Abstract
The propensity of isolates of the malaria parasite Plasmodium falciparum to delete a segment of chromosome 9 has provided positional information that has allowed us to identify a gene necessary for cytoadherence. It has been termed the cytoadherence-linked asexual gene (clag9). clag9 encodes at least nine exons and is expressed in blood stages. The hydrophobicity profile of the predicted CLAG9 protein identifies up to four transmembrane domains. We show here that targeted gene disruption of clag9 ablated cytoadherence to C32 melanoma cells and purified CD36. DNA-induced antibodies to the clag9 gene product reacted with a polypeptide of 220 kDa in the parental malaria clone but not in clones with a disrupted clag9 gene.
Of the human malaria parasites, Plasmodium falciparum is the most significant, being a major cause of morbidity and mortality in malaria-endemic areas. In addition, resistance to antimalarial drugs is widespread. Severe malaria is associated with the cytoadherence of infected red blood cells to the endothelial lining of capillaries and venules of various tissues, including the brain. Ligands on the surface of parasitized red cells can bind to a number of endothelial cell receptors, including CD36 (1), intercellular adhesion molecule-1 (2), thrombospondin (3), chondroitin-4-sulfate (4), vascular cell adhesion molecule-1 (5), E selectin (5), and platelet/endothelial cell adhesion molecule-1 (6). Therefore, intensive research has been aimed at understanding the mechanisms of cytoadherence, so that therapeutic agents blocking these interactions might eventually be designed (7, 8).
The importance of electron-dense structures (knobs) on the surface of the parasitized red blood cell to cytoadherence has long been recognized. A major constituent of knobs is the knob-associated histidine-rich protein (KAHRP) (9), localized under the red cell membrane. During in vitro culture, some lines of P. falciparum lose the ability to produce knobs (10), and they generally lose the ability to cytoadhere. This is a consequence of subtelomeric deletions of the region of chromosome 2 bearing the KAHRP gene (11). It is important to note, however, that there are exceptions. Clone B8, for example, can adhere to melanoma cells, although it is KAHRP-negative and knob-negative (12). Recently, a targeted recombinational knockout of the KAHRP gene has been used to demonstrate that KAHRP itself is essential for knobs and stable cytoadherence under physiological shear-stress levels (13).
P. falciparum erythrocyte membrane protein 1 (PfEMP1) is a variable molecule of approximately 250 kDa located on the surface of the parasitized red blood cell (14). PfEMP1 is now used as a collective term for any product of the multigene var family. It is clear that the parasite can undergo clonal antigenic variation by switching on the expression of different members of this set of about 50 polymorphic genes (15–17). Because switching can occur at up to 2% per generation in some instances (18), clonal parasite populations can express a mixture of PfEMP1 types even though only one (or at most a few) is expressed per cell. The PfEMP1 type expressed has an important role in determining the receptor specificity of the parasitized red blood cell (19–22).
Nevertheless, at least one more previously undefined gene product is also essential for cytoadherence. During in vitro cultivation, isolates of P. falciparum commonly undergo loss of cytoadherence, as measured by binding to C32 melanoma cells (10). We have associated this loss with subtelomeric deletions of chromosome 9 (23–25), where the independent deletion breakpoints are tightly clustered (26). Mixed parasite populations arise during propagation of clones because of such deletions. Binding these populations to melanoma cells resulted in the selection of parasites with the undeleted form of chromosome 9 in all lines tested (24, 27). We proposed that a gene essential for cytoadherence must be located in this region (24).
Clone ItG2 differs from a number of other parasite lines in that cytoadherence is stable over many generations (28), so it has been used to study cytoadherence in several laboratories. Studies (25) have shown that cytoadherent clones derived from ItG2 (e.g., B8) possess a chromosome 9 of intermediate size between that of cytoadherent isolate 1776 and its noncytoadherent derivative clone C10 (23). This is because of a deletion of intermediate size at the right end of the chromosome, as well as an internal deletion of about 15 kb that deletes an ORF at the site of the most common breakpoints in other isolates (26). The remaining segment of about 55 kb in ItG2 is colinear with its counterpart in the widely used stably cytoadherent clone of P. falciparum, 3D7; this segment, therefore, defines the cytoadherence locus on chromosome 9. Because no var genes are detectable in this region (26), it must contain a unique cytoadherence gene.
