Abstract
The activation of Janus kinases (JAKs) is crucial for propagation of the proliferative response initiated by many cytokines. The proliferation of various cell lines, particularly those of hematopoietic origin, is also modulated by mediators of oxidative stress such as nitric oxide and thiol redox reagents. Herein we demonstrate that nitric oxide and other thiol oxidants can inhibit the autokinase activity of rat JAK2 in vitro, presumably through oxidation of crucial dithiols to disulfides within JAK2. The reduced form of JAK2 is the most active form, and the oxidized JAK2 form is inactive. Nitric oxide pretreatment of quiescent Ba/F3 cells also inhibits the interleukin 3-triggered in vivo activation of JAK2, a phenomenon that correlates with inhibited proliferation. Furthermore, we observed that the autokinase activity of JAK3 responds in a similar fashion to thiol redox reagents in vitro and to nitric oxide donors in vivo. We suggest that the thiol redox regulation of JAKs may partially explain the generally immunosuppressive effects of nitric oxide and of other thiol oxidants.
The proliferation of lymphoid cells is controlled at multiple levels by both independent and interdependent regulatory mechanisms. External signals such as cytokines and antigens may normally initiate a proliferative response through binding to cell surface receptors, but the ultimate outcome of the transduced signal can be substantially modulated, or even abrogated, by prevalent intracellular conditions. In recent years, a considerable body of evidence has accumulated that demonstrates that the balance of the oxidative and reductive potentials within the cell (cellular redox state) can have profound consequences on signal transduction pathways (1). The proliferative response of lymphocytes to mitogens and cytokines is significantly impaired by thiol-oxidizing conditions (2); in many instances thiol oxidants can induce apoptosis (3, 4). Because some of the reactive oxygen species, such as hydrogen peroxide, that have the potential to act as thiol oxidants also have the potential to damage DNA, it is tempting to speculate that an evolutionary advantage of such oxidation-induced apoptosis would be to prevent the propagation of genetic infidelity. Thiol reducing agents, which by themselves do not have a mitogenic effect, typically exert a costimulatory effect on mitogen- and cytokine-stimulated proliferation (2, 5, 6). Nitric oxide (NO), which is produced in relatively large amounts by activated macrophages, also affects the ability of certain lymphoid cells to proliferate in response to cytokines (7–11). NO also has been implicated in both tumor-induced (12) and malarial immunosuppression (13).
Thiol redox regulation of lymphocyte proliferation may initially seem to be unrelated to the role of NO in immunosuppression, but in fact at least two common elements are shared by these two phenomena. (i) The first common element is the fundamental protein biochemistry. Thiol oxidants can oxidize vicinal dithiols (a pair of spatially proximal thiols) within proteins to the disulfide state, as can NO (14–17). (ii) Much of the literature describing both NO-mediated immunosuppression and thiol oxidant inhibition of proliferation describe the loss of lymphocyte proliferation, directly or indirectly, in response to cytokine stimulation (10, 11). The activation of Janus kinases (JAKs) is a crucial event in the propagation of cytokine-induced proliferative and differentiation signals (18). We therefore considered whether the thiol redox status of JAKs influenced the autokinase activity of the enzyme, which might thereby provide a mechanistic link between thiol redox biochemistry and physiology.
MATERIALS AND METHODS
Cell Culture.
Sf21 insect cells were maintained in IPL-41 medium supplemented with 10% heat-inactivated fetal bovine serum, 0.1% pluronic F-68, 50 units of penicillin per ml, and 50 μg of streptomycin per ml. Cells were infected at saturating multiplicities of infection with recombinant baculovirus expressing wild-type rat JAK2 (19), then harvested 120 hr after infection, and stored at −80°C.
The Ba/F3 cell line is a murine interleukin 3 (IL-3)-dependent pro-B cell line (20). Normal Ba/F3 cells or stably transfected interleukin 2 receptor β chain (IL-2Rβ)-expressing Ba/F3 cells (21) were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 5 mM Hepes (pH 7.3), 50 units of penicillin per ml, 50 μg of streptomycin per ml, and 5% WEHI-conditioned medium (as a source of IL-3).
The YT cell line is a human cell line that resembles natural killer cells (22). YT cells were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 5 mM Hepes (pH 7.3), 50 units of penicillin per ml, and 50 μg of streptomycin per ml.
Immunoprecipitation and Western Immunoblot Analysis.
Cell pellets were solubilized by resuspension and incubation in 1 ml of lysis buffer [1% Triton X-100/5 mM EDTA/50 mM NaCl/30 mM sodium pyrophosphate/50 mM sodium fluoride/0.2 mM sodium orthovanadate/1 mM phenylmethylsulfonyl fluoride/aprotinin (5 μg/ml)/pepstatin A (1 μg/ml)/leupeptin (2 μg/ml)/10 mM Tris⋅HCl, pH 7.6] for 1–2 hr at 4°C. Nuclei and insoluble material were removed by centrifugation for 10 min at 3,000 × g. Insect cell lysates, but not YT or Ba/F3 cell lysates, were preincubated with 0.1 vol of 10% protein A-Sepharose CL-4B (Pharmacia Biotech) for 1 hr at 4°C and centrifuged 15 min at 18,000 × g, and clarified supernatants were transferred to new vials for immunoprecipitation. Samples were immunoprecipitated by a 2-hr incubation at 4°C with rabbit anti-mouse JAK2 antiserum (Upstate Biotechnology, product 06–255; used in Ba/F3 and in baculoviral experiments), rabbit anti-mouse JAK3 antiserum (Upstate Biotechnology, product 06–342; used in Ba/F3 experiments), or rabbit anti-human JAK3 [directed against the peptide derived from amino acids 1104–1124 of human JAK3 (23); used in YT experiments], followed by a 30-min incubation at 4°C with 0.1 vol of protein A-Sepharose CL-4B. The centrifugal pellet was washed three times with 1 ml of lysis buffer, before SDS/PAGE and Western immunoblot analysis as described (19).
