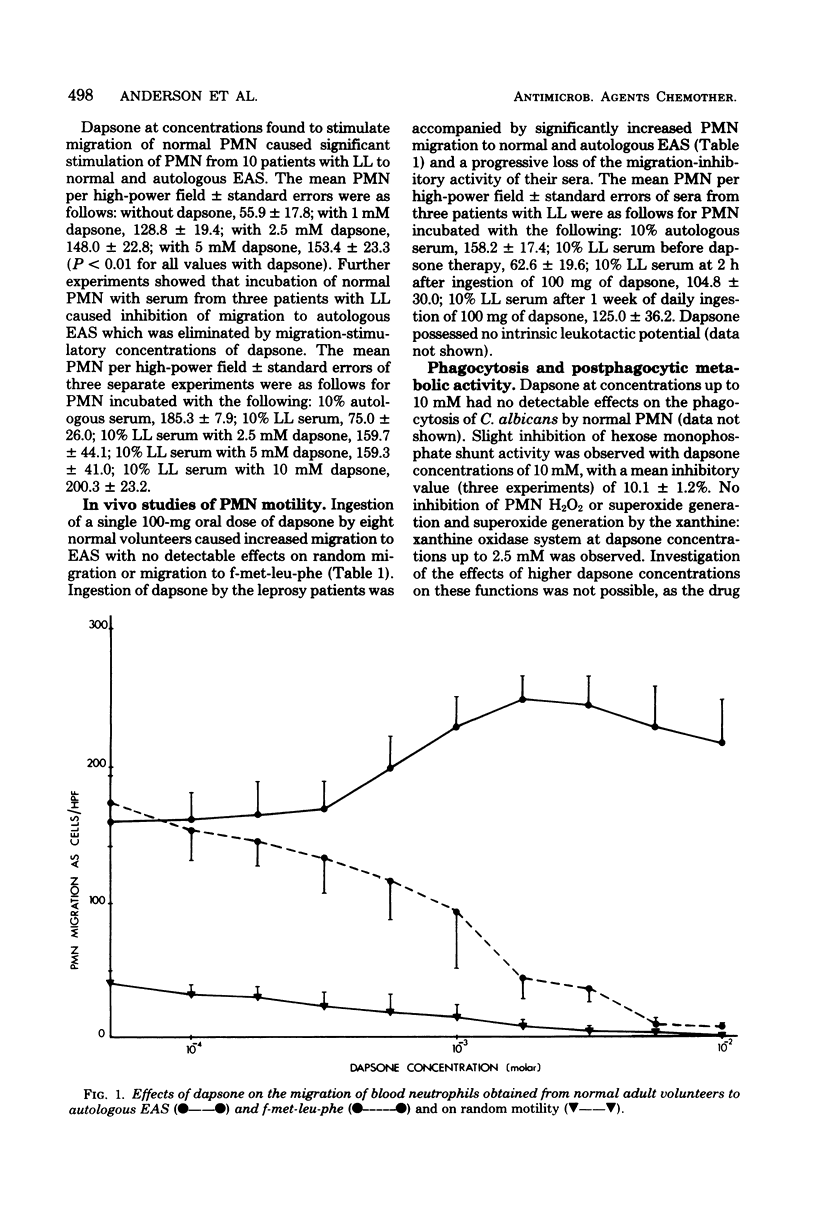
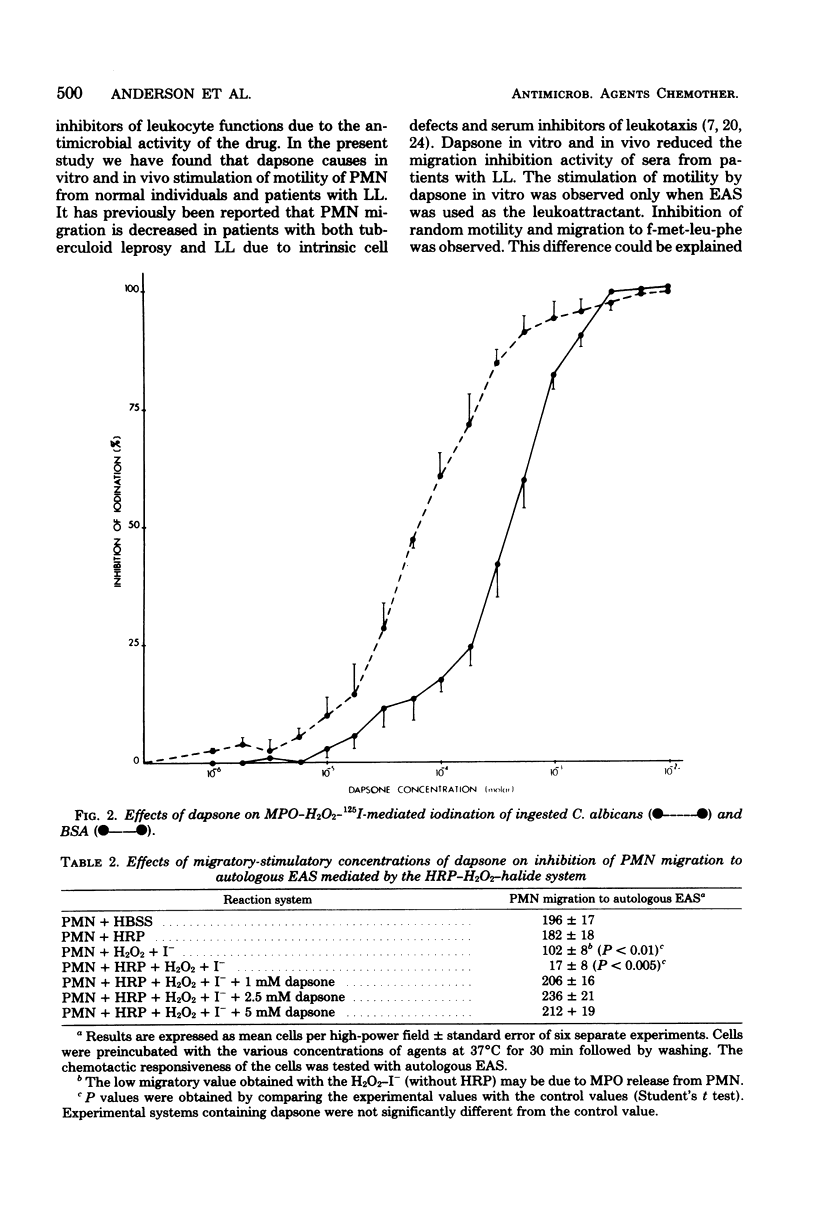
Abstract
The effects of dapsone on polymorphonuclear leukocyte functions and lymphocyte mitogen-induced transformation were assessed in vitro and in vivo in normal individuals and in newly diagnosed untreated patients with lepromatous leprosy. The effects of dapsone on the cell-free generation of superoxide by the xanthine: xanthine oxidase system and iodination of bovine serum albumin by horseradish peroxidase were also investigated. In normal individuals dapsone mediated stimulation of polymorphonuclear leukocyte migration in vitro and vivo. Dapsone had no effect on postphagocytic hexose monophosphate shunt activity in vivo. Similar effects were found in patients with lepromatous leprosy. Dapsone also decreased the inhibitory activity of serum from patients with lepromatous leprosy on normal polymorphonuclear leukocyte migration in vitro. Progressive loss of serum-mediated inhibition of migration was observed after ingestion of dapsone by the patients. Further experiments showed that stimulation of polymorphonuclear leukocyte motility was related to inhibition of lymphocyte transformation at high concentrations in vitro, but had slight stimulatory activity on phytohemagglutinin-induced transformation in controls and patients in vivo.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. O., Young E., McFadyen T., Fraser N. G., Duguid W. P., Meredith E. M. Absorption and excretion of 35S dapsone in dermatitis herpetiformis. Br J Dermatol. 1970 Dec;83(6):620–631. doi: 10.1111/j.1365-2133.1970.tb15756.x. [DOI] [PubMed] [Google Scholar]
- Anderson R., Gatner E. M., Imkamp F. M., Kok S. H. In vivo effects of propranolol on some cellular and humoral immune functions in a group of patients with lepromatous leprosy. Lepr Rev. 1980 Jun;51(2):137–148. doi: 10.5935/0305-7518.19800012. [DOI] [PubMed] [Google Scholar]
- Anderson R., Oosthuizen R., Theron A., Van Rensburg A. J. The in vitro evaluation of certain neutrophil and lymphocyte functions following the ingestion of 150 mg oral dose of levamisole: assessment of the extent and duration of stimulation of neutrophil chemotaxis, protein iodination and lymphocyte transformation. Clin Exp Immunol. 1979 Mar;35(3):478–483. [PMC free article] [PubMed] [Google Scholar]