We describe here the identification of a gene from this locus that is required for cytoadherence to C32 melanoma cells and CD36. The gene has been named the cytoadherence-linked asexual gene (clag9).
Materials and Methods
Mapping and Sequencing.
Mapping of the cytoadherence locus by long PCR (29) and sequencing has been described elsewhere (30). The sequence of the spliced form has been deposited in the GenBank database (accession no. AF055476).
Construction of the Plasmid pAC4-Clag9.
Oligonucleotides Cso1E (tccaattgtagtgctaatg) and S2B (cggaacattcttctgatgtgg) were used to generate an incomplete copy of clag9 (bases 313–1,021, GenBank accession no. AF055476), containing neither the 5′ nor 3′ end of the gene (Fig. 1). This PCR product was blunt-end cloned into the unique XhoI restriction site of plasmid pTgD-TS.C5/H3 (31). Transformants were screened by using primers LAV2 (ggaaacagctatgaccatgattacgccaagct) and DCH42 (ggttaacaaagaagaagctcagag). These primers span the XhoI site of pTgD-TS.C5/H3. Clones in which the clag9 fragment was inserted were sequenced to confirm the orientation with respect to the drug resistance cassette. Only clones that had the clag9 insert in the same orientation as the drug resistance cassette were used. This vector was named pAC4-Clag9.
Figure 1.
(A) Structure of the region of the chromosomal copy of
clag9 used for production of the vector pAC4-Clag9.
Arrows indicate primer locations. (▧) Region of
clag9 gene incorporated into the vector pAC4-Clag9.
(
) Region of clag9 gene 5′ and 3′ to that
incorporated into the vector pAC4-Clag9. (B) Structure
of the knockout construct pAC4-CLAG. Large arrows indicate the plasmid
backbone; small arrows indicate primer locations. (▤)
P. falciparum calmodulin 5′ untranslated
region. ( )
Toxoplasma gondi dihydrofolate reductase, thymidylate
synthase, gene. (▥) P. falciparum
histidine-rich protein two 3′ untranslated region. (C)
PCRs testing for the presence of vector integrated into the chromosomal
copy of clag9. Lanes 1–5, 3D7 DNA; lanes 6–10,
clag9 transfectant DNA 19 weeks after transfection.
Lanes 1 and 6, primers for the KAHRP gene. Lanes 2 and 7, primers Cso1
and S2EB (test for uninterrupted clag9). Lanes 3 and 8,
primers Cso1 and DCH42 (integration test). Lanes 4 and 9, primers LAV2
and S2EB (integration test). Lanes 5 and 10, negative controls.
)
Toxoplasma gondi dihydrofolate reductase, thymidylate
synthase, gene. (▥) P. falciparum
histidine-rich protein two 3′ untranslated region. (C)
PCRs testing for the presence of vector integrated into the chromosomal
copy of clag9. Lanes 1–5, 3D7 DNA; lanes 6–10,
clag9 transfectant DNA 19 weeks after transfection.
Lanes 1 and 6, primers for the KAHRP gene. Lanes 2 and 7, primers Cso1
and S2EB (test for uninterrupted clag9). Lanes 3 and 8,
primers Cso1 and DCH42 (integration test). Lanes 4 and 9, primers LAV2
and S2EB (integration test). Lanes 5 and 10, negative controls.
Parasites.
P. falciparum clone 3D7, obtained from D. Walliker (University of Edinburgh, Edinburgh, U.K.), was cultured essentially as described (32). Ring-stage parasites were subjected to electroporation in the presence of 150 μg of plasmid pAC4-Clag9 DNA as described (31, 33). Parasites resistant to pyrimethamine were obtained 28 days later.
Antibody Production.