Autokinase Assays.
Immunoprecipitated enzymes were assayed for autokinase activity after redox pretreatment and after the removal of aliquots for anti-JAK2 and anti-phosphotyrosine analyses. As described (19), immunoprecipitates were resuspended with 100 μl of kinase mixture [50 mM NaCl/5 mM MgCl2/5 mM MnCl2/0.1 mM Na3VO4/carrier-free [γ-32P]ATP (250 μCi/ml; 1 Ci = 37 GBq)/10 mM Hepes, pH 7.4] and incubated 30 min at room temperature (21–22°C). One milliliter of lysis buffer was added to the sample, which was centrifuged 10 min, and the pellet was washed twice with 1 ml of lysis buffer before analysis by SDS/PAGE and autoradiography.
Proliferation Assays.
Quiescent Ba/F3 cells were preincubated in IL-3-deficient medium containing various concentrations of DEA-NO (or diethylamine plus NaNO2, as a control), recovered, then distributed into microtiter wells containing or lacking IL-3 at 0.1 μg/ml, and assayed for proliferation (24) by measuring [3H]thymidine incorporation 18 hr after stimulation.
Pretreatment of Intact Cells with a NO Donor.
Quiescent Ba/F3 cells were preincubated in IL-3-deficient medium with various concentrations of DEA-NO for 30 min at 37°C. Cells were recovered, stimulated with or without IL-3 at 1 μg/ml for 5 min at 37°C, and then analyzed for JAK content and JAK tyrosine phosphorylation status as described above.
α-Lipoic acid and dihydrolipoic acid used in certain experiments were provided by Asta Medica, Frankfurt/Main, Germany.
Direct Treatment of JAK2 Immunoprecipitates with NO.
Baculovirally produced immunoprecipitated JAK2 was preincubated with 10 mM DTT to maximize autophosphorylation activity, suspended in dialysis bags in a gas scrubbing bottle, and then exposed directly to NO gas as described (16). Dissolved oxygen was first depleted by sparging the buffer (150 mM NaCl/5 mM EDTA/20 mM Hepes, pH 7.4) with nitrogen gas, and then a control sample and a buffer aliquot were removed under nitrogen flow. NO gas was slowly bubbled through the buffer, and then samples and buffer aliquots were removed simultaneously under nitrogen flow. Additional NO gas was bubbled through the buffer, and subsequent samples and buffer aliquots were removed under nitrogen flow. Buffer aliquots were assayed (25) for nitrite content as an indicator of NO exposure, and JAK2 immunoprecipitates were divided into equal aliquots. The samples were incubated with or without 10 mM DTT and then assayed for autokinase activity as described above.
RESULTS
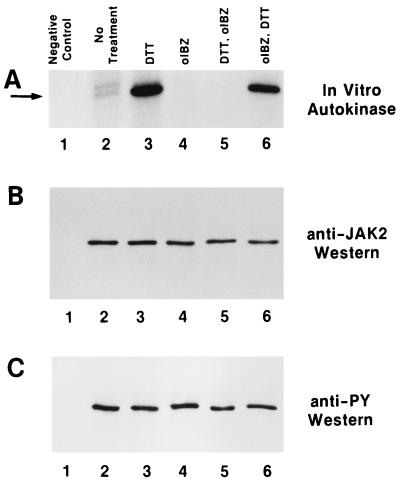
Pretreatment of baculovirally produced immunoprecipitated JAK2 (19) with DTT, which reduces disulfides to dithiols (26, 27), dramatically increased the autokinase activity of the enzyme (Fig. 1A, compare lanes 2 and 3). To verify that this phenomenon was related to the thiol redox status of the enzyme, JAK2 was pretreated with o-iodosobenzoate, which oxidizes vicinal dithiols to disulfides (28–30). Oxidative pretreatment of JAK2 resulted in the abolition of its autokinase activity (Fig. 1, compare lanes 2 and 4). Further, the activity of DTT-pretreated enzyme was abolished by subsequent treatment with o-iodosobenzoate (Fig. 1, lanes 3 and 5), although a high level of autokinase activity could be restored by DTT treatment of o-iodosobenzoate-pretreated JAK2 (Fig. 1, lanes 4 and 6). It is interesting to note that these treatments did not alter the pre-established phosphotyrosine status of the JAK2 (Fig. 1C), leading to the conclusion that although tyrosine phosphorylation of JAK2 may be a necessary prerequisite for enzymatic activity, it alone is not sufficient. The data presented in Fig. 1 are consistent with an interpretation that the maximally activated state of JAK2 requires critical cysteine residues to be present as reduced thiols, that JAK2 is inactive when these cysteines are oxidized to disulfides, and that these two states are reversible.
Figure 1.
Reversible modulation of JAK2 autokinase activity by thiol redox reagents. (A) Autokinase assays (19) of baculovirally produced immunoprecipitated rJAK2 with no additions (lane 2), 10 mM DTT pretreated (lane 3), 2.5 mM o-iodosobenzoate pretreated (lane 4), successive pretreatment with 10 mM DTT and then 2.5 mM o-iodosobenzoate (lane 5), and successive pretreatment with 2.5 mM o-iodosobenzoate and then 10 mM DTT (lane 6). Lane 1 is a negative control lacking recombinant rJAK2. The arrow points to the rJAK2 band. (B) Anti-JAK2 immunoblot of above samples before autokinase assay. (C) Anti-phosphotyrosine immunoblot of above samples before autokinase assay.