- Anderson R., Theron A. Effects of ascorbate on leucocytes: Part I. Effects of ascorbate on neutrophil motility and intracellular cyclic nucleotide levels in vitro. S Afr Med J. 1979 Sep 1;56(10):394–400. [PubMed] [Google Scholar]
- Becker E. L., Showell H. J., Henson P. M., Hsu L. S. The ability of chemotactic factors to induce lysosomal enzyme release. I. The characteristics of the release, the importance of surfaces and the relation of enzyme release to chemotactic responsiveness. J Immunol. 1974 Jun;112(6):2047–2054. [PubMed] [Google Scholar]
- Becker E. L., Sigman M., Oliver J. M. Superoxide production induced in rabbit polymorphonuclear leukocytes by synthetic chemotactic peptides and A23187. Am J Pathol. 1979 Apr;95(1):81–97. [PMC free article] [PubMed] [Google Scholar]
- Bullock W. E., Jr, Ho M. F., Chen M. J. Quantitative and qualitative studies of the local cellular exudative response in leprosy. J Reticuloendothel Soc. 1974 Nov;16(5):259–268. [PubMed] [Google Scholar]
- CORNBLEET T. Sulfozone (diasone) sodium for dermatitis herpetiformis. AMA Arch Derm Syphilol. 1951 Dec;64(6):684–687. doi: 10.1001/archderm.1951.01570120019003. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1979 Oct;64(4):913–920. doi: 10.1172/JCI109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Biological defense mechanisms. The effect of bacteria and serum on superoxide production by granulocytes. J Clin Invest. 1974 Jun;53(6):1662–1672. doi: 10.1172/JCI107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIASH E., FROHWIRT N. Spectrum of horse-heart cytochrome c. Biochem J. 1959 Mar;71(3):570–572. doi: 10.1042/bj0710570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough J. L., Maren T. H. Inhibition of dihydropteroate synthetase from Escherichia coli by sulfones and sulfonamides. Antimicrob Agents Chemother. 1973 Jun;3(6):665–669. doi: 10.1128/aac.3.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall A. C. Dapsone. Clin Exp Dermatol. 1979 Jun;4(2):139–142. doi: 10.1111/j.1365-2230.1979.tb01608.x. [DOI] [PubMed] [Google Scholar]