By using direct DNA injection into mice of constructs in vector pCtla4-hlgl (34), which targets expression products to lymphoid organs, we successfully generated antibodies to a fragment of CLAG9 (nucleotides 3,116–3,416; GenBank accession no. AF055476). Four individual mice were injected with 100 μg of purified DNA at each immunization, followed by two reimmunizations with 50 μg of the same segment expressed as a pGEX fusion protein in Escherichia coli.
Binding Assays.
Melanoma cell-binding assays were performed on the clag9 knockout line 11E and the parental 3D7 by using a published method (10).
Adhesion to purified CD36 was measured by using a modification of a published method (19). Triplicate spots of CD36 (0.5 μg/ml) were placed in plastic Petri dishes. Gelatin-enriched infected red blood cells were used at 20% parasitemia and 5% hematocrit in duplicate experiments. Results are expressed as the number of infected red blood cells bound per mm2 of surface area.
Northern Blotting.
Total RNA was extracted by using TRIzol (Life Technologies) from 10 ml of gelatin-synchronized trophozoites; 10-pellet volumes of prewarmed (37°C) TRIzol was added to the packed parasitized erythrocytes. The tube was gently shaken to remove clumps and incubated for 5 min at room temperature. Two-pellet volumes of chloroform were added and the tube agitated for 15 s and incubated for a further 3 min before being centrifuged at 1,200 × g for 30 min at 4°C. The aqueous phase consisting of approximately 0.6 of the original TRIzol volume was removed and precipitated with 0.7 volume of isopropanol, with a 2-h incubation on ice before being centrifuged at 7,500 × g for 30 min. The pellet was dried and then resuspended in molecular biology-grade formamide to approximately one-fifth the original erythrocyte pellet volume. The RNA concentration was determined on a spectrophotometer at 260 nm.
Approximately 10–20 μg of total RNA was heated to 65°C for 10 min and then separated by gel electrophoresis on a 1% TBE (Tris/borate/EDTA) gel containing 10 mM guanidine thiocyanate. The gel was then soaked in 50 mM NaOH for 30 min before transfer to Hybond N+ by dry blotting overnight. The blot was air dried. Prehybridization, hybridization, and washing were performed by using a MaxiNorthern Kit (Ambion, Austin, TX). The Northern blot was probed with a purified PCR product amplified from a pBluescript clone containing 3D7 clag9 cDNA corresponding to bases 313–1,021 (GenBank accession no. AF055476) by using M13 forward and reverse primers. This product was labeled with [α-32P]dATP produced by random priming according to the manufacturer's specifications (Giga Prime, Bresatec, Adelaide, Australia).
Western Blotting.
Gelatin-selected trophozoite-stage parasites (50 μl) were extracted on ice for 30 min with 2 ml of ice-cold TPP [PBS containing 1% Triton X-100 and protease inhibitors (Complete Mini; Boehringer Mannheim)]. The suspension was vortexed vigorously every 10 min. The solution was centrifuged at 7,500 × g for 10 min and the supernatant was discarded. The pellet was washed once in TPP. The pellet was then resuspended in 150 μl of 2% SDS/PBS and protease inhibitors. The solution was incubated for 45 min at room temperature with frequent vortexing and pipetting to solubilize the pellet. The solution was centrifuged for 15 min at 7,500 × g and the supernatant was stored at −70°C until used.
Triton X-100-insoluble/SDS-soluble fraction (20 μl) was added to 2 μl of 10× loading buffer and heated to 100°C for 10 min. This mixture was then fractionated under denaturing conditions on a 4–20% Tris-glycine polyacrylamide gradient gel. The fractionated protein was transferred to nitrocellulose by wet blotting and blocked overnight in 5% skim milk in Tris-buffered saline/Tween 20. Antisera to clag9, produced in mice as described (34), was used at a 1/2,000 dilution as the primary antibody. The secondary antibody was a horseradish peroxidase-labeled anti-mouse antibody (Transduction Laboratories, Lexington, KY) used at a 1/5,000 dilution.
Results
Identification of clag9.
