Abstract
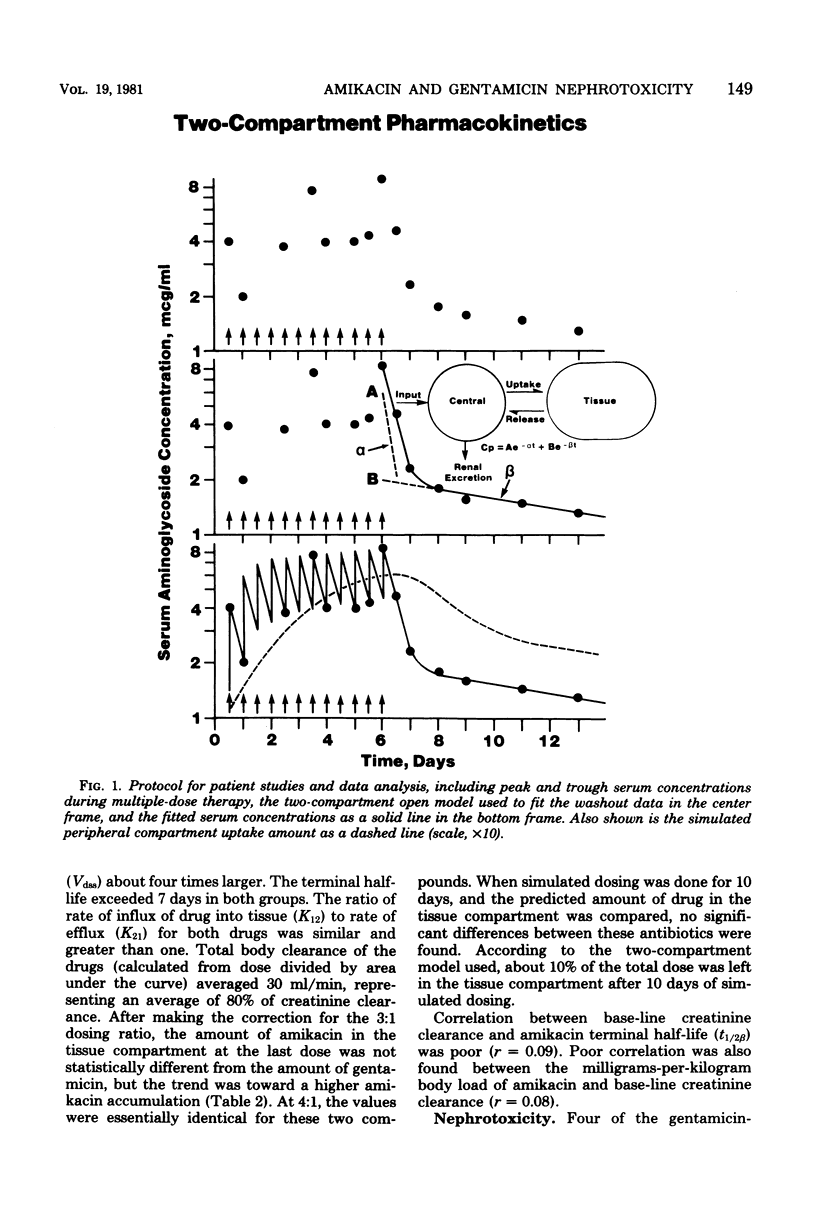
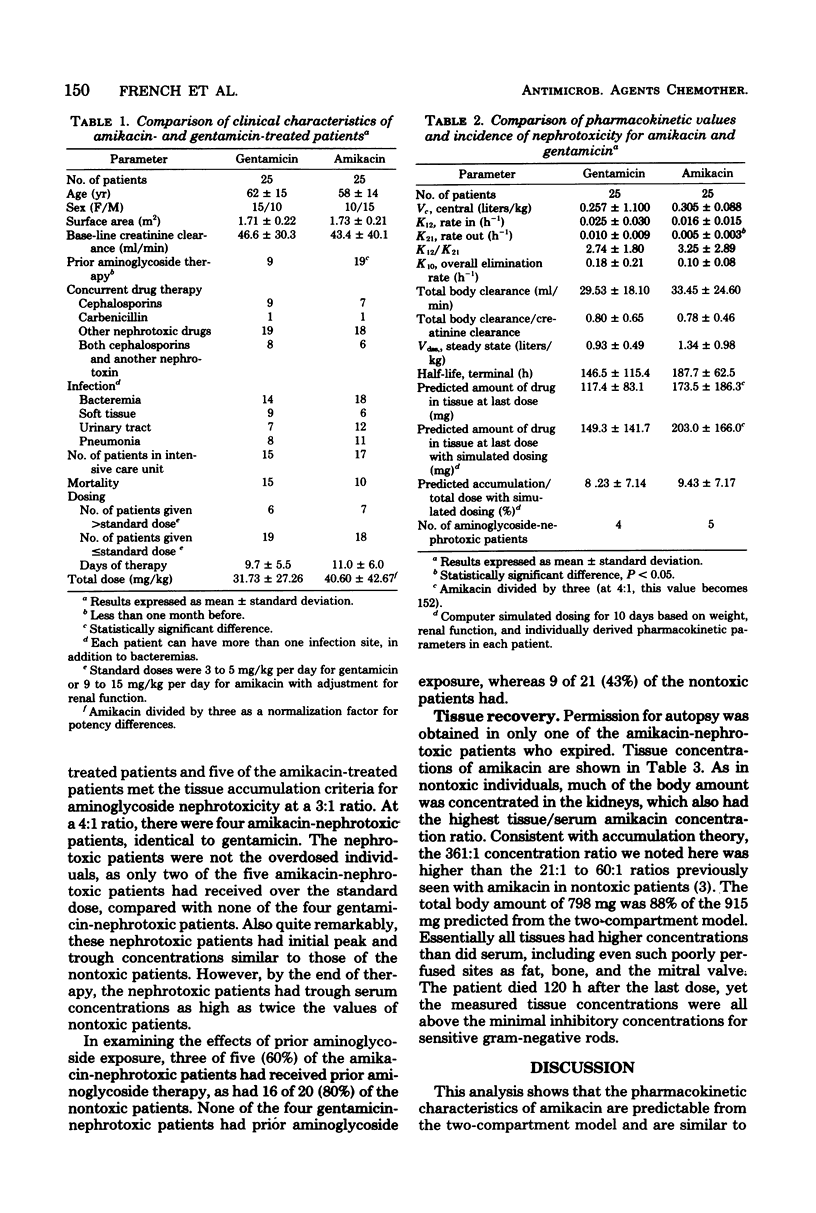
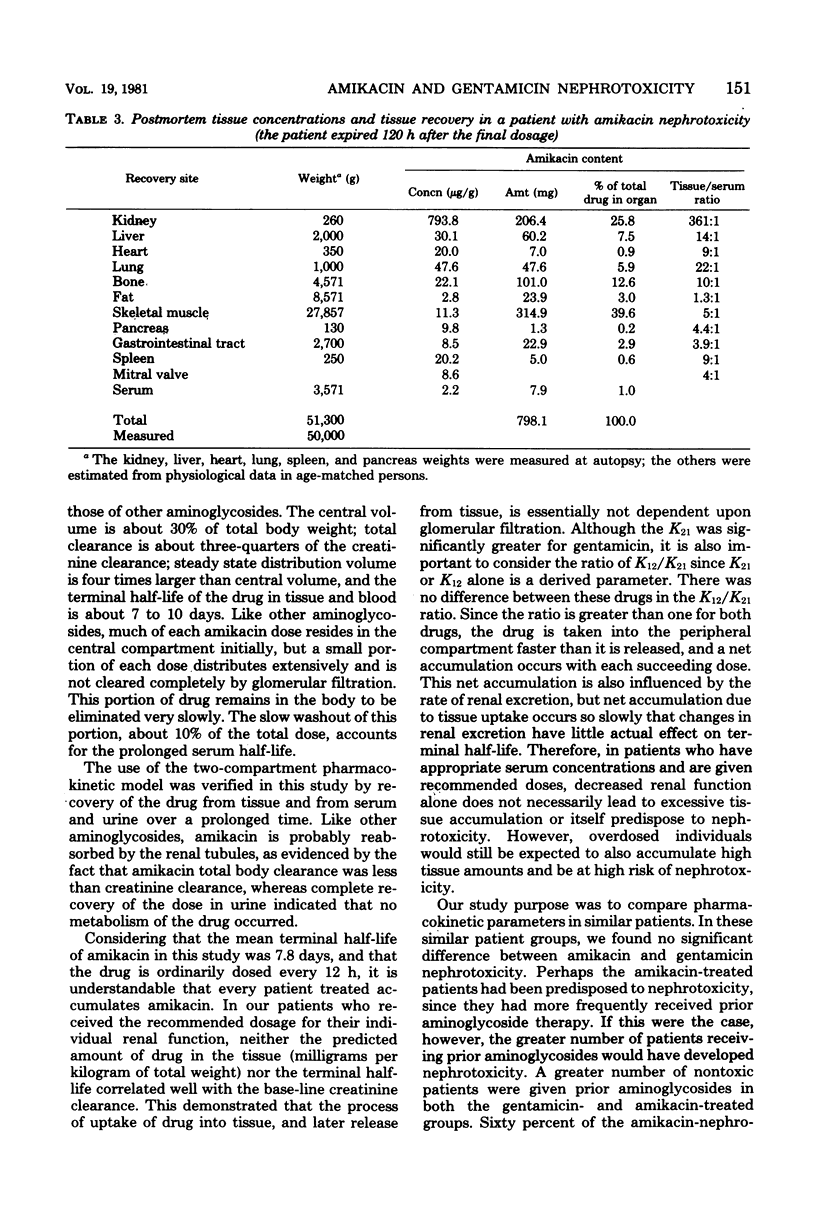
Twenty-five critically ill adults receiving blood level-adjusted doses of amikacin were prospectively studied with serum, urine, and, when possible, tissue amikacin concentrations. These data were fitted to a two-compartment pharmacokinetic model. Prolonged urine collections or postmortem tissues (or both) were used to confirm predicted tissue accumulation. Nephrotoxicity was also investigated. Patients were defined as having renal damage if they showed an increase in serum creatinine of greater than 0.5 mg/100 ml, an increase in urine beta 2-microglobulin of greater than 50 mg/day, and presence of urinary casts of greater than 500/ml. Renal damage was attributed to amikacin if there was, in addition to the above, tissue accumulation of amikacin of greater than 600 mg. These patients were matched with 25 patients treated with gentamicin during the same time period. There were no statistical differences between the gentamicin- and amikacin-treated patients in age, sex, weight, base-line creatinine clearance, concurrent cephalosporins or diuretics, treatment duration, site of infection, normalized (amikacin/gentamicin dosing ratio of 3:1) total dose, mortality, or tissue accumulation. More amikacin-treated patients (19 of 25) than gentamicin-treated patients (9 of 25) received prior aminoglycosides (P less than 0.01). The only pharmacokinetic parameter that differed between amikacin and gentamicin was a greater K21 for gentamicin. Nephrotoxicity was observed in 4 gentamicin was a greater K21 for gentamicin. Nephrotoxicity was observed in 4 gentamicin-treated patients (16%) and 5 amikacin-treated patients (20%). At a 3:1 dosing ratio, there were no significant differences between amikacin and gentamicin two-compartment pharmacokinetics and nephrotoxic potential in matched critically ill patients, but the trend of these data showed greater amikacin tissue accumulation. However, at an amikacin/gentamicin dosing ratio of 4:1, their tissue accumulation potential appeared to be almost identical.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke J. T., Libke R. D., Regamey C., Kirby W. M. Comparative pharmacokinetics of amikacin and kanamycin. Clin Pharmacol Ther. 1974 Jun;15(6):610–616. doi: 10.1002/cpt1974156610. [DOI] [PubMed] [Google Scholar]
- Dahlgren J. G., Anderson E. T., Hewitt W. L. Gentamicin blood levels: a guide to nephrotoxicity. Antimicrob Agents Chemother. 1975 Jul;8(1):58–62. doi: 10.1128/aac.8.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards C. Q., Smith C. R., Baughman K. L., Rogers J. F., Lietman P. S. Concentrations of gentamicin and amikacin in human kidneys. Antimicrob Agents Chemother. 1976 Jun;9(6):925–927. doi: 10.1128/aac.9.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlmeter G., Kamme G. Letter: Prolonged excretion of gentamicin in a patient with umimpaired renal function. Lancet. 1975 Feb 1;1(7901):286–286. doi: 10.1016/s0140-6736(75)91197-6. [DOI] [PubMed] [Google Scholar]
- Loirat P., Rohan J., Baillet A., Beaufils F., David R., Chapman A. Increased glomerular filtration rate in patients with major burns and its effect on the pharmacokinetics of tobramycin. N Engl J Med. 1978 Oct 26;299(17):915–919. doi: 10.1056/NEJM197810262991703. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Gengo F. M., Plaut M. E., Danner D., Mangione A., Jusko W. J. Urinary casts as an indicator of renal tubular damage in patients receiving aminoglycosides. Antimicrob Agents Chemother. 