- Paul B. B., Selvaraj R. J., Sbarra A. J. A sensitive assay method for peroxidases from various sources. J Reticuloendothel Soc. 1978 May;23(5):407–410. [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Root R. K., Metcalf J., Oshino N., Chance B. H2O2 release from human granulocytes during phagocytosis. I. Documentation, quantitation, and some regulating factors. J Clin Invest. 1975 May;55(5):945–955. doi: 10.1172/JCI108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Stossel T. P. Myeloperoxidase-mediated iodination by granulocytes. Intracellular site of operation and some regulating factors. J Clin Invest. 1974 May;53(5):1207–1215. doi: 10.1172/JCI107667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNEDDON I. B., WILKINSON D. S. Subcorneal pustular dermatosis. Br J Dermatol. 1956 Dec;68(12):385–394. doi: 10.1111/j.1365-2133.1956.tb12774.x. [DOI] [PubMed] [Google Scholar]
- Sher R., Anderson R., Glover A., Wadee A. A. Polymorphonuclear cell function in the various polar types of leprosy and erythema nodosum leprosum. Infect Immun. 1978 Sep;21(3):959–965. doi: 10.1128/iai.21.3.959-965.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Molin L., Dahlgren C. The inhibition of polymorphonuclear leukocyte cytotoxicity by dapsone. A possible mechanism in the treatment of dermatitis herpetiformis. J Clin Invest. 1978 Jul;62(1):214–220. doi: 10.1172/JCI109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D. M., Souhami R. Suppression of the arthus reaction in the guinea-pig by dapsone. Proc R Soc Med. 1975 May;68(5):273–273. [PMC free article] [PubMed] [Google Scholar]
- WINKELMANN R. K., DITTO W. B. CUTANEOUS AND VISCERAL SYNDROMES OF NECROTIZING OR "ALLERGIC" ANGIITIS: A STUDY OF 38 CASES. Medicine (Baltimore) 1964 Jan;43:59–89. doi: 10.1097/00005792-196401000-00003. [DOI] [PubMed] [Google Scholar]
- WOOD H. G., KATZ J., LANDAU B. R. ESTIMATION OF PATHWAYS OF CARBOHYDRATE METABOLISM. Biochem Z. 1963;338:809–847. [PubMed] [Google Scholar]
- Ward M., McManus J. P. Letter: Dapsone in Crohn's disease. Lancet. 1975 May 31;1(7918):1236–1237. doi: 10.1016/s0140-6736(75)92216-3. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Goralnick S., Bullock W. E. Defective leukotaxis in patients with lepromatous leprosy. J Lab Clin Med. 1976 Jun;87(6):1025–1032. [PubMed] [Google Scholar]
- Williams K., Capstick R. B., Lewis D. A., Best R. Anti-inflammatory actions of dapsone and its related biochemistry. J Pharm Pharmacol. 1976 Jul;28(7):555–558. doi: 10.1111/j.2042-7158.1976.tb02794.x. [DOI] [PubMed] [Google Scholar]