Previously, we had cloned the 55-kb region at the right end of chromosome 9 in yeast artificial chromosomes and generated a detailed map. Segments of up to 16 kb have been amplified by using primers derived from sequence tag sites mapped to this region (30). These individual fragments now cover the entire 55-kb region containing the putative cytoadherence locus. We generated subclones from DraI and RsaI fragments of these long PCR products to facilitate sequencing. We have sequenced about half of the region so far, and the remainder will be sequenced at the Sanger Centre (Hinxton, U.K.) in the Malaria Genome Sequencing Project by using yeast artificial chromosome 1,039 (30) which spans this region.
The sequence revealed a prime candidate gene: It is located just distal to the common breakpoint and encodes at least nine exons within a region of at least 7 kb (35). The exons and introns were initially distinguished by the considerably higher AT content of the introns and the ORFs of the exons. These predictions were then tested by reverse transcription–PCR primed by oligo dT from mRNA prepared from gelatin-purified trophozoite-stage parasites of clone 3D7. The PCR products amplified from this cDNA by using primers derived from two adjacent exons were of the sizes expected for the spliced products rather than the genomic size. We have confirmed this by sequencing the reverse transcription–PCR products across each splice junction found. We concluded on the basis of this and the data presented below that this gene on chromosome 9 is expressed in asexual stages of P. falciparum clone 3D7. The gene has therefore been named the cytoadherence-linked asexual gene of chromosome 9 (clag9).
In the GenBank nr database, no homolog with significant matching to clag9 was identified in any other organism. However, it has now become clear from sequence data determined at the Sanger Centre and The Institute for Genomic Research (Rockville, MD) in the Malaria Genome Sequencing Project that there are a limited number of related sequences on other chromosomes of P. falciparum. Although it is not yet clear how many of these are present, at least three genes with an identical exon–intron structure to clag9 have been found (35). One gene is located on chromosome 2 (clag2), and two genes are located on chromosome 3 (clag3.1 and clag3.2).
A hydrophobicity plot generated by toppred ii predicted several potential transmembrane domains in the protein CLAG9 (35). The prediction indicated with certainty that four of these domains would be localized in a membrane, presumably that of the red cell. However, other protein topography programs such as tmhmm are not entirely in agreement with this. The N-terminal one predicted by both the toppred ii and tmhmm programs, as a potential transmembrane region, is a putative signal sequence of 18 amino acids at the 5′ end of the gene, with a cleavage site between positions 18 and 19 (36). This signal sequence is also found in an identical position in all the other clag family genes for which sequence data are available.
Southern hybridization to HindIII fragments of DNA from a number of P. falciparum isolates and clones revealed two hybridizing fragments of 4 and 8.5 kb (expected because of the internal HindIII site). These fragments, which were present in isolates/clones with an intact chromosome 9 (CSL2 and 3D7) and in the cytoadherent ItG2 clone B8, did not vary in size between isolates/clones. The fragments were absent from those clones (D10, E12, C10, NF7, and 7G8) with large chromosome 9 deletions (data not shown). Pulsed-field electrophoresis confirmed that clag9 was located on chromosome 9 only (data not shown). Furthermore, clag9 could be amplified by PCR by using yeast artificial chromosomes 776 and 1,073 as templates (data not shown). Because these have been mapped on chromosome 9 in detail (30), there can be no doubt that clag9 is located in the deletable region of chromosome 9.
Targeted Gene Disruption of clag9.
Homologous recombination of the plasmid vector with the chromosomal copy of clag9 was initially detected by using a PCR test (Fig. 1). This test relied on PCR primer sequences in the chromosomal copy of clag9 that were outside, either 3′ or 5′ (S2EB and Cso1), to the segment of clag9 included in pAC4-Clag9, and vector-specific sequences (LAV2 and DCH42). Both of these primer sets generated DNA fragments of sizes consistent with integration but only after 18 weeks of culture. Because this culture contained both integrated and unintegrated versions of clag9, full-length clag9 could also still be detected by PCR (Fig. 1). Nonetheless, this line showed an 85% reduction in binding to melanoma cells.
