli A E, Jouaville L S, Rizzuto R, Rutter G A. J Biol Chem. 1999;274:13281–13291. doi: 10.1074/jbc.274.19.13281. [DOI] [PubMed] [Google Scholar]
- 49.Corton J M, Gillespie J G, Hawley S A, Hardie D G. Eur J Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- 50.Davies S P, Helps N R, Cohen P T, Hardie D G. FEBS Lett. 1995;377:421–425. doi: 10.1016/0014-5793(95)01368-7. [DOI] [PubMed] [Google Scholar]
- 51.Hawley S A, Davison M, Woods A, Davies S P, Beri R K, Carling D, Hardie D G. J Biol Chem. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura M, Fedorov S, Uyeda K. J Biol Chem. 1994;269:26100–26106. [PubMed] [Google Scholar]
- 53.Nishimura M, Uyeda K. J Biol Chem. 1995;270:26341–26346. doi: 10.1074/jbc.270.44.26341. [DOI] [PubMed] [Google Scholar]
- 54.Ludin K, Jiang R, Carlson M. Proc Natl Acad Sci USA. 1998;95:6245–6250. doi: 10.1073/pnas.95.11.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merrill G F, Kurth E J, Hardie D G, Winder W W. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- 56.Prentki M, Tornheim K, Corkey B E. Diabetologia. 1997;40:S32–S41. doi: 10.1007/s001250051395. [DOI] [PubMed] [Google Scholar]
- 57.MacFarlane W M, McKinnon C M, Felton-Edkins Z A, Cragg H, James R L, Docherty K. J Biol Chem. 1999;274:1011–1016. doi: 10.1074/jbc.274.2.1011. [DOI] [PubMed] [Google Scholar]
- 58.Brown M S, Goldstein J L. Cell. 1997;89:331–340. doi: 10.1016/s0092-8674(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 59.DeVit M J, Waddle J A, Johnston M. Mol Biol Cell. 1997;8:1603–1618. doi: 10.1091/mbc.8.8.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Russell R R, Bergeron R, Shulman G I, Young L H. Am J Physiol. 1999;277:H643–H649. doi: 10.1152/ajpheart.1999.277.2.H643. [DOI] [PubMed] [Google Scholar]