Although DTT and o-iodosobenzoate may be useful reagents to ascertain the biochemistry of active and inactive states of JAK2, they do not occur in the physiological setting of the cell. NO is a physiologically relevant reagent that can oxidize vicinal dithiols to disulfides (14). Of the nucleophilic centers available for nitrosylation in proteins, thiols are the most reactive (31), and S-nitrosothiols can rapidly form disulfides upon interaction with a neighboring thiol or thiolate anion (15). There is considerable debate as to whether S-nitrosothiol, the candidate intermediary structure in this oxidation process, can be formed from NO alone or whether it requires an intermediate product of the NO/O2 reaction (32), such as N2O3 (33). Other derivatives of NO such as peroxynitrite anion (34) also possess significant dithiol oxidizing potential. Because all such mechanistic scenarios implicate NO as a common thiol oxidant, we examined whether exposure of JAK2 to NO would result in a loss of autokinase activity (Fig. 2).
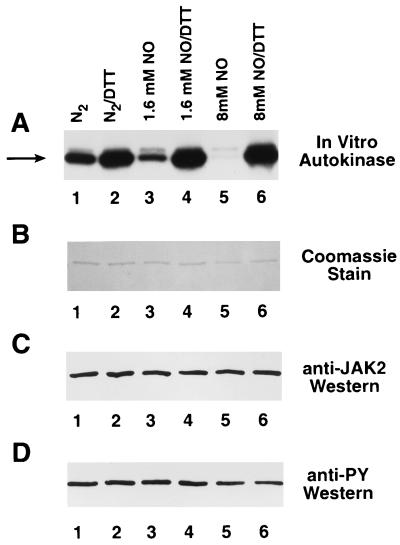
Figure 2.
DTT-reversible inhibition of JAK2 autokinase activity by NO. (A) Baculovirally produced immunoprecipitated JAK2 was preincubated with 10 mM DTT to maximize autophosphorylation activity, suspended in three dialysis bags in a gas scrubbing bottle, and then exposed directly to NO gas. A control sample (lanes 1 and 2) and two samples exposed to increasing amounts of NO (lanes 3 and 4 and lanes 5 and 6) were removed under nitrogen flow, as were corresponding buffer aliquots that were assayed (25) for nitrite content. The three sets of JAK2 immunoprecipitates were divided into equal aliquots. The samples were incubated with (lanes 2, 4, and 6) or without (lanes 1, 3, and 5) 10 mM DTT and then assayed for autokinase activity (19). The arrow points to JAK2. (B) Coomassie blue-stained gel of above samples after autokinase assay. (C) Anti-JAK2 Western of above samples before autokinase assay. (D) Anti-phosphotyrosine Western of above samples before autokinase assay.
Baculovirally produced immunoprecipitated JAK2 was exposed directly to NO gas as described (16) and then assayed for autokinase activity (Fig. 2). As the JAK2 was exposed to buffer equilibrated with increasing amounts of NO, the autokinase activity dropped significantly (Fig. 2A, lanes 1, 3, and 5). PhosphorImager (Molecular Dynamics Model SF) analysis of the intensity of these bands indicated that the autokinase activity decreased to approximately 49% and 8% of control as the nitrite content (equivalent to the initial NO content) of the buffer increased to 1.6 mM and 8 mM, respectively. More importantly, the autokinase activity could be completely restored by subsequent treatment of the samples with DTT (Fig. 2A, lanes 2, 4, and 6). The amount of JAK2 was approximately equal in all six samples (Fig. 2 B and C) and the phosphotyrosine content of the samples before autokinase assay (Fig. 2D) was the same regardless of pretreatment conditions. These results are consistent with a model in which NO oxidizes crucial vicinal dithiols within JAK2 to disulfides (14, 16), because the autokinase activity could be restored by retreatment with a well-defined sulfur reducing agent.
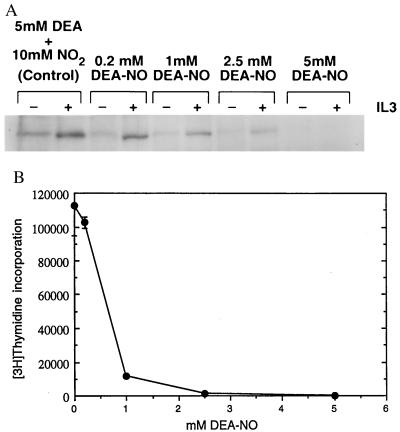
The demonstration that a thiol reductant (DTT) maximizes JAK2 activity and that either NO or another thiol oxidant (o-iodosobenzoate) inhibits JAK2 activity may begin to explain a number of observations regarding both immunosuppression and lymphocyte proliferation (2, 5, 7–9). To ascertain whether a correlation exists between the oxidative inhibition of JAK2 and proliferative response, we pretreated quiescent Ba/F3 cells with increasing concentrations of the NO donor DEA-NO (35). These cells were then stimulated with IL-3 and assayed for JAK2 tyrosine phosphorylation (Fig. 3A) and for proliferative response (Fig. 3B). As the amount of DEA-NO used in the pretreatment increased, the ability of IL-3 to activate JAK2 diminished, as demonstrated by the decreased level of tyrosine phosphorylation of the immunoprecipitated JAK2 (Fig. 3A). The decreased level of JAK2 tyrosine phosphorylation corresponded to a decrease in IL-3-stimulated proliferation as Ba/F3 cells were pretreated with increasing amounts of DEA-NO (Fig. 3B). Tyrosine phosphorylation of JAKs appears to be a necessary precondition for sustained catalytic competence; the catalytically competent JAKs described thus far have all been tyrosine-phosphorylated (18, 19, 37–40).