1979 Oct;16(4):468–474. doi: 10.1128/aac.16.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schentag J. J., Jusko W. J., Plaut M. E., Cumbo T. J., Vance J. W., Abrutyn E. Tissue persistence of gentamicin in man. JAMA. 1977 Jul 25;238(4):327–329. [PubMed] [Google Scholar]
- Schentag J. J., Jusko W. J., Vance J. W., Cumbo T. J., Abrutyn E., DeLattre M., Gerbracht L. M. Gentamicin disposition and tissue accumulation on multiple dosing. J Pharmacokinet Biopharm. 1977 Dec;5(6):559–577. doi: 10.1007/BF01059684. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Lasezkay G., Cumbo T. J., Plaut M. E., Jusko W. J. Accumulation pharmacokinetics of tobramycin. Antimicrob Agents Chemother. 1978 Apr;13(4):649–656. doi: 10.1128/aac.13.4.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schentag J. J., Lasezkay G., Plaut M. E., Jusko W. J., Cumbo T. J. Comparative tissue accumulation of gentamicin and tobramycin in patients. J Antimicrob Chemother. 1978 May;4 (Suppl A):23–30. doi: 10.1093/jac/4.suppl_a.23. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Sutfin T. A., Plaut M. E., Jusko W. J. Early detection of aminoglycoside nephrotoxicity with urinary beta-2-microglobulin. J Med. 1978;9(3):201–210. [PubMed] [Google Scholar]
- Smith C. R., Baughman K. L., Edwards C. Q., Rogers J. F., Lietman P. S. Controlled comparison of amikacin and gentamicin. N Engl J Med. 1977 Feb 17;296(7):349–353. doi: 10.1056/NEJM197702172960701. [DOI] [PubMed] [Google Scholar]
- Zaske D. E., Cipolle R. J., Strate R. J. Gentamicin dosage requirements: wide interpatient variations in 242 surgery patients with normal renal function. Surgery. 1980 Feb;87(2):164–169. [PubMed] [Google Scholar]