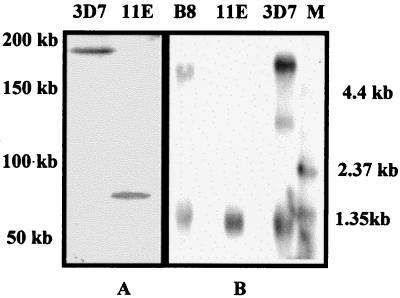
In addition, we have performed an independent knockout with a distinguishable insert that had additional clag9 sequence inserted 3′ to the drug-resistance cassette and obtained the same phenotype (data not shown). The change in phenotype clearly indicates that the integrants outgrew the nonintegrants in both cases, because the integrants were more stable during drug selection. This was confirmed when the culture was cloned by limiting dilution. The majority of the clones obtained were of the integrated genotype as determined by PCR analysis; two clones (11E and B1) were studied in depth. Both were free of nonintegrants. Southern blotting of a pulsed-field gel after BglI digestion demonstrated that the BglI site of the plasmid had in fact been introduced into clag9 as shown by the much smaller BglI fragment in Fig. 2A.
Figure 2.
(A) Southern blot of genomic DNA digested with BglI from 3D7 and knockout clone 11E hybridized with clag9 DNA 5′ to the sequence inserted into pAC 4-Clag9 after introduction of another BglI site and integration of vector into clag9 on chromosome 9. (B) Northern blot of 20 μg of total RNA of 3D7, the knockout clone, and ItG2 clone B8 hybridized sequentially with clag9 and MSP3 (34). “M” represents the marker lane. The higher band in 3D7 and B8 represents the clag9 signal, whereas the lower molecular weight band in each isolate represents the MSP3 signal.
Northern blotting revealed a single RNA species of about 5 kb in 3D7 that was not detectable in 11E (Fig. 2B), although the positive control (MSP3; ref. 37) hybridized with similar intensity to each RNA preparation (Fig. 2B). As expected, clag9 RNA was present in B8 (Fig. 2B) but not in chromosome 9 deletion clone C10 (not shown). Because the probe used in the Northern blot analysis corresponded to the segment inserted into the pAC4-Clag9 vector, it would have hybridized to mRNA made by either incomplete copy of clag9 produced by the targeted gene disruption if these were stable in the cells.
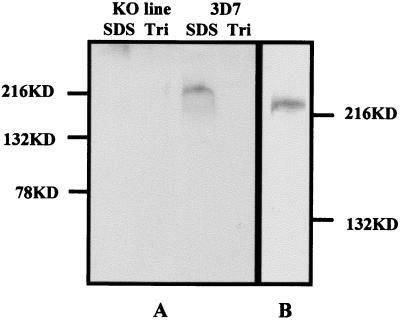
By using antibodies raised in mice, we applied immunoblotting to detect a protein with a molecular mass of ≈220 kDa, with a small smear presumably caused by degradation in 3D7. This protein was not detectable in 11E or the second knockout line (Fig. 3). This protein was present only in the SDS-soluble, Triton X-100-insoluble fraction, the same fraction as for PfEMP1.
Figure 3.
(A) Western blot of Triton-insoluble, SDS-soluble protein fraction of trophozoite-stage parasites from 3D7 and the knockout clone probed with antibodies raised in mice. SDS is the SDS-soluble fraction and Tri represents the Triton X-100-soluble fraction. (B) Western blot of a separate extraction of the Triton-insoluble, SDS-soluble protein fraction of 3D7 fractionated on a 6% Tris-glycine polyacrylamide gel and probed as in A.
11E and B1 showed only background levels of binding to melanoma cells (Table 1). Attempts to increase the level of binding of clone 11E to melanoma cells by up-selection four successive times failed and the time to grow to 1% parasitemia did not decrease during this process. It has been observed that parasites with chromosome 9 deletions cannot be up-selected for binding to melanoma cells (24, 27). This accords with loss of cytoadherence being unrelated to var switching. Clone 11E also shows a binding level to purified CD36 that is substantially lower than that of the parental line 3D7 (Table 1). Transmission electron microscopy revealed that all cells examined continued to express knobs. Reverse transcription–PCR also demonstrated that KAHRP mRNA was still expressed in 11E as expected from its knob-positive phenotype before cloning.