Figure 3.
Effect of DEA-NO pretreatment of Ba/F3 cells on IL-3 induction of both JAK2 tyrosine phosphorylation and proliferation. (A) Dose–response profile of DEA-NO effect on IL-3 stimulation of JAK2 tyrosine phosphorylation in Ba/F3 cells. Ba/F3 cells were made quiescent as described (36) and then preincubated in IL-3-deficient medium with 0.2 mM, 1 mM, 2.5 mM, or 5 mM DEA-NO (or 5 mM diethylamine plus 10 mM NaNO2 for the 0 control) for 30 min at 37°C. Cells were recovered, washed with IL-3-deficient medium, stimulated with (+) or without (−) 1 μg of IL-3 per ml for 5 min at 37°C, recovered, and lysed, and the JAK2 in the lysate was immunoprecipitated with anti-JAK2 and analyzed for phosphotyrosine content as described (36). (B) Dose–response profile of DEA-NO effect on IL-3 stimulation of Ba/F3 cell proliferation. Quiescent Ba/F3 cells were pretreated with various concentrations of DEA-NO and diluted to 106 cells per ml, and then 50-μl aliquots were distributed into microtiter wells containing or lacking 0.1 μg of IL-3 per ml and assayed for proliferation (24) by measuring [3H]thymidine incorporation 18 hr after stimulation.
Preliminary quantitative discrimination between the viable early apoptotic, and necrotic subpopulations of DEA-NO-pretreated IL-2Rβ-transfected Ba/F3 cells was obtained through fluorescence-activated cell sorting analysis done in parallel with proliferation assays (data not shown). Although their ability to proliferate in response to IL-3 had been significantly impaired, cells pretreated with 1 or 2 mM DEA-NO retained approximately 88% to 70%, respectively, of their original viability as judged by propidium iodide exclusion and a lack of surface-accessible annexin V. In contrast, the majority (approximately 90%) of cells pretreated with 5 mM DEA-NO were nonviable 18 hr after treatment whether they were incubated in the presence or absence of cytokine; such cells exhibited virtually no proliferative response to cytokine.
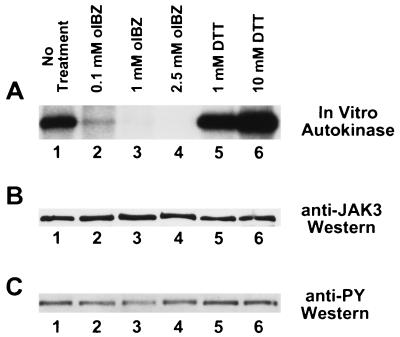
The high degree of structural similarity among the JAKs, particularly within the carboxyl-terminal JH1 catalytic domain, might lead one to expect that JAK2 and JAK3 respond to thiol redox reagents in a similar fashion. Moreover, most of the cellular responses involved in T cell proliferation should involve the activation of JAK3 rather than JAK2 (39, 40). We therefore examined the in vitro autokinase activity of human JAK3 immunoprecipitated from IL-2-stimulated YT cells and pretreated with 0.1–2.5 mM o-iodosobenzoate or with 1–10 mM DTT (Fig. 4). Significant inhibition of JAK3 autokinase activity was observed when the enzyme was pretreated with 0.1 mM o-iodosobenzoate, and the autokinase activity was essentially abolished when pretreated with higher concentrations of oxidant (Fig. 4A, lanes 1–4). Conversely, the JAK3 autokinase activity was increasingly stimulated when pretreated with increasing concentrations of DTT (Fig. 4A, lanes 1, 5, and 6). As was the case with JAK2 (Figs. 1 and 2), neither oxidative nor reductive treatments appeared to alter the pre-established phosphotyrosine status of JAK3 (Fig. 4C), emphasizing that tyrosine phosphorylation status may be a necessary, but insufficient, marker for JAK3 enzymatic activity.
Figure 4.
Modulation of JAK3 autokinase activity by thiol redox reagents. (A) Autokinase assays of immunoprecipitated human JAK3. YT cells were stimulated 10 min at 37°C with 100 nM IL-2 before lysis and immunoprecipitation with antiserum to human JAK3 (23). The washed JAK3 immunoprecipitate was incubated for 1 hr at 4°C with no additions (lane 1), 0.1 mM o-iodosobenzoate (lane 2), 1 mM o-iodosobenzoate (lane 3), 2.5 mM o-iodosobenzoate (lane 4), 1 mM DTT (lane 5), or 10 mM DTT (lane 6) before autokinase assay using the same conditions as in Figs. 1 and 2. (B) Anti-JAK3 immunoblot of above samples before autokinase assay. (C) Anti-phosphotyrosine immunoblot of above samples before autokinase assay.
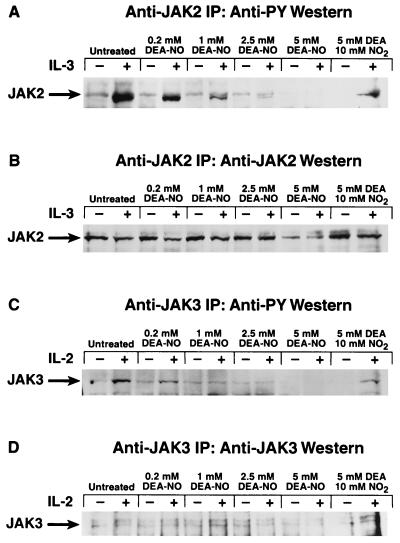
To determine whether the oxidative inhibition of JAK3 can occur in a physiological setting, stably transfected Ba/F3 cells that expressed the full-length IL-2Rβ chain (21) were made quiescent and then pretreated with increasing concentrations of DEA-NO. They then were stimulated with either IL-2 to induce tyrosine phosphorylation of JAK3 or with IL-3 to induce tyrosine phosphorylation of JAK2. As was the case with nontransfected Ba/F3 cells, DEA-NO pretreatment significantly inhibited the ability of IL-3 to induce JAK2 tyrosine phosphorylation (Fig. 5A). Pretreatment of the cells with DEA-NO at concentrations of 1 mM or higher blocked the induction of JAK3 tyrosine phosphorylation by IL-2 (Fig. 5C).
Figure 5.
Effect of DEA-NO pretreatment of IL-2Rβ-expressing Ba/F3 transfectants on cytokine induction of JAK tyrosine phosphorylation. (A) Dose–response profile of DEA-NO effect on IL-3 stimulation of JAK2 tyrosine phosphorylation in IL-2Rβ-expressing Ba/F3 transfectants. Stably transfected IL-2Rβ-expressing Ba/F3 cells (21) were made quiescent as described (36) and then preincubated in IL-3-deficient medium with 0.2 mM, 1 mM, 2.5 mM, or 5 mM DEA-NO (or 5 mM diethylamine plus 10 mM NaNO2 for the “0” control) for 30 min at 37°C. Cells were recovered, washed with IL-3-deficient medium, stimulated with (+) or without (−) 1 μg of IL-3 per ml for 5 min at 37°C, recovered, and lysed, and the JAK2 in the lysate was immunoprecipitated with anti-JAK2 and analyzed for phosphotyrosine content as described (36). (B) Anti-JAK2 immunoblot of above samples. (C) Dose–response profile of DEA-NO effect on IL-2 stimulation of JAK3 tyrosine phosphorylation in IL-2Rβ-expressing Ba/F3 transfectants. Experiment performed as in A, except that murine IL-2 was used to stimulate the cells and anti-JAK3 antibodies were used to immunoprecipitate the enzyme for phosphotyrosine analysis. (D) Anti-JAK3 immunoblot of above samples.
DISCUSSION
In this manuscript, we have presented direct evidence for a mechanism that modulates the catalytic activity of JAKs. The reduced forms of JAK2 and JAK3 are most active, and the oxidized forms of these enzymes are inactive, as judged by autokinase assays. The oxidizing and reducing pretreatments of purified enzymes dramatically affects catalytic activity without alteration of the pre-established phosphotyrosine status of either JAK2 or JAK3, emphasizing that although tyrosine phosphorylation of the JAKs may be a necessary prerequisite for catalytic competence, it alone is insufficient.
Our results suggest that the oxidative inhibition of JAKs may contribute to the overall mechanism whereby NO and thiol oxidants inhibit proliferation in many cell lines (2, 5, 7–9). Conversely, the enhancement of JAK activity by thiol reductants may help to explain the general requirement for thiol reductants in lymphocyte proliferation. Human peripheral blood mononuclear cells and phytohemagglutinin blasts did not proliferate in response to mitogens when cultured in thiol-free medium, and the proliferative response could be partially restored by medium supplementation with l-cysteine, 2-mercaptoethanol, or thioredoxin (2). Similarly, thiol reducing agents such as l-cysteine, 2-mercaptoethanol, and reduced glutathione are required for IL-2-induced proliferation of human natural killer cells (5) and of human peripheral blood lymphocytes (6). The oxidative inhibition of JAKs may explain how NO generated by activated macrophages suppresses the proliferation of splenic leukocytes (7–9), as well as provide a key molecular role for NO in tumor-induced immunosuppression (12) and in malarial immunosuppression (13).
The correlation between JAK’s thiol redox status and its activation state may help reconcile seemingly divergent physiological effects of thiol reductants. For example, thioredoxin expression is required for the interferon-γ-mediated growth arrest of HeLa cells (41), a signaling pathway that normally induces JAK2 and JAK1 activation (37). On the other hand, thioredoxin is highly expressed in the human T cell lymphotropic virus type I-transformed T cell lines (42), a condition that may act in synergy with the production of IL-15 (43) to promote the constitutive activation of JAK3 observed in such cell lines (44), which in turn can account for the IL-2-independent proliferation of T cell lines (45). Both of these observations are consistent with a model in which an internal thiol-reducing environment maximizes the signal propagation through JAKs, regardless of whether the outcome of that signal is growth-promoting or growth-arresting.
Although we have been able to readily reverse the oxidative inhibition of JAK autokinase activity in vitro simply by retreating the enzyme with a reducing dithiol, similar attempts to restore cytokine responsiveness to DEA-NO-pretreated cells by subsequent treatment with either monothiol (reduced glutathione, N-acetylcysteine, or 2-mercaptoethanol) or dithiol (DTT or dihydrolipoic acid) reducing agents did not meet with such success. This lack of ready reversibility reflects the delicate balance of distinct intracellular and extracellular redox environments required for successful cytokine-mediated signal transduction. The extracellular environment must be oxidizing to maintain the structural integrity of cytokines, such as IL-3 (46, 47), which exist in a structure composed of a core four-helical bundle constrained by interhelical disulfide bridges (48–62). Further, inducible trans-receptor disulfide bridges are crucial to the biological responsiveness of several cytokine receptors, such as the receptors for IL-3 (63), IL-6, leukemia inhibitory factor, and ciliary neurotrophic factor (64). In our attempts to reverse the in vivo consequences of oxidative stress, it appears that residual extracellular dithiols have impaired the ability of cytokines to initiate signal transduction, as confirmed by control experiments (data not shown).