Table 1.
Binding and inhibition studies of the CLAG9 knockout clones 11E, B1, and the parental line 3D7
| Parasite line | No. of IRBC per
100 C32 cells
|
No. of IRBC per mm2 purified
CD36
|
||
|---|---|---|---|---|
| Exp. 1 | Exp. 2 | Exp. 1 | Exp. 2 | |
| 3D7 | 145 | 192 | 2,490 | 3,600 |
| 148 | 183 | 3,165 | 5,300 | |
| 11E | 8 | 20 | 49 | 93 |
| 12 | 15 | 51 | 197 | |
| B1 | 6 | 6 | ND | ND |
| 2 | 1 | ND | ND | |
ND, not determined; IRBC, infected red blood cells.
Discussion
Using positional information as an approach to the identification of genes of P. falciparum has not often been possible because only two experimental crosses have ever been performed (38, 39). However, positional information led to identification of the cytoadherence locus on chromosome 9 by virtue of subtelomeric deletions that ablated the ability of the parasitized red cells to bind to C32 melanoma cells (23–26). This has resulted in the identification of clag9 as a candidate gene because it lies within the region defined as the cytoadherence locus, it is expressed in blood stages, and its structure as a membrane protein credibly fits this role.
We have used the transfection system recently developed for P. falciparum (31, 33) to produce a targeted gene disruption of clag9 in the stably cytoadherent line 3D7and have shown that the resulting parasites do not bind to melanoma cells or CD36 and cannot be up-selected for binding to melanoma cells, as shown for all in vitro deletions of chromosome 9.
At least three classes of models for the action of CLAG9 can be considered. First, CLAG9 may be a component of the PfEMP1/KAHRP complex involved in cytoadherence. Second, it may be an entirely separate entity that itself mediates binding, or third, it may have a role in the regulation, transport, or chaperoning of PfEMP1. Preliminary immunofluorescence data with live parasites and two distinct polyclonal antisera (including one as described above) has indicated that CLAG9 may be located on the surface of parasitized erythrocytes. Anti-CLAG9 antibodies also appear to inhibit the binding of parasitized erythrocytes to melanoma cells, and unlike the chromosome 9 deletion lines (24) C10 and HB3, the knockout clone 11E retains its ability to agglutinate in the presence of hyperimmune sera. Nevertheless, the cellular location of CLAG9 must be determined unambiguously to start to distinguish between these models, and studies by immuno-electron microscopy and immunoprecipitation will facilitate this.
It is becoming increasingly clear that well established components such as PfEMP1 and KAHRP are only part of the cytoadherence picture. There are no data that exclude a role for a number of other genes. For example, the results of protease sensitivity studies were explained by postulating a protease-insensitive common ligand in addition to the protease-sensitive variable PfEMP1 (19). Furthermore, other distinct variable gene families in P. falciparum such as subtelomeric, variable open reading frames (STEVOR) (40) and repetitive interspersed family (RIF) (41) have been discovered. Additionally, there is considerable evidence that modification of the erythrocyte protein band three plays a role (42). Another candidate molecule with properties of a ligand has been termed Sequestrin (43), but targeted gene disruption of this failed to disrupt cytoadherence to CD36 (our unpublished results). Currently, no coherent model can accommodate all of these observations. The identification of clag9 as an essential gene contributing to cytoadherence to CD36 should facilitate clarification of this picture. The observation that chromosome 9 deletions, although unable to bind to CD36, can bind to endothelial cells (27) suggests that different members of the clag gene family (35) may have different specificities.
The properties of CLAG9 described here suggest that if it is indeed localized at the red cell surface, it may well be a candidate molecule for an “antidisease” rather than “antiparasite” vaccine.
Acknowledgments
We thank Sue Keyes and Chris Newbold for their advice on Northern blotting. This work was supported by generous donations from Mark Nicholson, Alice Hill, and The Tudor Foundation, in conjunction with grants from the Australian National Health and Medical Research Council, the Northern Territory Government, and the Howard Hughes Medical Institute.