When assessing the consequences of perturbation of the cytosolic redox state on signal transduction processes, one also must consider the potential contribution of many other enzymes and signal transducing proteins whose activities may be modulated by either NO (17) or thiol oxidants (1). A classic example of such a molecule is the nuclear transcription factor NF-κB, whose activation can be initiated by multiple stimuli, including oxidative stimuli and low levels of intracellular thiols (65–68). A limited list of redox-sensitive signaling molecules also includes ribonucleotide reductase (69, 70), which is partially inactivated by NO, Fos and Jun, whose DNA binding are enhanced by sulfhydryl reduction and inhibited by sulfhydryl oxidation (68, 71), and Ras (72), whose guanine nucleotide exchange activity is stimulated by either S-nitrosylation of Cys-118 (72) or by thiol oxidants (73). In contrast to the JAKs, kinases such as Lck (74) and Ltk (75) are activated when oxidized, and certain mitogen-activated protein kinases are activated by NO donor compounds (76). This differential response may be important in explaining why macrophage-generated NO has been reported to inhibit T cell proliferation without inhibiting T cell activation (10), as well as why NO donors inhibit proliferation of Th1-like (WEP 999), but not Th2-like (WEP 988) cells (11). Given that kinase-defective dominant negative JAK2 expression can block the erythropoietin-mediated inhibition of apoptosis (38), it is conceivable that the oxidative inhibition of JAKs also may be relevant to the role of thiol redox regulation in such processes as the activation of programmed cell death (3, 4) and disease progression in HIV-infected individuals (77).
Acknowledgments
We are gratefully indebted to Dr. J. E. Saavedra for the gift of DEA-NO, to Dr. O. M. Z. Howard for both the provision of Ba/F3 transfectants and for critical review of this manuscript, and to Dr. J. J. Oppenheim and Dr. S. K. Durum for review of this manuscript.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: JAK, Janus kinase; IL, interleukin; IL-2Rβ, interleukin 2 receptor β chain; DEA-NO, diethylamine/nitric oxide [sodium-l-(N,N-diethylamino)diazene-1,2-diolate].
References
- 1.Powis G, Gasdaska J R, Baker A. Adv Pharmacol. 1997;38:329–359. doi: 10.1016/s1054-3589(08)60990-4. [DOI] [PubMed] [Google Scholar]
- 2.Iwata S, Hori T, Sato N, Ueda-Taniguchi Y, Yamabe T, Nakamura H, Masutani H, Yodoi J. J Immunol. 1994;152:5633–5642. [PubMed] [Google Scholar]
- 3.Sato N, Iwata S, Nakamura K, Hori T, Mori K, Yodoi J. J Immunol. 1995;154:3194–3203. [PubMed] [Google Scholar]
- 4.Slater A F G, Stefan C, Nobel I, van den Dobbelsteen D J, Orrenius S. Toxicol Lett. 1995;82/83:149–153. doi: 10.1016/0378-4274(95)03474-9. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi A, Bloom E T. J Immunol. 1993;151:5535–5544. [PubMed] [Google Scholar]
- 6.Smyth M J. J Immunol. 1991;146:1921–1927. [PubMed] [Google Scholar]
- 7.Hibbs J B, Jr, Taintor R R, Vavrin Z, Rachlin E M. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- 8.Mills C D. J Immunol. 1991;146:2719–2723. [PubMed] [Google Scholar]
- 9.Albina J E, Abate J A, Henry W L., Jr J Immunol. 1991;147:144–148. [PubMed] [Google Scholar]
- 10.Upham J W, Strickland D H, Bilyk N, Robinson B W S, Holt P G. Immunology. 1995;84:142–147. [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor-Robinson A W, Liew F Y, Severn A, Xu D, McSorley S J, Garside P, Padron J, Phillips R S. Eur J Immunol. 1994;24:980–984. doi: 10.1002/eji.1830240430. [DOI] [PubMed] [Google Scholar]
- 12.Lejeune P, Lagadec P, Onier N, Pinard D, Ohshima H, Jeannin J-F. J Immunol. 1994;152:5077–5083. [PubMed] [Google Scholar]
- 13.Rockett K A, Awburn M M, Rockett E J, Cowden W B, Clark I A. Parasite Immunol. 1994;16:243–249. doi: 10.1111/j.1365-3024.1994.tb00346.x. [DOI] [PubMed] [Google Scholar]
- 14.Pryor W A, Church D F, Govindan C K, Crank G. J Org Chem. 1982;47:156–159. [Google Scholar]
- 15.Arnelle D R, Stamler J S. Arch Biochem Biophys. 1995;318:279–285. doi: 10.1006/abbi.1995.1231. [DOI] [PubMed] [Google Scholar]
- 16.Duhé R J, Nielsen M D, Dittman A H, Villacres E C, Choi E-J, Storm D R. J Biol Chem. 1994;269:7290–7296. [PubMed] [Google Scholar]
- 17.Stamler J S. Cell. 1994;78:931–936. doi: 10.1016/0092-8674(94)90269-0. [DOI] [PubMed] [Google Scholar]
- 18.Ihle J N. Nature (London) 1995;377:591–594. doi: 10.1038/377591a0. [DOI] [PubMed] [Google Scholar]
- 19.Duhé R J, Farrar W L. J Biol Chem. 1995;270:23084–23089. doi: 10.1074/jbc.270.39.23084. [DOI] [PubMed] [Google Scholar]
- 20.Palacios R, Steinmetz M. Cell. 1985;41:727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- 21.Howard O M Z, Kirken R A, Garcia G G, Hackett R H, Farrar W L. Biochem J. 1995;306:217–224. doi: 10.1042/bj3060217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yodoi J, Teshigawara K, Nikaido T, Fukui K, Noma T, Honjo T, Takigawa M, Sasaki M, Minato N, Tsudo M, Uchiyama T, Maeda M. J Immunol. 1985;134:1623–1630. [PubMed] [Google Scholar]
- 23.Malabarba M G, Rui H, Deutsch H H J, Chung J, Kalthoff F S, Farrar W L, Kirken R A. Biochem J. 1996;319:865–872. doi: 10.1042/bj3190865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills G B, Cragoe E J, Gelfand E W, Grinstein S. J Biol Chem. 1985;260:12500–12507. [PubMed] [Google Scholar]
- 25.Ding A H, Nathan C F, Stuehr D J. J Immunol. 1988;141:2407–2412. [PubMed] [Google Scholar]
- 26.Cleland W W. Biochemistry. 1964;3:480–483. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- 27.Konigsberg W. Methods Enzymol. 1972;25:185–188. doi: 10.1016/S0076-6879(72)25015-7. [DOI] [PubMed] [Google Scholar]
- 28.Hellerman L, Chinard F P, Ramsdell P A. J Am Chem Soc. 1941;63:2551–2553. [Google Scholar]
- 29.Chinard F P, Hellerman L. Methods Biochem Anal. 1954;1:1–26. doi: 10.1002/9780470110171.ch1. [DOI] [PubMed] [Google Scholar]
- 30.Cecil R, McPhee J R. Adv Protein Chem. 1959;14:255–389. doi: 10.1016/s0065-3233(08)60613-0. [DOI] [PubMed] [Google Scholar]
- 31.Simon D I, Mullins M E, Jia L, Gaston B, Singel D J, Stamler J S. Proc Natl Acad Sci USA. 1996;93:4736–4741. doi: 10.1073/pnas.93.10.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wink D A, Nims R W, Darbyshire J F, Christodoulou D, Hanbauer I, Cox G W, Laval F, Laval J, Cook J A, Krishna M C, DeGraff W G, Mitchell J B. Chem Res Toxicol. 1994;7:519–525. doi: 10.1021/tx00040a007. [DOI] [PubMed] [Google Scholar]
- 33.Kharitonov V G, Sundquist A R, Sharma V S. J Biol Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- 34.Radi R, Beckman J S, Bush K M, Freeman B A. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 35.Maragos C M, Morley D, Wink D A, Dunams T M, Saavedra J E, Hoffman A, Bove A A, Isaac L, Hrabie J A, Keefer L K. J Med Chem. 1991;34:3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 36.Evans G A, Goldsmith M A, Johnston J A, Xu W, Weiler S R, Erwin R, Howard O M Z, Abraham R T, O’Shea J J, Greene W C, Farrar W L. J Biol Chem. 1995;270:28858–28863. doi: 10.1074/jbc.270.48.28858. [DOI] [PubMed] [Google Scholar]
- 37.Watling D, Guschin D, Müller M, Silvennoinen O, Witthuhn B A, Quelle F W, Rogers N C, Schindler C, Stark G R, Ihle J N, Kerr I M. Nature (London) 1993;366:166–170. doi: 10.1038/366166a0. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang H, Niu Z, He T-C, Patel S V, Wojchowski D M. J Biol Chem. 1995;270:14500–14504. doi: 10.1074/jbc.270.24.14500. [DOI] [PubMed] [Google Scholar]
- 39.Johnston J A, Kawamura M, Kirken R A, Chen Y-Q, Blake T B, Shibuya K, Ortaldo J R, McVicar D W, O’Shea J J. Nature (London) 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- 40.Witthuhn B A, Silvennoinen O, Miura O, Lai K S, Cwik C, Liu E T, Ihle J N. Nature (London) 1994;370:153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- 41.Deiss L P, Kimchi A. Science. 1991;252:117–120. doi: 10.1126/science.1901424. [DOI] [PubMed] [Google Scholar]
- 42.Makino S, Masutani H, Maekawa N, Konishi I, Fuji S, Yamamoto R, Yodoi J. Immunology. 1992;76:578–583. [PMC free article] [PubMed] [Google Scholar]
- 43.Bamford R N, Battiata A P, Burton J D, Sharma H, Waldmann T A. Proc Natl Acad Sci USA. 1996;93:2897–2902. doi: 10.1073/pnas.93.7.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Migone T-S, Lin J-X, Cereseto A, Mulloy J C, O’Shea J J, Franchini G, Leonard W J. Science. 1995;269:79–81. doi: 10.1126/science.7604283. [DOI] [PubMed] [Google Scholar]
- 45.Miyazaki T, Kawahara A, Fujii H, Nakagawa Y, Minami Y, Liu Z-J, Oishi I, Silvennoinen O, Witthuhn B A, Ihle J N, Taniguchi T. Science. 1994;266:1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- 46.Feng Y, Klein B K, McWherter C A. J Mol Biol. 1996;259:524–541. doi: 10.1006/jmbi.1996.0337. [DOI] [PubMed] [Google Scholar]
- 47.Feng Y, Klein B K, Vu L, Aykent S, McWherter C A. Biochemistry. 1995;34:6540–6551. doi: 10.1021/bi00019a036. [DOI] [PubMed] [Google Scholar]
- 48.Abdel-Meguid S S, Shieh H-S, Smith W W, Dayringer H E, Violand B N, Bentle L A. Proc Natl Acad Sci USA. 1987;84:6434–6437. doi: 10.1073/pnas.84.18.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powers R, Garrett D S, March C J, Frieden E A, Gronenborn A M, Clore G M. Science. 1992;256:1673–1677. doi: 10.1126/science.256.5064.1673. [DOI] [PubMed] [Google Scholar]