Abbreviations
- clag
cytoadherence-linked asexual gene
- KAHRP
knob-associated histidine-rich protein
- PfEMP1
P. falciparum erythrocyte membrane protein 1
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF055476).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040561197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040561197
References
- 1.Barnwell J W, Asch A S, Nachman R L, Yamaya M, Aikawa M, Ingravallo P. J Clin Invest. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Nature (London) 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 3.Roberts D D, Sherwood J A, Spitalnik S L, Panton L J, Howard R J, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Nature (London) 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 4.Rogerson S J, Chaiyaroj S C, Ng K, Reeder J C, Brown G V. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ockenhouse C F, Tegoshi T, Maeno Y, Benjamin C, Ho M, Kan K E, Thway Y, Win K, Aikawa M, Lobb R R. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Treutiger C J, Heddini A, Fernandez V, Muller W A, Wahlgren M. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 7.Newbold C, Craig A G, Kyes S, Berendt A R, Snow R W, Peshu N, Marsh K. Ann Trop Med Parasitol. 1997;91:551–557. doi: 10.1080/00034989760923. [DOI] [PubMed] [Google Scholar]
- 8.Berendt A R, Craig A G. Nat Med. 1997;3:1315–1316. doi: 10.1038/nm1297-1315. [DOI] [PubMed] [Google Scholar]
- 9.Kilejian A. Proc Natl Acad Sci USA. 1979;76:4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Udeinya I J, Graves P M, Carter R, Aikawa M, Miller L H. Exp Parasitol. 1983;56:207–214. doi: 10.1016/0014-4894(83)90064-4. [DOI] [PubMed] [Google Scholar]
- 11.Corcoran L M, Forsyth K P, Bianco A E, Brown G V, Kemp D J. Cell. 1986;44:87–95. doi: 10.1016/0092-8674(86)90487-3. [DOI] [PubMed] [Google Scholar]
- 12.Biggs B A, Gooze L, Wycherley K, Wilkinson D, Boyd A W, Forsyth K P, Edelman L, Brown G V, Leech J H. J Exp Med. 1990;171:1883–1892. doi: 10.1084/jem.171.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crabb B S, Cooke B M, Reeder J C, Waller R F, Caruana S R, Davern K M, Wickham M E, Brown G V, Coppel R L, Cowman A F. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 14.Leech J H, Barnwell J W, Miller L H, Howard R J. J Exp Med. 1984;159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baruch D I, Pasloske B L, Singh H B, Bi X, Ma X C, Feldman M, Taraschi T F, Howard R J. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 16.Su X-Z, Heatwole V M, Wertheimer S P, Guinet F, Herrfeldt J A, Peterson D S, Ravetch J A, Wellems T E. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 17.Smith J D, Chitnis C E, Craig A G, Roberts D J, Hudson-Taylor D E, Peterson D S, Pinches R, Newbold C I, Miller L H. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roberts D J, Craig A G, Berendt A R, Pinches R, Nash G, Marsh K, Newbold C I. Nature (London) 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gardner J P, Pinches R A, Roberts D J, Newbold C I. Proc Natl Acad Sci USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith J D, Kyes S, Craig A G, Fagan T, Hudson-Taylor D, Miller L H, Baruch D I, Newbold C I. Mol Biochem Parasitol. 1998;97:133–148. doi: 10.1016/s0166-6851(98)00145-5. [DOI] [PubMed] [Google Scholar]
- 21.Baruch D I, Ma X C, Singh H B, Bi X, Pasloske B L, Howard R J. Blood. 1997;90:3766–3775. [PubMed] [Google Scholar]