- 50.Redfield C, Smith L J, Boyd J, Lawrence G M P, Edwards R G, Smith R A G, Dobson C M. Biochemistry. 1991;30:11029–11035. doi: 10.1021/bi00110a004. [DOI] [PubMed] [Google Scholar]
- 51.Mott H R, Driscoll P C, Boyd J, Cooke R M, Weir M P, Campbell I D. Biochemistry. 1992;31:7741–7744. doi: 10.1021/bi00148a040. [DOI] [PubMed] [Google Scholar]
- 52.McKay D B. Science. 1992;257:412–413. doi: 10.1126/science.257.5068.412. [DOI] [PubMed] [Google Scholar]
- 53.Somers W, Stahl M, Seehra J S. EMBO J. 1997;16:989–997. doi: 10.1093/emboj/16.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu G-Y, Yu H-A, Hong J, Stahl M, McDonagh T, Kay L E, Cumming D A. J Mol Biol. 1997;268:468–481. doi: 10.1006/jmbi.1997.0933. [DOI] [PubMed] [Google Scholar]
- 55.Robinson R C, Grey L M, Staunton D, Vankelecom H, Vernallis A B, Moreau J-F, Stuart D I, Heath J K, Jones E Y. Cell. 1994;77:1101–1116. doi: 10.1016/0092-8674(94)90449-9. [DOI] [PubMed] [Google Scholar]
- 56.Wlodaver A, Pavlovsky A, Gustchina A. FEBS Lett. 1992;309:59–64. doi: 10.1016/0014-5793(92)80739-4. [DOI] [PubMed] [Google Scholar]
- 57.Hill C P, Osslund T D, Eisenberg D. Proc Natl Acad Sci USA. 1993;90:5167–5171. doi: 10.1073/pnas.90.11.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang F, Basinski M B, Beals J M, Briggs S L, Churgay L M, Clawson D K, DiMarchi R D, Furman T C, Hale J E, Hsiung D P, Schoner B E, Smith D P, Zhang X Y, Wery J P, Schevitz R W. Nature (London) 1997;387:206–209. doi: 10.1038/387206a0. [DOI] [PubMed] [Google Scholar]
- 59.Kline A D, Becker G W, Churgay L M, Landen B E, Martin D K, Muth W L, Rathnachalam R, Richardson J M, Schoner B, Ulmer M, Hale J E. FEBS Lett. 1997;407:239–242. doi: 10.1016/s0014-5793(97)00353-0. [DOI] [PubMed] [Google Scholar]
- 60.Snouwaert J N, Leebeek F W G, Fowlkes D M. J Biol Chem. 1991;266:23097–23102. [PubMed] [Google Scholar]
- 61.Rock F L, Li X, Chong P, Ida N, Klein M. Biochemistry. 1994;33:5146–5154. doi: 10.1021/bi00183a018. [DOI] [PubMed] [Google Scholar]
- 62.Sprang S R, Bazan J F. Curr Opin Struct Biol. 1993;3:815–827. [Google Scholar]
- 63.Stomski F C, Sun Q, Bagley C J, Woodcock J, Goodall G, Andrews R K, Berndt M C, Lopez A F. Mol Cell Biol. 1996;16:3035–3046. doi: 10.1128/mcb.16.6.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davis S, Aldrich T H, Stahl N, Pan L, Taga T, Kishimoto T, Ip N Y, Yancopoulos G D. Science. 1993;260:1805–1808. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- 65.Staal F J T, Roederer M, Herzenberg L A, Herzenberg L A. Proc Natl Acad Sci USA. 1990;87:9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anderson M T, Staal F J T, Gitler C, Herzenberg L A, Herzenberg L A. Proc Natl Acad Sci USA. 1994;91:11527–11531. doi: 10.1073/pnas.91.24.11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Westendorp M O, Shatrov V A, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer P H, Dröge W, Lehmann V. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schenk H, Klein M, Erdbrügger W, Dröge W, Schulze-Osthoff K. Proc Natl Acad Sci USA. 1994;91:1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kwon N S, Stuehr D J, Nathan C F. J Exp Med. 1991;174:761–767. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lepoivre M, Fieschi F, Coves J, Thelander L, Fontecave M. Biochem Biophys Res Commun. 1991;179:442–448. doi: 10.1016/0006-291x(91)91390-x. [DOI] [PubMed] [Google Scholar]
- 71.Abate C, Patel L, Rauscher F J, III, Curran T. Science. 1990;249:1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- 72.Lander H M, Milbank A J, Tauras J M, Hajjar D P, Hempstead B L, Schwartz G D, Kraemer R T, Mirza U A, Chait B T, Burk S C, Quilliam L A. Nature (London) 1996;381:380–381. doi: 10.1038/381380a0. [DOI] [PubMed] [Google Scholar]
- 73.Lander H M, Ogiste J S, Teng K K, Novogrodsky A. J Biol Chem. 1995;270:21195–21198. doi: 10.1074/jbc.270.36.21195. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura K, Hori T, Sato N, Sugie K, Kawakami T, Yodoi J. Oncogene. 1993;8:3133–3139. [PubMed] [Google Scholar]
- 75.Bauskin A R, Alkalay I, Ben-Neriah Y. Cell. 1991;66:685–696. doi: 10.1016/0092-8674(91)90114-e. [DOI] [PubMed] [Google Scholar]
- 76.Lander H M, Jacovina A T, Davis R J, Tauras J M. J Biol Chem. 1996;271:19705–19709. doi: 10.1074/jbc.271.33.19705. [DOI] [PubMed] [Google Scholar]
- 77.Baruchel S, Wainberg M A. J Leukocyte Biol. 1992;52:111–114. doi: 10.1002/jlb.52.1.111. [DOI] [PubMed] [Google Scholar]