- 22.Baruch D I, Gormely J A, Ma C, Howard R J, Pasloske B L. Proc Natl Acad Sci USA. 1996;93:3497–3502. doi: 10.1073/pnas.93.8.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirley M W, Biggs B A, Forsyth K P, Brown H J, Thompson J K, Brown G V, Kemp D J. Mol Biochem Parasitol. 1990;40:137–146. doi: 10.1016/0166-6851(90)90087-3. [DOI] [PubMed] [Google Scholar]
- 24.Day K P, Karamalis F, Thompson J, Barnes D A, Peterson C, Brown H, Brown G V, Kemp D J. Proc Natl Acad Sci USA. 1993;90:8292–8296. doi: 10.1073/pnas.90.17.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barnes D A, Thompson J, Triglia T, Day K, Kemp D J. Mol Biochem Parasitol. 1994;66:21–29. doi: 10.1016/0166-6851(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 26.Bourke P F, Holt D C, Sutherland C J, Kemp D J. Mol Biochem Parasitol. 1996;12:25–36. doi: 10.1016/0166-6851(96)02715-6. [DOI] [PubMed] [Google Scholar]
- 27.Chaiyaroj S C, Coppel R L, Magowan C, Brown G V. Mol Biochem Parasitol. 1994;67:21–30. doi: 10.1016/0166-6851(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 28.Graves P M, Carter R, Keystone J S, Seeley D C. Am J Trop Med Hyg. 1984;33:212–219. doi: 10.4269/ajtmh.1984.33.212. [DOI] [PubMed] [Google Scholar]
- 29.Barnes W M. Proc Natl Acad Sci USA. 1994;91:2216–2220. doi: 10.1073/pnas.91.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holt D C, Bourke P F, Mayo M, Kemp D J. Mol Biochem Parasitol. 1998;97:229–233. doi: 10.1016/s0166-6851(98)00123-6. [DOI] [PubMed] [Google Scholar]
- 31.Crabb B S, Cowman A F. Proc Natl Acad Sci USA. 1996;93:7289–7294. doi: 10.1073/pnas.93.14.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trager W, Jensen J B. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 33.Wu Y, Kirkman L A, Wellems T E. Proc Natl Acad Sci USA. 1996;93:1130–1134. doi: 10.1073/pnas.93.3.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyle J S, Brady J L, Lew A M. Nature (London) 1998;392:408–410. doi: 10.1038/32932. [DOI] [PubMed] [Google Scholar]
- 35.Holt D C, Gardiner D L, Thomas E A, Mayo M, Bourke P F, Sutherland C J, Carter R, Myers G, Kemp D J, Trenholme K R. Int J Parasitol. 1999;29:939–944. doi: 10.1016/s0020-7519(99)00046-6. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 37.McColl D J, Silva A, Foley M, Kun J F, Favaloro J M, Thompson J K, Marshall V M, Coppel R L, Kemp D J, Anders R F. Mol Biochem Parasitol. 1994;68:53–67. doi: 10.1016/0166-6851(94)00149-9. [DOI] [PubMed] [Google Scholar]
- 38.Wellems T E, Panton L J, Gluzman I Y, doRosario V E, Gwadz R W, Walker-Jonah A, Krogstad D J. Nature (London) 1990;345:253–255. doi: 10.1038/345253a0. [DOI] [PubMed] [Google Scholar]
- 39.Walliker D, Quakyi I A, Wellems T E, McCutchan T F, Szarfman A, London T, Corcoran L M, Burkot T R, Carter R. Science. 1987;236:1661–1666. doi: 10.1126/science.3299700. [DOI] [PubMed] [Google Scholar]
- 40.Cheng Q, Lawrence G, Reed C, Stowers A, Ranford-Cartwright L, Creasey A, Carter R, Saul A. Am J Trop Med Hyg. 1997;57(4):495–500. doi: 10.4269/ajtmh.1997.57.495. [DOI] [PubMed] [Google Scholar]
- 41.Weber J L. Mol Biochem Parasitol. 1998;29:117–124. doi: 10.1016/0166-6851(88)90066-7. [DOI] [PubMed] [Google Scholar]
- 42.Winograd E, Sherman I W. J Cell Biol. 1989;108:23–30. doi: 10.1083/jcb.108.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ockenhouse C F, Klotz F W, Tandon N N, Jamieson G A. Proc Natl Acad Sci USA. 1991;88:3175–3179. doi: 10.1073/pnas.88.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]